Choosing an optimal algorithm for the assessment of the condition of regional lymph nodes in patients with breast cancer using modern ultrasound diagnostics
Objective. To develop a diagnostic algorithm for assessing the condition of the axillary lymphatic collector in patients with early stages of breast cancer.Khakurinova N.D., Snitkin V.М., Sholokhov V.N., Petrovsky А.V., Valiev R.K., Avtomonov D.Е., Samoylenko I.V.
Materials and methods. The study included 118 patients with clinical stage T1–2 and the absence of palpable axillary lymph nodes (N0).Results. Elastometric evaluation showed that the shear wave velocity in the axillary lymph nodes averaged 1.54 (±0.44) m/s in the case of a benign lesion and 2.94 (±1.19) m/s in a metastatic lesion. Thus, the shear wave velocity in the axillary lymph nodes in patients with metastatic nodes differed significantly from that in patients without metastatic lymph nodes. During the ROC analysis, a threshold value of the shear wave velocity was calculated; the model showed sensitivity in detecting metastases in lymph nodes at the rate of 1.85 m/s in 90% (27 out of 30 patients), and specificity in 72.7% (64 out of 88 patients) of cases. The accuracy of the method was 77.1%, positive predictive value was 52.9%, and negative predictive value was 95.5%.
Conclusion. The comprehensive ultrasound assessment revealed 27 patients with metastases in the axillary lymph nodes compared to 22 patients with nodes detected by B-mode ultrasonography (90% and 73%, respectively). The patients underwent lymphadenectomy, sentinel lymph node biopsy in this case was not performed.
Keywords
Recent advances in the field of radiation and drug treatment of breast cancer, which outweigh any advantages of aggressive surgical approach in patients with the initial stages of the disease, direct modern scientific research towards further minimization of surgical trauma. Therefore, it is necessary to conduct adequate staging in planning treatment tactics. The assessment of the condition of the regional lymph nodes plays an important role in this process. For preoperative diagnosis of the regional (mainly axillary) lymph nodes, various non-invasive imaging methods are used, but the most common and affordable method is the ultrasound examination; moreover, this study makes it possible to perform simultaneously a targeted biopsy of a suspicious lymph node, if necessary. The assessment of the condition of regional lymph nodes in patients with early stages of breast cancer helps to perform staging of the tumor and determine the volume of surgical intervention; patients who might undergo intraoperative biopsy of signal lymph nodes can avoid this procedure after the evaluation of regional lymph nodes. In this regard, there is a question of developing an optimal algorithm for diagnosing the condition of the regional lymphatic collector using the opportunities of the ultrasound method. According to some scientists [1–21], «methodological limit» of ultrasound assessment and fine needle aspiration biopsy (FNAB) has ranged approximately between 50 to 80% in the last two decades; its specificity reaches 100%, and the frequency of false-negative results ranges from 14 to 30% and its average level is 20–25%. Thus, most patients have to undergo a sentinel lymph node biopsy (SLNB), metastases in axillary lymph nodes are detected in no more than 20% of the patients, and up to 50% of these patients will have micrometastases, which do not have clinical significance due to the modern adjuvant treatment options. However, it is possible to improve these indicators with the introduction of modern ultrasound technologies into medical practice.
Materials and Methods
The study included 118 patients with clinical stage T1–2 and the absence of palpable axillary lymph nodes (N0). In this research, we wanted to demonstrate the results of the assessment of the condition of the axillary lymph nodes, which were obtained using various methods of ultrasound diagnostics. In order to evaluate the effectiveness of determining metastatic lymph nodes based on the results of preoperative diagnostics, information content indicators were identified: sensitivity, specificity, and accuracy. The formulas for calculating statistical indicators were used. Ultrasound imaging of axillary tissues was performed using a standard technique, in B-mode, with the high-frequency sensors with operating frequency ranging from 5 to 13 MHz. The first stage of the study was a polypositional examination with the use of standard techniques. Ultrasound imaging was supplemented by a color Doppler assessment to determine the degree of lymph node vascularization. At the second stage, shear wave elastography was used to obtain objective results; it reduced the number of artifacts and provided preliminary, visual information about the degree of stiffness of the lymph nodes. At the third stage of the study, elastometry of previously identified and marked lymph nodes was performed. When performing elastometry, the peripheral part of the lymph node and its central parts were examined successively, and at least three measurements were performed with the calculation of the average value. The result was considered negative, if the average shear wave velocity was below 1.85 m/s, i.e. there was no metastatic lesion. If the shear wave velocity was ≥1.85 m/s, the result was considered doubtful or positive and it was suggestive of a metastatic lesion of the lymph node. The fourth stage was a percutaneous puncture biopsy of pre-marked lymph nodes in patients whose results were considered doubtful or positive in terms of metastatic lesion. All patients underwent axillary lymphadenectomy or sentinel lymph node biopsy on a regional (axillary) lymphatic collector simultaneously with breast surgery. The results of non-invasive methods were compared with the results of pathomorphological studies.
