Non-contraceptive benefits of combined oral contraceptives: opportunities for the correction of vaginal microbiocenosis
Objective. To evaluate the role of combined estrogen and progestin oral contraceptives in the correction of vaginal microbiocenosis in patients with recurrent bacterial vaginosis.Kutsenko I.I., Kravtsova E.I., Rubinina E.R.
Materials and Methods. The assessment of the disease manifestations, characteristics of the vaginal microflora and local immunity was performed in patients with recurrent bacterial vaginosis before and after the treatment while they were taking various oral contraceptives.
Results. Lactobacillus-deficient flora and decreased local vaginal immunity affecting nonspecific protection of biological media, secretory function and cellular mechanisms were revealed in patients with recurrent bacterial vaginosis.
Conclusion. Combined oral contraceptives with estradiol valerate and dienogest have an advantage over ones with ethinyl estradiol in case of contraception in patients with bacterial vaginosis.
Keywords
Introduction
Since the invention of combined oral contraceptives (COCs), these drugs have been constantly improved, mainly in the dosage of ethinyl estradiol and changes in the progestin component. To date, modern COCs contain the 3rd generation progestins and ethinyl estradiol, which have minimal side effects and a number of additional non-contraceptive properties that allow a clinician to select individual therapy taking into account the presence of a range of genital and extragenital pathological conditions in patients. The effect of COC on the vaginal microflora has been repeatedly discussed. Since the vaginal microflora is largely hormone-dependent, it is logical to assume that use of COC should have a positive effect on the local microbiota. However, along with the studies confirming the positive role of COCs, there is evidence of a possible increase in frequency of vaginal candidiasis, as well as the risk of developing bacterial vaginosis [1, 2 ,3, 4]. It is known that the synthesis of glycogen in the cells of the vagina occurs with the action of estrogen. Progesterone causes desquamation and destruction of the epithelial cells of the vagina, followed by the release of glycogen from these cells. Glycogen breaks down into maltose and dextrose, which serve as substrates for lactobacilli. In the process of interaction of lactobacilli and glycogen, lactic acid is formed, which maintains the necessary pH level of vaginal secretion and creates a natural filter. The estrogen-dependent ability of lactobacilli to adhere to epithelial cells, the production of hydrogen peroxide and antibiotic-like substances prevent the reproduction and growth of pathogenic and opportunistic microorganisms [2, 5, 6]. A decrease in the estrogenic saturation of the vaginal epithelium leads to favorable conditions for the development of bacterial vaginosis. Among women of reproductive age, bacterial vaginosis is diagnosed with a frequency of 4% to 87%, and the recurrence rate within six months to a year after treatment reaches 30% [7]. The recurrence of bacterial vaginosis is due to the lack of the nonspecific immunity of the vagina, in particular its nonspecific, humoral and cellular links; it also can be directly associated with estriol [8, 9]. Probably, the absence of bioidentity in ethinyl estradiol did not allow us to unambiguously answer the question about the effect of COC with ethinyl estradiol on the vaginal biotope. The appearance of estradiol valerate as COC component, a bioidentical synthetic estrogen, which is metabolized in a woman’s body to estriol, has made it possible to continue research in this direction.
The objective of the study is to evaluate the role of combined estrogen and progestin oral contraceptives in the correction of vaginal microbiocenosis in patients with recurrent bacterial vaginosis.
Materials and Methods
The study group included 106 patients aged from 25 to 35 years with recurrent bacterial vaginosis (2–4 episodes a year), voluntary informed consent was obtained. The control group consisted of 35 healthy patients aged 18–35 years.
The criterion for inclusion in the main group was the presence of recurrent bacterial vaginosis. The exclusion criteria were the presence of HIV infection, viral hepatitis, syphilis, gonococcal, chlamydia, ureaplasma, mycoplasma, viral (cytomegalovirus, herpes simplex virus, human papilloma virus), trichomonas infection, fungal infection, the presence of IUD, pregnancy, and other gynecological pathologies, acute extragenital pathology, exacerbation of chronic extragenital pathology, diabetes mellitus type 1 and 2, grade 3 obesity.
