Lipid markers of metastatic lesions in regional lymph nodes in patients with breast cancer
Aim. To assess the possibility of diagnosis of lymph node metastasis of breast cancer by lipid profile in normal and malignant breast tissue. Materials and methods. Semiquantitative evaluation of the lipids in tissue was performed using HPLC-MS/MS of tissue organic extracts. The lipids were identified by accurate molecular masses and tandem mass-spectra (MS/MS). To create the logistic regression based on the Akaike information criteria, the lipids with significantly lipid level difference were used (p<0.05 by Mann-Witney U-test). Results. The obtained diagnostic models based on normal tissue had sensitivity 81% and specificity 79%, the diagnostic model based on malignant tissue had sensitivity 78% and specificity 81% respectively. The lipids, which were selected as markers of lymph node metastases, belonged to sphingomyelins, ether lipids, phosphotidylcholines and phosphotidylethanoamines. Conclusion. The study confirmed that ether lipids and sphingomyelins may be the indicators of metastasis and demonstrated the possibility to use them for metastasis diagnosis.Tokareva A.O., Chagovets V.V., Rodionov V.V., Kometova V.V., Rodionova M.V., Starodubtseva N.L., Frankevich V.E.
Keywords
For a long time, breast cancer remains the most common malignant tumor in women, and it has the highest mortality rate among other cancers. In the Russian Federation, the incidence of breast cancer is higher than on average in the world and is growing every year [1]. Until present, surgery remains the leading method in treatment of patients with breast cancer. Moreover, the last decades have been marked by the reduction of volume of surgery, both on the mammary gland – from classical mastectomy to organ-sparing surgery, and on the organs of regional metastases – from complete lymph node dissection to sentinel lymph node biopsy. Sentinel node biopsy significantly reduced early and late postoperative complications. However, the rate of complications after sentinel lymph node biopsy remain rather high (up to a quarter of cases) [2]. It is suggested not to perform this procedure if no evidence of the metastatic process is obtained at the stage of preoperative diagnosis.
Ultrasound examination is a standard method of preoperative assessment of axillary lymph nodes in patients with breast cancer. The sensitivity and specificity of ultrasound in detecting metastases in regional lymph nodes in breast cancer are on average 85% and 90% respectively, and are defined by the level of the equipment and the competence of the operator [3]. The attempts to improve these parameters by using magnetic resonance imaging and positron emission tomography were not successful. The sensitivity and specificity of diagnostics with the use of magnetic resonance tomography are around 88% and 90% respectively. However, this test is contraindicated in patients with allergies, cardio pacemakers, and renal failure. Also, the accuracy of the assessment is highly dependent on randomly occurring image artifacts, due to which the sensitivity may decline to 60% [4]. The disadvantages of positron emission tomography include low sensitivity in the diagnosis of metastases in the axillary nodes [5]. The search for biomarkers of metastases in regional lymph nodes based on clinical, instrumental and molecular data, such as the woman's age, size and histological subtype of the primary tumor, lymphovascular invasion, HER2 status and others, remains challenging. [6, 7].
The most common approach to the search for molecular markers of a malignant process is mass spectrometric and nuclear magnetic resonance analysis of the metabolome and proteome of tumor tissue and blood plasma. The lipids are biologically active compounds that regulate a number of important cellular processes: proliferation, apoptosis and angiogenesis [8]. The differences in lipid profile help to identify the pathological processes in the tissue [9]. High performance liquid chromatography with mass spectrometry (HPLC-MS) is the most informative method for lipid analysis [10].
The aim of study was to assess the possibility of chromatography-mass spectrometric analysis of the primary tumor and the surrounding tissues in the diagnosis of metastasis to regional lymph nodes in breast cancer.
Materials and methods
Semiquantitative evaluation of the lipids in the tissues was carried out using HPLC-MS/MS of tissue organic extracts. The lipids were identified by accurate molecular mass and tandem mass-spectra (MS/MS). To create the logistic regression based on the Akaike information criteria, the lipids with significantly lipid level difference (p<0.05) defined by Mann–Whitney U-test were chosen.
44 patients with breast cancer were uncluded in the study in the National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov of the Ministy of Health of the Russian Federation. The exclusion criteria were neoadjuvant therapy and malignant neoplasms of other localization prior to breast cancer diagnosis. The study was approved by the Ethic Committee of the Center. All patients have signed the informed consent.