Statistical analysis
Statistical analysis of the obtained data was performed using standard methods of mathematical and statistical processing with PC software (MSOffice Excel and IBM SPSS v. 20). When describing quantitative data, the mean value and standard deviation (SD) were estimated, normality of distribution was determined using the Shapiro-Wilk Test. The quantitative data presented in this paper had a normal distribution. Qualitative indicators are presented in both absolute and relative values (%). For the evaluation of the effectiveness of determining metastatic lymph nodes based on the results of preoperative diagnostics, information content indicators were identified: sensitivity, specificity, and accuracy. The formulas for calculating statistical indicators were used. Sensitivity, specificity, disease prevalence, positive and negative prognostic values, and accuracy are presented as percentages. Confidence intervals (CI) for sensitivity, specificity, and accuracy are determined using Clopper–Pearson confidence intervals. The differences between groups in event frequencies were evaluated using the Z-test, and the Mann–Whitney U-test was used for continuous variables. For all criteria and tests, the critical significance level was assumed to be 0.05, i.e. the differences were considered statistically significant at p<0.05. ROC analysis with AUC calculation was performed to determine the cut-off points with a given sensitivity and specificity, positive and negative prognostic values.
Results
Thus, the evaluation of the changes in the axillary lymph nodes on the basis of ultrasound data in B-mode showed that sensitivity of this ultrasound method was 73.3% (22 out of 30, 95% CI: 54.1–87.7%), and the specificity was 94.3% (83 out of 88, 95% CI: 87.2–98.1%). The data are presented in Table 1. The accuracy of the method was 88.9% (95% CI: 81.9–94.0%). Positive predictive value (PPV) was 81.4% (95% CI: 64.6–91.4%), negative predictive value (NPV) was 91.2% (95% CI: 81.1–90.7%).
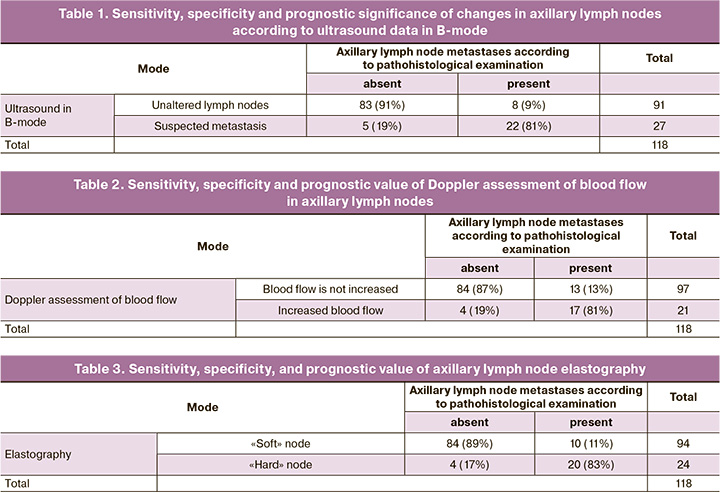
Table 2 shows the frequency of detection of abnormally active blood flow in the axillary lymph nodes according to Doppler assessment. The prognostic value of this particular ultrasound method was also further analyzed, since we have information about the presence or absence of metastases in the axillary lymph nodes according to morphological studies. The sensitivity of this ultrasound method was 56.7% (17 of 30, 95% CI: 37.4–74.5%), and its specificity was 95.5% (84 of 88, 95% CI: 88.7–98.8%). The accuracy of the method was 85.6% (95% CI: 77.9–91.4%). PPV was 80.9% (95% CI: 60.8–92.1%), NPV was 86.6% (95% CI: 81.1–90.7%).