A study of vaginal microbiocenosis was conducted in all patients of the main and control groups. Bacterial vaginosis was verified on the basis of Amsel’s criteria (vaginal pH was determined using the test for self-diagnosis of vaginal acidity Premium Diagnostics with a color scale from 4.0 to 7.0) and microscopy of a Gram-stained vaginal smear using the Nugent method. Vaginal biocenosis was studied in women using real-time PCR or quantitative PCR (qPCR) (Femoflor 16), including facultative anaerobic microorganisms, obligate anaerobic microorganisms, mycoplasmas and fungi. The type of dominant lactoflora was determined by the method for genotyping qPCR.
In the vaginal secretion, the indicators of local immunity were investigated: lysozyme was studied using the method of competitive enzyme immunoassay (Human Lysozyme ELISA kit EL3010-1 (Assay Max, USA)); sIgA, IL-6, IL-10 were studied using enzyme-linked immunosorbent assay (ELISA) (ELISA-Best test system) with the calculation of the pro-inflammatory index (PII), as IL-6 to IL-10 ratio.
Bacterial vaginosis (BV) treatment was carried out according to a single scheme including decontamination and contamination therapy. As decontamination therapy, clindamycin at a dose of 300 mg was administered orally twice a day for seven days, and a drug combining micronized metronizazole 750 mg and micronized miconazole nitrate 200 mg was used intravaginally, one suppository per night daily for seven days. As decontamination therapy, a probiotic containing lyophilized culture of lactobacilli L. casei rhamnosus Doderleini (1108 CFU per one capsule) was taken; one capsule during 14 days was administered daily intravaginally. After completing the course of decontamination-contamination, a follow-up examination was performed to confirm the effectiveness of therapy. In all cases the sexual partner was examined by a urologist, and, if necessary, treated according to the diagnosis. In the future, due to the need for contraception, the predominant number of people observed (80 patients) were prescribed combined hormonal contraceptives. Depending on the prescribed drug, the patients were divided into three clinical groups. Group 1 (n=27) used four-phase COC containing estradiol valerate and dienogest, group 2 (n=27) used monophasic drug containing 30 μg ethinyl estradiol and dienogest, and group 3 (n=26) used monophasic COC containing 30 μg ethinyl estradiol and 3 mg drospirenone. Patients who refused to take hormonal contraception were included in clinical group 4 (n=26). Monitoring of the surveyed contingent lasted for six months; the number of relapses, the dynamics of the state of local immunity and vaginal microbiocenosis were assessed.
The statistical analysis of the data was performed using the characterization of descriptive statistics. Preliminary analysis of the distribution of numerical data in the samples was carried out using graphical construction, the test for normality of distribution was performed using the Shapiro-Wilk test. To describe the quantitative data with a normal distribution, the arithmetic mean (M) and standard deviation (SD) were used. When describing features with a distribution that is different from the normal, the average values were described as a median and a flat deviation. When performing the main task of comparing two independent groups on the same basis, the two-tailed Student’s t-test for independent variables and the Mann-Whitney U-test were used. To compare the two samples in percent, the φ* – Fisher angular transformation was used. Statistical analysis of the data was performed using standard methods of mathematical statistical processing using Microsoft Office Excel 2013 and Statistica 6.0 software. Differences between groups were considered statistically significant at p < 0.05.
Results and Discussion
The mean age of the examined patients was 25.7 (5.3) years. In the main group, a positive amine test and the presence of key cells in smear microscopy were detected in all patients (100%), the vaginal pH was 5.3 (5; 5.5), which was statistically significantly higher than 4.0 (4; 4.4), p < 0.001 in patients of the control group. When interpreting the results of assessment of the vaginal biotope on the Nugent scale in patients of the main group, the number of points was 9 (8; 9), which corresponded to the parameters defined as bacterial vaginosis (7–10 points) and was statistically highly significantly different from the results of patients in the control group 2 (1; 2), p < 0.001.
According to the study of the vaginal biocenosis in patients of the main clinical group, 99 cases (93.4%) were interpreted as marked dysbiosis, 87 cases (82%) – aerobic dysbiosis, 19 cases (18%) – mixed dysbiosis; cases were classified using the algorithms for interpreting the results of the Femoflor-16 test. Total bacterial number (TBN) in patients with bacterial vaginosis averaged 106.8 CFU/sample, the absolute number of lactobacilli is 103.6 CFU/ml, the relative number of lactobacilli on average is -2.6 log of the TBN. In the control group, the TBN was 107.4 CFU/ml, with a relative number of lactobacilli of 107.2 CFU/ml, which corresponded to -0.1 log of the TBN.