More than half of the patients (55%) had metastases at least in one lymph node. In patients with regional lymph nodes metastases stage pT1N1M0 were diagnosed in 5 patients (21%), stages pT2N1-3M0 – in 18 (75%), stage pT3N3M0 – in 1 (4%). In the group of patients without regional lymph nodes metastases, half of them had stage pT1N0M0 of the disease, another half – pT2N0M0.
2 samples of breast tissue were collected from each patient: the tissue from the tumor area and normal tissue away from the tumor. Each sample was histologically verified. The analysis of the lipid composition of the tissue was performed with the use of HPLC-MS according to the previously developed protocol for determining the lipid composition of the tissue [9, 11–14]. In particular, the dried lipid extract was redissolved in the acetonitrile/isopropanol mixture (1/1), separated by Dionex UltiMate 3000 chromatograph (Thermo Scientific, Germany), and further detected by Maxis Impact qTOF mass spectrometer (Bruker Daltonics, Germany) in the positive and negative ion modes formed by electrospray ionizaton. Additionally, to clarify the identification of substances, tandem mass spectrometry with a scanning window 5 Da was performed.
The resulting .d files were converted into ms2 files containing information on the ion fragmentation at each moment of time (the .d files that contained information on tandem mass spectrometry were transformed) and MzXml containing information on the mass spectrum of molecular ions at each moment time of chromatographic analysis. For this purpose accessible MsConvert software (Proteowizard, 3.0.9987) was used. The MxXml was processed in MzMine, isolating the ion peaks and normalizing them to the total ion current. Ms2 files were used for lipids identification by LipidMatch scripts correlating the time and ions masses in the table generated by MzMine containing the information on the fragmentation spectrum of a given ion at a given moment of time in ms2 file format. To assess the correspondence between ion fragmentation and lipid fragmentation spectra, the specific fragments library in the software package was used [15]. Lipid nomenclature matches LipidMaps [16].
Statistical analysis
The statistical analysis was performed using the scripts in the R programming language (version 3.3.3) in the RStudio environment (1.383 GNU) [17, 18]. The Shapiro-Wilk test was used to check the clinical data of the patients and the histological characteristics of the tissues relating to the numerical characteristics (p> 0.05). Statistically significant differences for the normally distributed variables were determined with the use of the Student's t-test (the accepted critical value p <0.05). The nonparametric Mann-Whitney test was used to test the values that did not correspond to the normal distribution and to determine the presence of statistically significant difference (the accepted critical value p <0.05). To assess the differences in factorial histological characteristics of tissues in patients with and without metastasis, Pearson's χ2 criterion was used (the accepted critical value p <0.05). The nonparametric Mann–Whitney test (accepted critical value p <0.05) was used for testing the identified lipids for the presence of significant differences in the level in the presence and absence of metastases separately for the tumor tissues and for the tissues in normal breast.
Categorical data were described using the absolute number (N) and percentages of the total number of patients in the group (P) as N (P%). Normally distributed quantitative data were described using the arithmetic mean (M) and the standard deviation (SD) as M (SD). Quantitative data with a distribution differing from normal were presented as the median (Me) and quartiles Q1 and Q3 in the Me format (Q1; Q3).
The lipids with statistically significant level changes in the group were used to create a diagnostic model based on the logistic regression, optimized by stepwise adding the variables and verification of the Akaike information criterion [19]. Tissue lipid levels were used as variables, and the diagnosis of the presence/absence of metastases was used as the response variables. To assess the quality of a potential diagnostic model based on the logistic regression, N logistic regressions were constructed based on N different samples containing (N–1) objects with a subsequent test on the object, which was not participating in the construction of regression, where N is the number of all objects in a pair of clinical groups. Sensitivity and specificity were assessed as the number of true positive test results/total number of patients with metastases and the number of true negative test results/ total number of patients without metastases respectively. The positive and negative predictive value of the results was assessed as the number of true positives results/the number of positive results and true negative results/the number of negative results.