Then, ultrasound examination in B-mode and M-mode was followed by elastography, the result of which was recorded as an integral assessment, namely, «soft» lymph node or «hard» lymph node (Table 3).
On the basis of the obtained results, we can conclude that the sensitivity of the method was 66.7% (20 out of 30, 95% CI: 47.2–82.7%), and the specificity was 95.5% (84 out of 88). The accuracy of the method was 88.4% (95% CI: 80.9–93.36%). PPV was 83.3% (from 65.0% to 93.1%), NPV was 89.3% (from 83.5% to 93.3%).
All patients also underwent elastometry and measurement of the shear wave velocity in the tissues of the axillary region (both in the lymph node and in the surrounding tissues). As a demonstration, we present the results of shear wave elastometry in one of our patients (Fig. 1).
We evaluated the obtained average values of the shear wave velocity in the axillary lymph nodes and surrounding tissues in 118 patients, depending on the presence or absence of metastatic lesions of the axillary lymphatic collector according to the morphological study (Table 4).
Thus, shear wave velocity in axillary lymph nodes in patients with metastatic nodes was statistically significantly different from that in patients without lymph node metastases.
We used ROC analysis to determine the threshold value of the shear wave velocity in the lymph node, which allowed us to distinguish metastatic and normal lymph nodes at the preoperative stage with acceptable sensitivity and specificity. During the analysis, a threshold value of the shear wave velocity of 1.85 m/s was calculated; the model showed sensitivity in detecting metastases in lymph nodes in 90% of cases (27 out of 30 patients, 95% CI: 73.5–97.9%), and specificity in 72.7% of cases (64 out of 88 patients, 95% CI: 62.2–81.7%). The accuracy of the method was 77.1% (95% CI: 68.5–84.4%). PPV was 52.9% (95% CI: 43.9–61.8%), and NPV was 95.5% (95% CI: 87.9–98.4%) (Table 5).
The analysis of ROC curves allows us to select other options for threshold values for the diagnostic method that meet the task of this work, namely, to find out whether ultrasound with other techniques used at the preoperative stage can avoid lymphadenectomy or SLNB procedure in at least some patients with clinically undetectable metastases in axillary lymph nodes. In this regard, we tried to sacrifice the sensitivity of detecting metastases in the lymph nodes in order to increase NPV; in other words, we tried to find the values of the shear wave velocity when metastases in the axillary lymph nodes are not detected with the maximum probability (100%). Such a threshold value in our ROC model was the value of the shear wave velocity of 1.05 m/s (Table 6).
 When the threshold value of the shear wave velocity of 1.05 m/s was used, this model showed 100% sensitivity in detecting metastases in lymph nodes (95% CI: 88.4–100%), while the specificity was 9% (95% CI: 2.6–14.6%). PPV was 27.3% (95% CI: 26.1–28.4%), and NPV was 100%. Unfortunately, the developed ROC models did not allow us to find more acceptable combinations of NPV (which is extremely important in our work), sensitivity and specificity of the test. Thus, if we had applied the threshold value of the shear wave velocity of 1.85 m/s in patients without clinically determined lymph node metastases with a negative study result (velocity less than 1.85 m/s), we would have missed 3 cases of metastatic lesion in the group of 67 patients (NPV is 95.5%, (95% CI: 87.9–98.4%)), but 67 patients out of 108 could have avoided surgery (SLNB or axillary lymphadenectomy) on the lymphatic collector. Trying to achieve a 100% NPV, we have to reduce the specificity of the method, as a result only 8 out of 108 patients could avoid manipulation (SLNB or axillary lymphadenectomy) on the lymphatic collector. The obtained data demonstrate that ultrasonic characteristics such as shear wave velocity, integral estimation of B-mode data, Doppler assessment, elastography, and integral estimation of all techniques have approximately similar and very reliable predictive models.