In the control group in 32 patients (91%), the only predominant type of lactobacilli was L. crispatus. In 3 cases (2.8 %), we noted an increase in L. iners, L. jensenii, L. gasseri, but it was not dominant in any of the cases. In the group of patients with recurrent BV, L. iners was identified in 58 cases (54.7%), and in 46 patients (43%) their growth was dominant. L. crispatus was detected in all patients, and the dominant growth was observed in only 36 patients (34%); L. jensenii and L. gasseri dominated in 10 (9.4%) and 15 (14%) cases, respectively. Vaginal microflora in patients with BV was characterized by an excessively high concentration of obligate and facultative anaerobic opportunistic microorganisms (> 107 CFU/ml). Among the obligate anaerobic organisms, Gardnerella vaginalis dominated, it was detected in all patients; Atopobium vaginae was found in 45 patients (42.5%), Lachnobacterium spp./Clostridium spp. was revealed in 32 cases (30.2%), Mobiluncus spp./Corynebacterium spp. was found in 28.3%. Facultative and obligate microorganisms in excess of 104 CFU/ml were represented by Enterobacteriaceae spp. – 12 patients (11.3%), Streptococcus spp. – 4.7% of patients, Staphylococcus spp. – 4 patients (3.8%) .
The local immunity of vaginal secretions in patients with recurrent BV was characterized by a statistically significant decrease in the level of lysozyme, it was 17.3 (16.2; 17.8) μg/L versus 52.6 (48.7; 53.4) μg/L in the control group, p < 0.001. There was also a statistically significant decrease in sIgA, performing the function of immune exclusion, to 21.6 (17.5; 26.6) mg/L versus 78.4 (78.4; 88.4) mg/L in the control group, p < 0.001. Despite the absence of an inflammatory reaction in patients with recurrent BV, there was an imbalance in the synthesis of cytokines involved in the development of the inflammatory response. Thus, the level of IL-6 was statistically significantly increased to 106.3 (103.7; 109.1) pg/ml versus 90.2 (90.2; 94.8) pg/ml in control group, p < 0.001. At the same time, there was a statistically significant decrease in IL-10 to 38.5 (37.8; 39.6), p < 0.001, which was reflected in the PII of the vaginal secret, which was 2.7 (2.4; 2.9) in the test group, against 1.5 (1.3; 1.5) in the control group, p < 0.001.
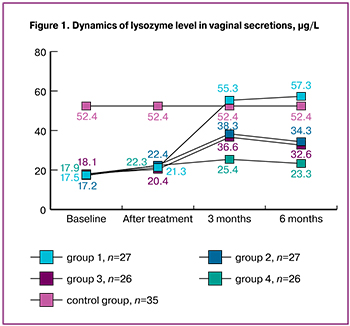 In general, according to our research, recurrent BV was characterized by a pronounced decrease in lactobacilli in the vaginal secretion, with a prevalence of L. iners in most women, a high concentration of obligate and facultative anaerobes, as well as an impairment of the local immunity of the vagina, affecting the nonspecific protection of biological media, secretory function and cellular mechanisms, which corresponds to the data of previous studies [4, 5]. At the end of the treatment, Amsel’s criteria in all 106 patients (100%) were absent, and the pH of the vagina was 4.0 (4; 4.4). The results of the assessment of the vaginal biotope on the Nugent scale revealed a decrease in the number of points to 5 (5; 6), which corresponded to the definition of intermediate microflora (4–6 points). The degree of epithelium colonization by facultative and obligate microorganisms decreased to 104 CFU/ml and less, and the colonization of lactic acid bacteria increased to 10 6-7 CFU/ml. At the same time, we noted some improvement in the local immunity of the vaginal secretions.
In general, according to our research, recurrent BV was characterized by a pronounced decrease in lactobacilli in the vaginal secretion, with a prevalence of L. iners in most women, a high concentration of obligate and facultative anaerobes, as well as an impairment of the local immunity of the vagina, affecting the nonspecific protection of biological media, secretory function and cellular mechanisms, which corresponds to the data of previous studies [4, 5]. At the end of the treatment, Amsel’s criteria in all 106 patients (100%) were absent, and the pH of the vagina was 4.0 (4; 4.4). The results of the assessment of the vaginal biotope on the Nugent scale revealed a decrease in the number of points to 5 (5; 6), which corresponded to the definition of intermediate microflora (4–6 points). The degree of epithelium colonization by facultative and obligate microorganisms decreased to 104 CFU/ml and less, and the colonization of lactic acid bacteria increased to 10 6-7 CFU/ml. At the same time, we noted some improvement in the local immunity of the vaginal secretions.
