Во всем мире рак молочной железы (РМЖ) занимает лидирующее место в структуре онкологических заболеваний среди женской популяции. Доля РМЖ среди всех злокачественных образований в 2017 г. составила 11,5%, среди женского населения – 21,1%. За последние десятилетия в мире, в том числе и на территории Российской Федерации, отмечается тенденция к увеличению числа пациентов с РМЖ в возрастной группе 30–45 лет; в связи с чем для улучшения качества жизни пациенток необходимо сохранять молочную железу [1].
В результате выявления РМЖ на ранних стадиях, а также своевременного проведения неоадъювантной полихимиотерапии стало возможным выполнение органосохраняющих операций (ОСО) и онкопластических резекций, чтобы улучшить качество жизни данной категории больных [2, 3].
Успех выполнения ОСО заключается в удалении сектора молочной железы с опухолевым узлом, но обязательным условием является достижение отрицательных краев резекций. В литературе встречаются данные, подтверждающие, что хорошие отдаленные результаты можно получить и при выполнении ОСО в сочетании с лучевой терапией, в адъювантном режиме, по сравнению с радикальной мастэктомией [4–7].
Важнейшим критерием для выполнения ОСО и онкопластических резекций является исследование краев резекций. По последним данным известно, что для достижения чистоты краев резекций при протоковой карциноме in situ (DCIS) в настоящее время рекомендуют отступ до 2 мм, а при инвазивной форме – до 1 мм [8].
Несмотря на существующие данные о безопасности и преимуществах выполнения ОСО, от 20 до 30% пациентов с инвазивным или неинвазивным РМЖ обычно подвергаются повторным операциям [9–12].
Увеличение количества повторных резекций, тем более мастэктомий, приводит к тяжелой психологической и физической травме у женщин, что значительно ухудшает качество жизни. В связи с этим важен вопрос о выборе правильной интраоперационной маркировки краев резекций [13, 14].
В статье мы рассмотрим современные методы интраоперационного исследования краев резекций.
Известно, что патоморфологическое исследование является золотым стандартом в онкологии. Ткань железы обрабатывают, разрезают, окрашивают гематоксилином и эозином и исследуют под микроскопом. Этот метод является точным, но выполняется в течение нескольких дней после операции. После патоморфологического заключения при обнаружении положительных или тесных краев решается вопрос о повторной операции.
Срочное гистологическое исследование замороженных срезов является наиболее часто используемым методом оценки краев резекций при ОСО и считается стандартом медицинской помощи во многих клиниках Европы, США и России [15].
Выполнение данного исследования занимает от 20 до 30 минут, в течение которых пациентка находится под наркозом. Процедура представляет собой замораживание небольших кусочков ткани молочной железы, затем подготовку срезов, окрашивание и интерпретацию их под микроскопом. Технически, при некорректно проведенном заборе и подготовке материала, возможны погрешности, особенно при работе с жировой тканью; кроме того, замороженные срезы несколько менее эффективны для исследования DCIS и более крупных опухолей [16–18].
При правильно выполненном исследовании метод имеет чувствительность 83% и специфичность 95%. Анализы срочного гистологического и планового морфологического исследования, как правило, дают аналогичные заключения. К сожалению, выполнение данного исследования невозможно ввести в рутинное применение. Имеются технические сложности в его выполнении: отсутствие хорошо обученного персонала, неправильное взятие материала, длительность выполнения исследования и высокая стоимость данного метода [19].
Учитывая вышеизложенные недостатки срочного гистологического метода, срочное цитологическое исследование становится приоритетным для большинства клиник.
Метод срочного цитологического исследования основан на том, что хирург берет соскобы со всех сторон резецированной молочной железы (латеральной, медиальной, нижней, верхней, фасциальной), обычно от 4 до 6 краев. Этот метод имеет ту же точность и прогностическую ценность, что и гистологическое исследование, но занимает меньше времени (около 10–15, а не 30 минут). Однако быстрая, но точная интерпретация требует наличия специалиста, обученного правильному забору материала, так как непосредственно при цитологическом исследовании выявляется материал, в котором зачастую превалирует жировая ткань, которая делает материал неинформативным для оценки краев резекций [16].
Чувствительность метода 72% и специфичность 97%, но встречаются и ложные результаты, которые могут быть получены вследствие повреждения краев коагуляцией [19].