When the threshold value of the shear wave velocity of 1.05 m/s was used, this model showed 100% sensitivity in detecting metastases in lymph nodes (95% CI: 88.4–100%), while the specificity was 9% (95% CI: 2.6–14.6%). PPV was 27.3% (95% CI: 26.1–28.4%), and NPV was 100%. Unfortunately, the developed ROC models did not allow us to find more acceptable combinations of NPV (which is extremely important in our work), sensitivity and specificity of the test. Thus, if we had applied the threshold value of the shear wave velocity of 1.85 m/s in patients without clinically determined lymph node metastases with a negative study result (velocity less than 1.85 m/s), we would have missed 3 cases of metastatic lesion in the group of 67 patients (NPV is 95.5%, (95% CI: 87.9–98.4%)), but 67 patients out of 108 could have avoided surgery (SLNB or axillary lymphadenectomy) on the lymphatic collector. Trying to achieve a 100% NPV, we have to reduce the specificity of the method, as a result only 8 out of 108 patients could avoid manipulation (SLNB or axillary lymphadenectomy) on the lymphatic collector. The obtained data demonstrate that ultrasonic characteristics such as shear wave velocity, integral estimation of B-mode data, Doppler assessment, elastography, and integral estimation of all techniques have approximately similar and very reliable predictive models.
Conclusion
Among 118 patients with clinically undetectable lymph nodes in the axillary region, the comprehensive ultrasound assessment revealed 27 patients with metastases in the axillary lymph nodes compared to 22 patients with nodes detected by B-mode ultrasonography (90% and 73%, respectively), and these patients underwent axillary lymphadenectomy, but sentinel lymph node biopsy in this case was not performed.
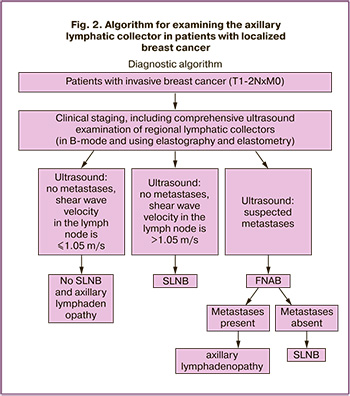
Thus, we have developed an algorithm for examining the axillary lymphatic collector in patients with localized breast cancer (Fig. 2).
References
- Yang T., Niu J., Dang Y., Zhou Y., Cao Y., Zeng M., Lv M. An innovative ultrasound strain elastographic method for the differential diagnosis of breast tumors. Ultrasound Med. Biol. 2019; 45(1): 56-67. https://dx.doi.org/10.1016/j.ultrasmedbio.2018.08.025.
- Graziano L., Bitencourt A.G., Cohen M.P., Guatelli C.S., Poli M.R., Souza J.A., Marques E.F. Elastographic evaluation of indeterminate breast masses on ultrasound. Rev. Bras. Ginecol. Obstet. 2017; 39(2): 72-9. https://dx.doi.org/10.1055/s-0036-1597753.
- Lee S.H., Moon W.K., Cho N., Chang J.M., Moon H.G., Han W. et al. Shear-wave elastographic features of breast cancers: comparison with mechanical elasticity and histopathologic characteristics. Invest. Radiol. 2014; 49(3): 147-55. https://dx.doi.org/10.1097/RLI.0000000000000006.
- Evans A., Whelehan P., Thomson K., McLean D., Brauer K., Purdie C. et al. Invasive breast cancer: relationship between shear-wave elastographic findings and histologic prognostic factors. Radiology. 2012; 263(3): 673-7. https://dx.doi.org/10.1148/radiol.12111317.
- Park Y.M., Fornage B.D., Benveniste A.P., Fox P.S., Bassett R.L.Jr., Yang W.T. Strain elastography of abnormal axillary nodes in breast cancer patients does not improve diagnostic accuracy compared with conventional ultrasound alone. AJR Am. J. Roentgenol. 2014; 203(6): 1371-8. https://dx.doi.org/10.2214/AJR.13.12349.
- Choi J.J., Kang B.J., Kim S.H., Lee J.H., Jeong S.H., Yim H.W. et al. Role of sonographic elastography in the differential diagnosis of axillary lymph nodes in breast cancer. J. Ultrasound Med. 2011; 30(4): 429-36. https://dx.doi.org/10.7863/jum.2011.30.4.429.