Results
No statistically significant difference in terms of patients the age, size and location of the tumor lesions, and HER2 status was noted in the groups of patients with and without metastases in the axillary lymph nodes (Table 1) Statistically more often (p<0,046) non-specific tumor types (30%) and specific histologic type of tumors (35%) were found in the group of patients without metastases. Mixed tumors were the most frequent histological variants (41.7%) in the group of patients with metastases. Multifocal tumors with a high degree of malignancy (G3 = 55%) and proliferative activity (mean level Ki67 = 30.35%) were often found in the group of patients without metastases to regional lymph nodes.
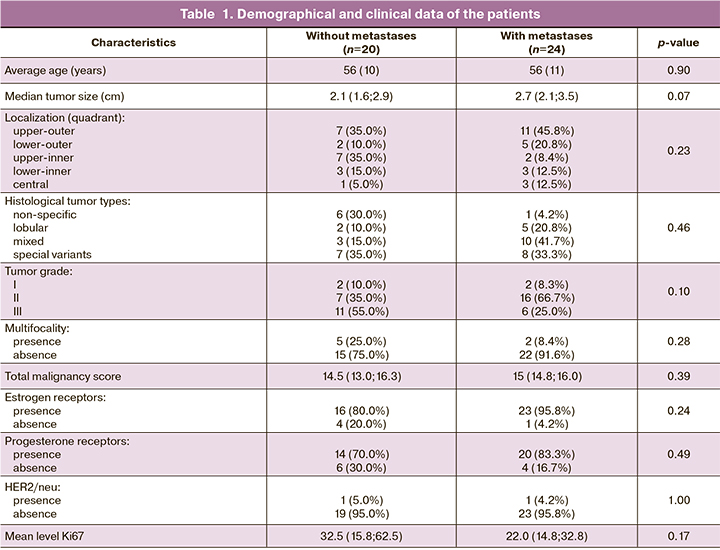
The analysis showed 6 compounds in positive and 12 compounds in negative ion modes in normal breast tissue, which are most important for the potential diagnosis of metastases in the regional lymph nodes, as well as 4 compounds - in positive and 5 compounds in negative ion modes in the tumor tissue, which have the same predictive properties.
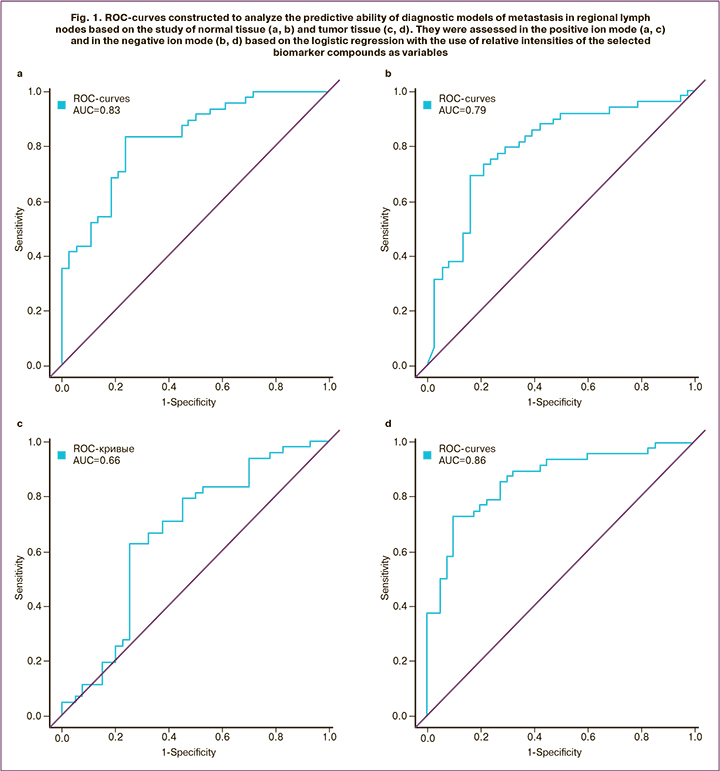
The highest diagnostic quality was demonstrated by the models obtained in the positive ion mode in normal tissues – the regression equation (1)* with sensitivity 81% and specificity 78%; and in the negative ion mode in tumor tissues – the regression equation (2)** with sensitivity 79% and specificity 81 % (Fig. 1, Table 2, 3). For detection of normal breast tissue (1), the predictive value of a positive result was 78%, and of the negative – 80%. For detection of tumor tissue (2), these values were 81% and 79% respectively. The values of the area under the curve (AUC) 0.83 and 0.86 indicate a very good quality of diagnostic models.