На протяжении последних 5 лет в различных клиниках США, Европы используются инструментальные методы интраоперационной оценки краев резекций, которые, по мнению многих авторов, могут стать альтернативой срочному гистологическому и цитологическому исследованиям. Это позволит снизить процент повторных операций у больных РМЖ и тем самым улучшит качество жизни пациенток. Ниже рассмотрим наиболее востребованные из них [20–23].
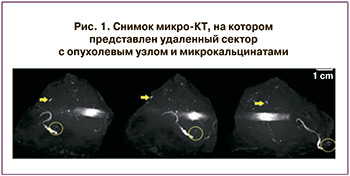 Mикрoкомпьютерная томография (микро-КT) (3D-объемы) является формой рентгенологической визуализации опухоли, имеет более высокое разрешение в масштабе микрометров. Это важно, поскольку позволяет выявить границы опухолевого узла в 3D-формате и четко показывает ход микрокальцинатов при их наличии (рис. 1). Благодаря способности микро-КТ создавать трехмерное изображение, возможно добиться визуализации микрокальцинатов в удаленном секторе [24].
Mикрoкомпьютерная томография (микро-КT) (3D-объемы) является формой рентгенологической визуализации опухоли, имеет более высокое разрешение в масштабе микрометров. Это важно, поскольку позволяет выявить границы опухолевого узла в 3D-формате и четко показывает ход микрокальцинатов при их наличии (рис. 1). Благодаря способности микро-КТ создавать трехмерное изображение, возможно добиться визуализации микрокальцинатов в удаленном секторе [24].
Метод микро-КТ проводят больным РМЖ, которым выполняют ОСО для определения ширины краев резекций в удаленном секторе молочной железы. Суть методики заключается в том, что рентгеновские снимки удаленного сектора выполняют под разными углами (как правило, 180 градусов), затем накладывают друг на друга для создания объемного трехмерного снимка (рис. 2) [24, 25]. В дальнейшем это 3D-изображение можно использовать для определения ширины краев резекций.
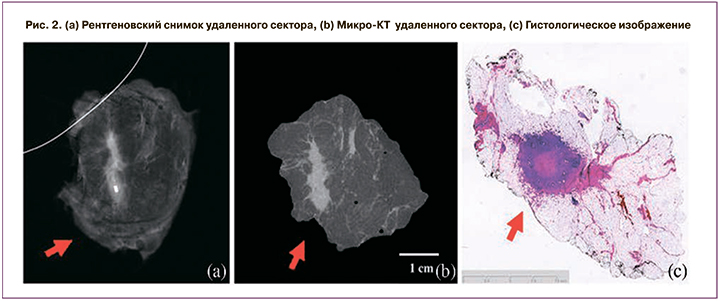
На маммографическом снимке (2, а) мы видим, что опухолевый узел находится далеко от края резекции. Второй снимок (2, б) – это микро-КТ, где изображены положительные края резекций. По данным морфологического исследования (2, c), опухолевый узел ближе к краю, что более схоже с данными среза микро-КТ, чем на 2-мерном рентгенографическом изображении. Преимущество метода заключается в том, что маммографическое изображение ограничено одним или двумя проекциями, в то время как микро-КТ является полностью объемной.
На рис. 3 представлены магнитно-резонансные томограммы (МРТ) молочной железы без патологии, а также при различных клинических состояниях и фото тех же участков под микроскопом.
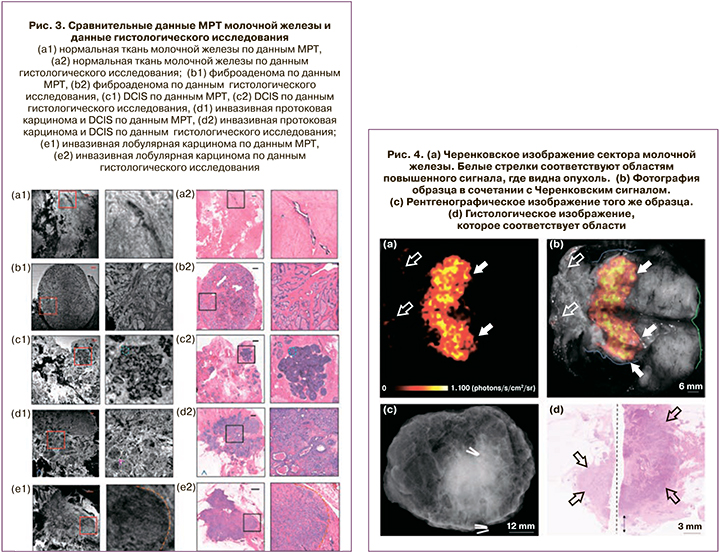
МРТ позволяет с наилучшим разрешением и полнотой объемного изображения показать точность локализации пораженных участков [26–29].