- Taylor K., O'Keeffe S., Britton P.D., Wallis M.G., Treece G.M., Housden J. et al.Ultrasound elastography as an adjuvant to conventional ultrasound in the preoperative assessment of axillary lymph nodes in suspected breast cancer: a pilot study. Clin. Radiol. 2011; 66(11): 1064-71. https://dx.doi.org/10.1016/j.crad.2011.05.015.
- Tsai W.C., Lin C.K., Wei H.K., Yu B.L., Hung C.F., Cheng S.H., Chen C.M. Sonographic elastography improves the sensitivity and specificity of axilla sampling in breast cancer: a prospective study. Ultrasound Med. Biol. 2013; 39(6): 941-9. https://dx.doi.org/10.1016/j.ultrasmedbio.2012.12.013.
- Youk J.H., Gweon H.M., Son E.J., Han K.H., Kim J.A. Diagnostic value of commercially available shear-wave elastography for breast cancers: integration into BI-RADS classification with subcategories of category 4. Eur. Radiol. 2013; 23(10): 2695-704. https://dx.doi.org/10.1007/s00330-013-2873-3.
- Park C.S., Kim S.H., Jung N.Y., Choi J.J., Kang B.J., Jung H.S. Interobserver variability of ultrasound elastography and the ultrasound BI-RADS lexicon of breast lesions. Breast Cancer. 2015; 22(2): 153-60. https://dx.doi.org/10.1007/s12282-013-0465-3.
- Yoon J.H., Kim M.H., Kim E.K., Moon H.J., Kwak J.Y., Kim M.J. Interobserver variability of ultrasound elastography: how it affects the diagnosis of breast lesions. AJR Am. J. Roentgenol. 2011; 196(3): 730-6. https://dx.doi.org/10.2214/AJR.10.4654.
- Berg W.A., Mendelson E.B., Cosgrove D.O., Dore C.J., Gay J., Henry J.P., Cohen-Bacrie C. Quantitative maximum shear-wave stiffness of breast masses as a predictor of histopathologic severity. AJR Am. J. Roentgenol. 2015; 205(2): 448-55. https://dx.doi.org/10.2214/AJR.14.13448.
- Cosgrove D.O., Berg W.A., Dore C.J., Skyba D.M., Henry J.P., Gay J. et al. Shear wave elastography for breast masses is highly reproducible. Eur. Radiol. 2012; 22(5): 1023-32. https://dx.doi.org/10.1007/s00330-011-2340-y.
- Tourasse C., Denier J.F., Awada A., Gratadour A.C., Nessah-Bousquet K., Gay J. Elastography in the assessment of sentinel lymph nodes prior to dissection. Eur. J. Radiol. 2012; 81(11): 3154-9. https://dx.doi.org/10.1016/j.ejrad.2012.04.031.
- Kilic F., Velidedeoglu M., Ozturk T., Kandemirli S.G., Dikici A.S., Er M.E. et al. Ex vivo assessment of sentinel lymph nodes in breast cancer using shear wave elastography. J. Ultrasound Med. 2016; 35(2): 271-7. https://dx.doi.org/10.7863/ultra.15.03039.
- Zhao Q., Sun J.W., Zhou H., Du L.Y., Wang X.L., Tao L. et al. Pre-operative conventional ultrasound and sonoelastography evaluation for predicting axillary lymph node metastasis in patients with malignant breast lesions. Ultrasound Med. Biol. 2018; 44(12): 2587-95. https://dx.doi.org/10.1016/j.ultrasmedbio.2018.07.017.
- Youk J.H., Son E.J., Kim J.A., Gweon H.M. Pre-operative evaluation of axillary lymph node status in patients with suspected breast cancer using shear wave elastography. Ultrasound Med. Biol. 2017; 43(8): 1581-6. https://dx.doi.org/10.1016/j.ultrasmedbio.2017.03.016.
- Seo M., Sohn Y.M. Differentiation of benign and metastatic axillary lymph nodes in breast cancer: additive value of shear wave elastography to B-mode ultrasound. Clin. Imaging. 2018; 50: 258-63. https://dx.doi.org/10.1016/j.clinimag.2018.04.013.
- Gennisson J.L., Deffieux T., Fink M., Tanter M. Ultrasound elastography: principles and techniques. Diagn. Interv. Imaging. 2013; 94(5): 487-95.