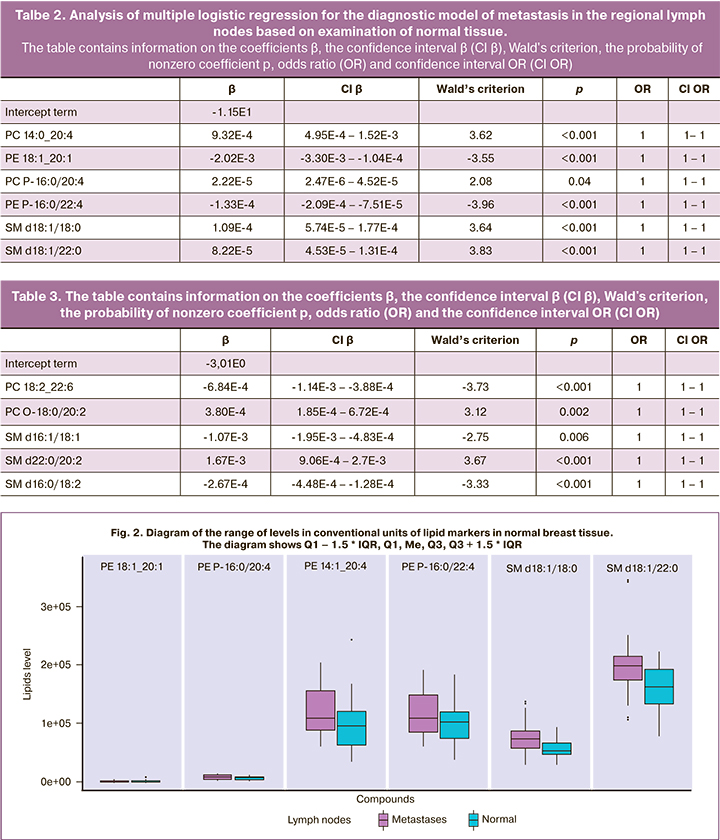
The odds ratio equal to 1 in relation to all investigated predictors in all logistic regressions is due to the fact that in the adopted system of units, a single change in the lipid level is insignificant for the accuracy used in the study.
The lipids identified as diagnostic markers of lymph node metastasis belonged to the classes of phosphatidylcholines (PC 14:0_20:4, PC 18:2_22:6, PC P-16:0/20: 4, plasmanyl-PC O-18:0/20:2), phosphatidylethanolamines (PE 18:1_20:1, PE P-16: 0/22:4), ether lipids (PC P-16:0/20:4, PE P-16:0/22:4, PC O-18:0/20:2) and sphingomyelins (SM d18:1/18:0, SM d18:1/22:0, SM d18:1/22:0, SM d22:0/20:2, SM d16:0/18:2) (Fig. 2, 3).
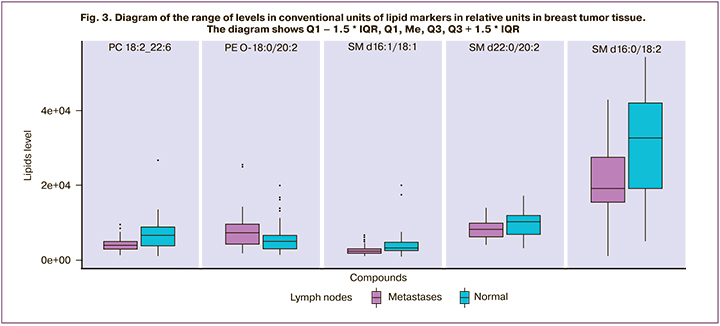
The above box plot diagrams show that the vectors of changes of the levels of sphingomyelins in normal and tumor tissues associated with metastases are opposite (it grows in normal tissue and decreases in tumor tissue), and the level of essential lipids increases in the presence of metastases in both types of tissues.