По данным современной литературы, использование МРТ с сильным магнитным полем (9,4 Тл), является более эффективным для определения краев резекции при интрадуктальной карциноме и менее эффективным – при DCIS [30].
Данный метод имеет ряд недостатков, таких как необходимость иметь материально-техническую базу и высококвалифицированного специалиста, высокую стоимость процедуры, длительность интраоперационного исследования. Разработка автономных МРТ-сканеров с высокой степенью защиты связана с меньшим риском облучения как пациенток, так и медицинского персонала, а также открывает возможности для рутинного интраоперационного использования данного метода [29].
Черенковская люминесцентная томография используется для планирования объемов хирургического вмешательства, особенно при ОСО (рис. 4). Метод представляет собой новый способ визуализации, аналогичный биолюминесцентной визуализации, который захватывает видимые фотоны, испускаемые черенковским излучением. В основном это оптическая визуализация радиотрецеров, которые испускают заряженные частицы, движущиеся быстрее, чем фазовая скорость света в этой конкретной среде [31–33].
Черенковские фотоны генерируют, когда заряженные частицы (например, позитроны, испускаемые ПЭТ-агентами) движутся со скоростью, превышающей скорость света в среде. Испускаемые черенковские фотоны могут быть обнаружены с помощью камер или технологий небольших детекторов. При этом используется 18F-фтордезоксиглюкоза (FDG) для интраоперационной оценки краев опухоли при операциях на молочной железе.
Эта технология является многообещающей, особенно с учетом того, что она обладает всеми преимуществами стандартной ПЭТ-визуализации при минимальной дополнительной работе. Это исследование было впервые опубликовано в декабре 2016 г., но данный метод находится на ранних стадиях клинического исследования [34].
Еще одна методика маркировки краев резекций на сегодняшний день – радиочастотная визуализация – Margin Probe, которая активно применяется в Израиле, США и в Германии [35–37].
 Принцип работы Margin Probe основан на регистрации взаимодействия живых тканей с электромагнитным полем (рис. 5). Сверхчувствительный детектор определяет малейшую разницу между биоэлектрическими свойствами здоровых и пораженных тканей, таким образом, выявляя проблемные участки. Для оценки полностью удаленного сектора требуется ~5 минут [38–40].
Принцип работы Margin Probe основан на регистрации взаимодействия живых тканей с электромагнитным полем (рис. 5). Сверхчувствительный детектор определяет малейшую разницу между биоэлектрическими свойствами здоровых и пораженных тканей, таким образом, выявляя проблемные участки. Для оценки полностью удаленного сектора требуется ~5 минут [38–40].
При сравнении 753 случаев, полученных на 76 образцах молочной железы, выявлено, что чувствительность метода составляет 70% и специфичность 70% для всех типов злокачественных новообразований молочной железы, включая DCIS [41]. Исследование, включавшее 596 пациентов, где в 50% выполненных операций использовалось устройство Margin Probe, показало значительное уменьшение количества необходимых повторных резекций. Это исследование привело к утверждению FDA (США 2013 г.) Margin Probe как устройства для маркировки краев резекций.
Еще одним популярным методом оценки краев резекций является флуоресцентная визуализация. Для маркировки краев опухоли при РМЖ выполняют инъекцию флуорофора до операции с последующей визуализацией во время операции и исследованием резецированного образца.
Исследование, проводимое в медицинском центре Лейденского университета, рассматривало флуоресцентную визуализацию в ближней инфракрасной области с метиленовым синим, основываясь на том факте, что метиленовый синий больше поглощается в опухолевых клетках и быстрее вымывается в доброкачественных тканях. Этот подход выявил опухоли у 20 из 24 (83%) пациентов.
На рис. 6 можно обнаружить, что флуоресцентное излучение не выявлено на поверхности резецированного образца или вдоль стенок хирургической полости, но было обнаружено после того, как образец был разрезан и опухоль была обнаружена [41].