- Shiina T., Nightingale K.R., Palmeri M.L., Hall T.J., Bamber J.C., Barr R.G. et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 1: basic principles and terminology. Ultrasound Med. Biol. 2015; 41(5): 1126-47. https://dx.doi.org/10.1016/j.ultrasmedbio.2015.03.009.
- Sigrist R.M.S., Liau J., Kaffas A.E., Chammas M.C., Willmann J.K. Ultrasound elastography: review of techniques and clinical applications. Theranostics. 2017; 7(5): 1303-29. https://dx.doi.org/10.7150/thno.18650.
Received 02.07.2020
Accepted 07.10.2020
About the Authors
Nafset D. Khakurinova, Oncologist, Department of the day hospital (chemotherapeutic and surgical methods of treatment), Research Institute of Clinical Oncology,N.N. Blokhin National Medical Research Center of Oncology, Ministry of Health of the Russian Federation (N.N. Blokhin NMRCO).
Tel.: +7(499)324-98-95. E-mail: nafset2701@mail.ru. 115478, Russia, Moscow, Kashirskoye highway, 23.
Vyacheslav M. Snitkin, post-graduate student of the Department of ultrasound diagnostics of the Research Institute of Clinical and Experimental Radiology, N.N. Blokhin National Medical Research Center of Oncology, Ministry of Health of the Russian Federation (N.N. Blokhin NMRCO). Tel.: +7(499)324-98-95. E-mail: snitkinvm@yandex.ru.
115478, Russia, Moscow, Kashirskoye highway, 23.
Vladimir N. Sholokhov, MD, Professor, leading researcher of the Department of ultrasound diagnostics of the Research Institute of Clinical and Experimental Radiology,
N.N. Blokhin National Medical Research Center of Oncology, Ministry of Health of the Russian Federation (N.N. Blokhin NMRCO).
Tel.: +7(499)324-98-95. E-mail: vnshell@mail.ru. 115478, Russia, Moscow, Kashirskoye highway, 23.
Alexander V. Petrovsky, PhD, deputy director for the development of cancer care in the regions, Research Institute of Clinical Oncology, N.N. Blokhin National Medical Research Center of Oncology, Ministry of Health of the Russian Federation (N.N. Blokhin NMRCO). Tel.: +7(499)324-98-95. E-mail: alexpetrovsky@hotmail.com.
115478, Russia, Moscow, Kashirskoye highway, 23.
Ramiz K. Valiev, PhD., Head of the Oncological Department of surgical methods of treatment No. 13, Research Institute of Clinical Oncology, N.N. Blokhin National Medical Research Center of Oncology, Ministry of Health of the Russian Federation (N.N. Blokhin NMRCO).
Tel.: +7(499)324-98-95. E-mail: info@ronc.ru. 115478, Russia, Moscow, Kashirskoye highway, 23.
Dmitry E. Avtomonov, PhD., assistant of the Department of Oncology, Institute of Clinical Medicine named after N.V. Sklifosovsky, I.M. Sechenov First Moscow State Medical University, Ministry of Health of the Russian Federation. Tel.: +7(925)866-68-72. E-mail: info@ronc.ru. 115478, Russia, Moscow, Kashirskoye highway, 23.
Igor V. Samoylenko, PhD, senior researcher, Oncological Department of surgical methods of treatment No. 12 (oncodermatology), N.N. Blokhin National Medical Research Center of Oncology, Ministry of Health of the Russian Federation (N.N. Blokhin NMRCO). Тel.: +7(499)324-90-24, +7(903)627-05-41. Е-mail: igor.samoylenko@gmail.com.
115478, Russia, Moscow, Kashirskoye highway, 23.
For citation: Khakurinova N.D., Snitkin V.M., Sholokhov V.N., Petrovsky A.V., Valiev R.K., Avtomonov D.E., Samoylenko I.V. Choosing an optimal algorithm for the assessment of the condition of regional lymph nodes in patients with breast cancer using modern ultrasound diagnostics.
Akusherstvo i Ginekologiya / Obstetrics and gynecology. 2020; 10: 118-124 (in Russian)
https://dx.doi.org/10.18565/aig.2020.10.118-124