Discussion
Sphingomyelins participate in the events that trigger apoptosis with the involvement of sphingomyelinases, which separate sphingolipids into ceramides, inducing activation of the phospholipase protein that suppress cell growth and division [20, 21].The level of SMPD3 gene responsible for the expression of neutral sphingomyelinase is increased in tumor tissues compared to normal breast tissues [20]. In addition, the mice with a deactivated acid sphingomyelinase, which were injected with melanoma cells, had significantly less pronounced metastasis than the mice with normal genome [22]. Roy et al. report lower levels of sphingomyelins in the metastatic bone cancer cells compared to primary malignant bone neoplasms [23]. Also, the study by Peng comparing the metabolomic profile of two types of colon cancer cell lines, showed a significantly higher level of sphingomyelins in the cancer cell line that is less prone to metastasis [24].
Ether-phospholipids are the lipid markers of neoplastic tissue damage [25]. It was noted, that the cell lines with a high metastatic potential, have a higher content of phosphatidylcholides and phosphatidylethanolamines with an ether bond compared with the cell lines with a low metastatic potential [26].
Conclusion
Metastatic lesions of regional lymph nodes are associated with changes in the lipid composition of breast tumor and normal tissues. The changes in the levels of sphingomyelin differ in normal and tumor tissue. This may indicate a disturbance of the metabolic pathways associated with apoptosis. Therefore, the lipid profile in normal and tumor breast tissues may be useful to predict metastatic lesions in regional lymph nodes in patients with breast cancer.
References
- Каприн А.Д., Старинский В.В., Петрова Г.В., ред. Злокачественные новообразования в России в 2017 году (заболеваемость и смертность). М.: МНИОИ им. П.А. Герцена - филиал ФГБУ «НМИРЦ» Минздрава России; 2018.
- Lucci A., McCall L.M., Beitsch P.D., Whitworth P.W., Reintgen D.S., Blumencranz P.W. et al. Surgical complications associated with sentinel lymph node dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the American College of Surgeons Oncology Group trial Zoo11. J. Clin. Oncol. 2007; 25(24): 3657-63. https://dx.doi.org/10.1200/JCO.2006.07.4062.
- Sukhikh G.T., Sencha A.N., eds. Multiparametric ultrasound diagnosis of breast diseases. Springer; 2018.
- Zhou M., Lu B., Lv G., Tang Q., Zhu J., Li J., Shi K. Differential diagnosis between metastatic and non-metastatic lymph nodes using DW-MRI: a meta-analysis of diagnostic accuracy studies . J. Cancer Res. Clin. Oncol. 2015; 141(6): 1119-30. 10.1007/s00432-014-1895-9.
- Ulaner G.A. PET/CT for patients with breast cancer: Where is the clinical impact? Am. J. Roentgenol. 2019; 213(2): 254-65. https://dx.doi.org/10.2214/AJR.19.21177.
- Voogd A.C., Coebergh J.W.W., Repelaer van Driel O.J., Roumen R.M.H., van Beek M.W.P.M., Vreugdenhil A., Crommelin M.A. The risk of nodal metastases in breast cancer patients with clinically negative lymph nodes: A population-based analysis. Breast Cancer Res. Treat. 2000; 62(1): 63-9. https://dx.doi.org/10.1023/a:1006447825160.
- Viale G., Zurrida S., Maiorano E., Mazzarol G., Pruneri G., Paganelli G. et al. Predicting the status of axillary sentinel lymph nodes in 4351 patients with invasive breast carcinoma treated in a single institution. Cancer. 2005; 103(3): 492-500. https://dx.doi.org/10.1002/cncr.20809.
- Bandu R., Mok H.J., Kim K.P. Phospholipids as cancer biomarkers: mass spectrometry-based analysis. Mass Spectrom. Rev. 2018; 37(2):107-38. https://dx.doi.org/10.1002/mas.21510.
- Adamyan L., Starodubtseva N., Borisova A., Stepanian A., Chagovets V., Salimova D. et al. Direct mass spectrometry differentiation of ectopic and eutopic endometrium in patients with endometriosis. J. Minim. Invasive Gynecol. 2018; 25(3): 426-33. https://dx.doi.org/10.1016/j.jmig.2017.08.658.
- Han X. Lipidomics for studying metabolism. Nat. Rev. Endocrinol. 2016; 12(11): 668-79. https://dx.doi.org/10.1038/nrendo.2016.98.