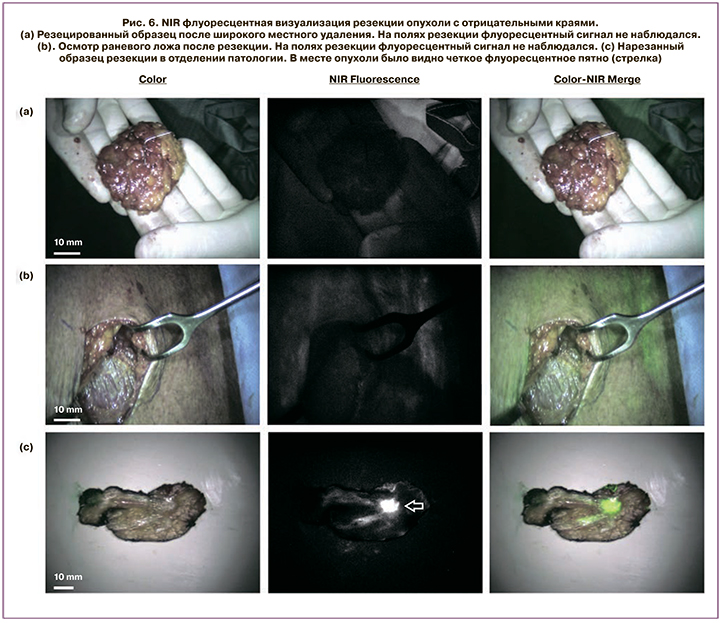
Несомненно, данный метод показывает значительную ценность, предоставляя хороший хирургический обзор иссекаемой ткани и краев, но требует контрастного введения до или во время операции. Точность распространения контраста зависит от ткани и сосудистой проходимости в опухоли и в нормальных тканях.
Микроскопия с ультрафиолетовым поверхностным возбуждением (MUSE) использует низкую глубину проникновения ультрафиолетового света для возбуждения флуорофоров на поверхности окрашенной ткани. Метод эффективен при оценке краев резекций при ОСО для инвазивной формы.
MUSE использует флуоресцентный краситель для окрашивания тканей. Это быстрый, недорогой метод, который не повреждает образцы ткани и не требует этапов, связанных с обычной гистологической обработкой, фиксацией, встраиванием или секционированием. Но он бесполезен, если необходимы данные о глубине поражения (например, при оценке DCIS), поскольку основывается на малой глубине проникновения ультрафиолетового света и в результате окрашивает поверхность резецированного образца [42].
Поскольку MUSE все еще находится в начальной стадии оценки эффективности метода, при исследовании границ опухоли молочной железы для получения показателей чувствительности и специфичности необходимы дополнительные широкомасштабные исследования. MUSE предлагает отличную информацию для выявления границы инвазивного рака, но требует дальнейшего, более глубокого изучения, прежде чем ее можно будет рассматривать как решение проблемы положительной границы [43].

Световая микроскопия – еще один метод интраоперационной оценки краев резекций, эта технология обеспечивает отличное разрешение и информацию о поверхности ткани, как представлено на рис. 7, но еще не была применена к достаточно обширному исследованию для получения измерений чувствительности и специфичности при оценке краев резекций. Данный метод применим для микроскопии поверхности с широкими краями, удаленного сектора (с неровностями поверхности ~200 мкм), быстрой интраоперационной оценки краев резекций (12,5 см2) и оптической объемной оценки – пункционные биопсии (1 мм в диаметре, 2 см в длину) [44]. Тем не менее это дорогостоящая процедура из-за длительного времени сканирования.
Использование углеродных наночастиц при ОСО для непальпируемых новообразований молочной железы. Этот метод оценки краев резекций зарекомендовал себя как наиболее успешный у пациенток с маленьким размером молочных желез. Это исследование было одобрено институциональным контрольным советом Фучжоуской больницы общего профиля [45].
В исследование были включены пациентки от 24 до 58 лет, которым выполнялась ОСО. В отделении хирургии Фучжоуского госпиталя больным были маркированы опухолевые узлы углеродными наночастицами. Максимальный диаметр первичного очага, определяемый при ультразвуковом исследовании перед операцией, варьировал от 0,6 до 1,8 см (в среднем 1,1 см). Все случаи были подтверждены как инфильтрирующий протоковый РМЖ с биопсией и соответствовали показаниям (NCCN) для резекции молочной железы.
 Текущий ретроспективный анализ включал 16 пациенток с непальпируемым РМЖ, которым проводилась предоперационная маркировка с использованием углеродных наночастиц, а также 3 пациенток с маркировкой углеродных наночастиц с последующим неоадъювантным лечением и дальнейшей ОСО.
Текущий ретроспективный анализ включал 16 пациенток с непальпируемым РМЖ, которым проводилась предоперационная маркировка с использованием углеродных наночастиц, а также 3 пациенток с маркировкой углеродных наночастиц с последующим неоадъювантным лечением и дальнейшей ОСО.
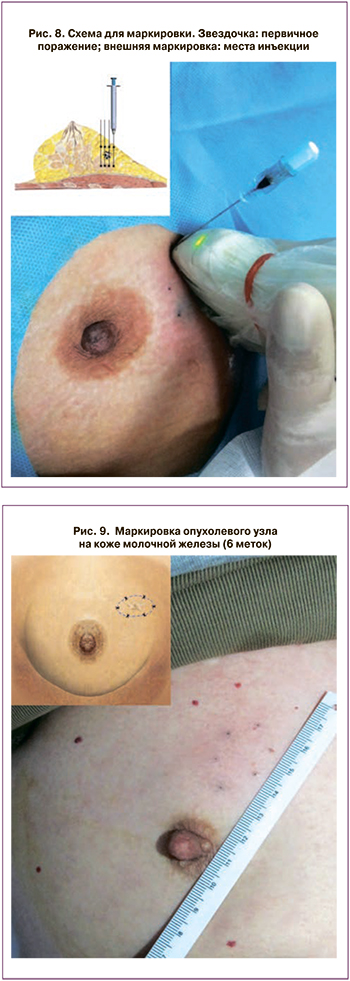
В зависимости от стадии пациенты были распределены следующим образом: T1N0M0 в 7, T1N1M0 в 2, T2N0M0 в 4 и T2N1M0 в 3 случаях. Наночастицу инъецировали в 12 местах (рис. 8) на расстоянии 0,5 см от видимого края при цветном ультразвуковом исследовании вдоль 6 дорожек, разделенных на 60 градусов (по 2 участка на каждую дорожку) с использованием 23-калибровочной иглы (рис. 9). Места инъекций были на расстоянии 0,5 см от видимого края первичного поражения [45].
Края резекций были интактны во всех 16 случаях (и в 3 случаях с неоадъювантным лечением). За период наблюдения рецидива или метастазирования не наблюдалось (от 2 до 22 месяцев наблюдения; медиана 6 месяцев).
Результаты исследования позволили с точностью маркировать границы непальпируемого РМЖ перед операцией и тем самым увеличили шансы на выполнение ОСО у женщин с маленьким размером молочных желез.
Таким образом, преимущества этого метода включают в себя: техническую осуществимость; четкое визуальное руководство во время операции и, как следствие, минимизированный риск рецидива и сохранения большего объема тканей молочной железы. Эти особенности важны для женщин с небольшим размером молочной железы и, следовательно, необходимостью сохранить как можно больше здоровой ткани молочной железы.
Заключение
На сегодняшний день, несмотря на развитие методов определения маркировки краев резекций при ОСО, нет единого стандарта, который обладал бы высокой точностью и специфичностью и мог бы использоваться в большинстве клиник. Идеальная система исследования краев резекций должна быть быстрой, недорогой и простой в использовании, с чувствительностью около 95% и специфичностью около 85%.
В большинстве клиник возможно использование нескольких методов для маркировки краев резекций. Но все они имеют значительные клинические и технические ограничения, которые препятствуют широкому распространению.
В идеале метод определения краев резекций должен быстро обнаруживать злокачественные клетки в краях, снижая процент положительных краев, чтобы сократить процент ререзекций.
Полное удаление опухолевого узла при ОСО должно выполняться согласно всем онкологическим критериям, но с учетом эстетического результата. Окрашивание красителями (например, метиленовым синим, чернилами), используемыми для маркировки краев резекций с окружающими здоровыми тканями, затрудняет работу морфологов.
Метки (например, проволочный проводник, зонд и радионуклид) обычно отмечают только очаг поражения и, таким образом, не являются приоритетными для маркировки краев резекций.
В данной статье были рассмотрены наиболее перспективные методы обнаружения краев изображения и глубины проникновения для установления четких границ при ОСО у больных РМЖ.