- Chagovets V.V., Wang Z., Kononikhin A.S., Starodubtseva N.L., Borisova A., Salimova D., Popov I.A., Kozachenko A.V., Chingin K., Chen H., Frankevich V.E., Adamyan L.V., Sukhikh G.T. Endometriosis foci differentiation by rapid lipid profiling using tissue spray ionization and high resolution mass spectrometry. Sci. Rep. 2017; 7(1): 2546. https://dx.doi.org/10.1038/s41598-017-02708-x.
- Tokareva A.O., Chagovets V.V., Starodubtseva N.L., Nazarova N.M., Nekrasova M.E., Kononikhin A.S., Frankevich V.E., Nikolaev E.N., Sukhikh G.T. Feature selection for OPLS discriminant analysis of cancer tissue lipidomics data. J. Mass Spectrom. 2020; 55(1): e4457. https://dx.doi.org/10.1002/jms.4457.
- Sukhikh G., Chagovets V., Wang X., Rodionov V., Kometova V., Tokareva A.,Kononikhin A., Starodubtseva N., Chingin K., Chen H., Frankevich V. Combination of low-temperature electrosurgical unit and extractive electrospray ionization mass spectrometry for molecular profiling and classification of tissues. Molecules. 2019; 24(16): 2957. https://dx.doi.org/10.3390/molecules24162957.
- Chagovets V., Wang Z., Kononikhin A., Starodubtseva N., Borisova A., Salimova D.,Popov I., Kozachenko A., Chingin K., Chen H., Frankevich V., Adamyan L., Sukhikh G. A comparison of tissue spray and lipid extract direct injection electrospray ionization mass spectrometry for the differentiation of eutopic and ectopic endometrial tissues. J. Am. Soc. Mass Spectrom. 2017; 29(2): 323-30. https://dx.doi.org/10.1007/s13361-017-1792-y.
- Koelmel J.P., Kroeger N.M., Ulmer C.Z., Bowden J.A., Patterson R.E., Cochran J.A. et al. Lipid match: An automated workflow for rule-based lipid identification using untargeted high-resolution tandem mass spectrometry data. BMC Bioinformatics. 2017; 18(1): 331. https://dx.doi.org/10.1186/s12859-017-1744-3.
- Sud M., Fahy E., Cotter D., Brown A., Dennis E.A., Glass C.K. et al. LMSD: LIPID MAPS structure database. Nucleic Acids Res. 2007; 35 (Database issue): D527-32. https://dx.doi.org/10.1093/nar/gkl838.
- R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. Vienna, Austria; 2018.
- R team R Studio: Integrated Development for R. 2016.
- Akaike H. Information theory and an extension of the maximum likelihood principle. Selected Papers of Hirotugu Akaike. Springer; 1998: 199-213.
- Shamseddine A.A., Airola M.V., Hannun Y.A. Roles and regulation of neutral sphingomyelinase-2 in cellular and pathological processes. Adv. Biol. Regul. 2015; 57: 24-41. https://dx.doi.org/10.1016/j.jbior.2014.10.002.
- Revill K., Wang T., Lachenmayer A., Kojima K., Harrington A., Li J. et al. Genome-wide methylation analysis and epigenetic unmasking identify tumor suppressor genes in hepatocellular carcinoma. Gastroenterology. 2013; 145 (6): 1424-35. e1-25. https://dx.doi.org/10.1053/j.gastro.2013.08.055.
- Carpinteiro A., Becker K.A., Japtok L., Hessler G., Keitsch S., Požgajovà M. et al. Regulation of hematogenous tumor metastasis by acid sphingomyelinase. EMBO Mol. Med. 2015; 7(6): 714-34. https://dx.doi.org/10.15252/emmm.201404571.
- Roy J., Dibaeinia P., Fan T.M., Sinha S., Das A. Global analysis of osteosarcoma lipidomes reveal altered lipid profiles in metastatic versus nonmetastatic cells. J. Lipid Res. 2019; 60(2): 375-87. https://dx.doi.org/10.1194/jlr.M088559.
- Peng W., Tan S., Xu Y., Wang L., Qiu D., Cheng C. et al. LC-MS/MS metabolome analysis detects the changes in the lipid metabolic profiles of dMMR and pMMR cells. Oncol. Rep. 2018; 40(2): 1026-34. https://dx.doi.org/10.3892/or.2018.6510.
- Smith R.E., Lespi P., Di Luca M., Bustos C., Marra F.A., De Alaniz M.J.T., Marra C.A. A reliable biomarker derived from plasmalogens to evaluate malignancy and metastatic capacity of human cancers. Lipids. 2008; 43(1): 79-89. https://dx.doi.org/10.1007/s11745-007-3133-6.
- Fallani A., Mannori G., Ruggieri S. Composition of ether-inked sub-classes of glycerophospholipids in clones with a different metastatic potential isolated from a murine fibrosarcoma line (T3 cells). Int. J. Cancer. 1995; 62(2): 230-2.https://dx.doi.org/10.1002/ijc.2910620220.
Received 25.02.2020
Accepted 07.07.2020
About the Authors
Alisa O. Tokareva, specialist of the Laboratory of Proteomics and Metobolomics of the Human Reproduction, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov, Ministry of Health of the Russian Federation; V.L. Talrose Institute for Energy Problems of Chemical Physics,N.N. Semenov Federal Center of Chemical Physic, Russian Academy of Sciences; Ph.D. student, Moscow Institute of Physics and Technology, Moscow, Russia.
Tel.: +7(965)128-68-86. E-mail: alisa.tokareva@phystech.edu.
117997, Russia, Moscow, Academika Oparina, 4; 119334, Russia, Moscow, Leninsky prospect 38/2; 141701, Russia, Dolgoprudny, Institutskiy per., 9.
Vitaliy V. Chagovets, Ph.D. in Physical and Mathematical Sciences, Senior researcher of the Laboratory of Proteomics and Metobolomics of the Human Reproduction,
National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov, Ministry of Health of the Russian Federation.
Tel.: +7(926)562-65-90. E-mail: vvchagovets@gmail.ru. 117997, Russia, Moscow, Academika Oparina, 4.
Valeriy V. Rodionov, M.D., Head of the Department of Breast Pathology, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov, Ministry of Health of the Russian Federation. Tel.: +7(926)562-65-90. E-mail: V_Rodionov@oparina4.ru.
117997, Russia, Moscow, Academika Oparina, 4.
Vlada V. Kometova, PhD in Medical Sciences, Senior researcher of the Anatomical Pathology Department, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov, Ministry of Health of the Russian Federation. Tel.: +7(926)562-65-90. E-mail: v_kometova@oparina4.ru.
117997, Russia, Moscow, Academika Oparina, 4.
Maria V. Rodionova, PhD in Medical Sciences, Oncologist of the Department of Breast Pathology, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov, Ministry of Health of the Russian Federation. Tel.: +7(926)562-65-90. E-mail: m_Rodionova@oparina4.ru.
117997, Russia, Moscow, Academika Oparina, 4.
Natalia L. Starodubtseva, PhD in Biological Sciences, Head of the Laboratory of Proteomics and Metobolomics of the Human Reproduction, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov, Ministry of Health of the Russian Federation; V. L. Talrose Institute for Energy Problems of Chemical Physics, N.N. Semenov Federal Center of Chemical Physic, Russian Academy of Sciences. Tel.: +7(916)463-98-67. E-mail: aurum19@mail.ru.
117997, Russia, Moscow, Academika Oparina, 4; 119334, Russia, Moscow, Leninsky prospect 38/2.
Vladimir E. Frankevich, Ph.D. in Physical and Mathematical Sciences, Systems Biology in Reproduction, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov, Ministry of Health of the Russian Federation. Tel.: +7(495)438-07-88. E-mail: v_frankevich@oparina4.ru.
117997, Russia, Moscow, Academika Oparina, 4.
For citation: Tokareva A.O., Chagovets V.V., Rodionov V.V., Kometova V.V., Rodionova M.V., Starodubtseva N.L., Frankevich V.E. Lipid markers of metastatic lesions in regional lymph nodes in patients with breast cancer.
Akusherstvo i Ginekologiya/ Obstetrics and gynecology. 2020; 8: 133-140 (in Russian).
https://dx.doi.org/10.18565/aig.2020.8.133-140