Thus, the level of lysozyme increased to 19.4 (19.2; 20.5) μg/L, the level of secretory sIgA increased to 27.6 (27.6; 29.5) mg/L, and the PII of the vaginal secretion decreased to 1.7 (1.5; 1.9).
 Moreover, all indicators of local immunity were statistically significantly different from the results of healthy women (lysozyme – 52.6 (48.7; 53.4) µg/L, sIgA – 78.4 (78.4; 88.4) mg/L, PII – 1.5 (1.3; 1.6), p < 0.001 for all indicators. (Fig. 1, 2, 3).
Moreover, all indicators of local immunity were statistically significantly different from the results of healthy women (lysozyme – 52.6 (48.7; 53.4) µg/L, sIgA – 78.4 (78.4; 88.4) mg/L, PII – 1.5 (1.3; 1.6), p < 0.001 for all indicators. (Fig. 1, 2, 3).
It was noted that in patients of group 1, the use of four-phase COCs with estradiol valerate and dienogest dynamically increased the level of lysozyme, secretory immunoglobulin sIgA and decreased PII of vaginal secretion, and by the 6th month of observation, the results practically reached or even exceeded the indicators of the control group in some parameters (group 1, lysozyme – 55.9 (52.2; 62.6) µg/L, sIgA – 78.4 (73.1; 88.4) mg/L, PII – 1.5 (1.4; 1.7) (Fig.1, 2, 3).
When using monophasic drug containing 30 μg ethinyl estradiol and dienogest (group 2) and monophasic COC containing 30 μg ethinyl estradiol and 3 mg drospirenone (group 3), the parameters of local immunity improved by the sixth month, the level of lysozyme increased to 32.6 (30.7; 34.6) μg/L in group 2 and up to 31.5 (29.1; 33.7) in group 3. The level of sIgA increased to 44.3 (38.5; 48.6) mg/L in group 2 and to 42.6 (37.7; 47.6) in group 3; PII decreased to 1.9 (1.7; 1.8) in group 2 and up to 1.8 (1.6; 1.7) in group 3. It was noted that all indicators characterizing the state of local immunity units of the vagina were statistically significantly different from group 4, where COCs were not used ( lysozyme – 17.9 (17.5; 19.6) µg/L, sIgA – 18.5 (16.7; 19.6) mg/L, PII – 2.3 (2.2; 2.4), p < 0.001 for all indicators), but they did not reach the indicators of the results of the control group, p < 0.001 (Fig. 1, 2, 3).
When genotyping lactobacilli of the vagina 6 months after the end of observation, we found a change in the dominant type of lactobacilli in 13 patients (48.1%) in group 1, in 6 patients (22.2%) in group 2 and in 5 patients (19.2%) in group 3. In group 4, the type of dominant lactobacilli flora did not change in any woman. During the entire observation period, we noted a single case of relapse in 1 patient of group 1 (3.7%), in 5 (18.5%) and 6 (19.2%) patients in groups 2 and 3, respectively, and in 16 patients (64%) in group 4, which correlated with the above state of local immunity of the vagina and the prevailing domination of L. iners (Table 1).
 Conclusions
Conclusions
Standard decontamination and contamination therapy for recurrent bacterial vaginosis leads to a temporary improvement in the vaginal microbiocenosis, with little or no effect on the state of local defense reactions.
Combined oral contraceptives containing ethinyl estradiol improve local immunity and help to reduce the recurrence of bacterial vaginosis.
Four-phase COC containing estradiol valerate and dienogest has an advantage over COC with ethinyl estradiol when contraception is necessary for a patient with bacterial vaginosis, probably due to the pharmacodynamic characteristics of estradiol valerate. Ethinyl estradiol, being a synthetic drug, directly affects competitive target cells. Estradiol valerate as a synthetic, but bioidentical drug is metabolized to estriol, which directly interacts with target cells together with endogenous estriol. This ensures its more effective influence on the proliferative processes occurring in the vaginal epithelium, indirectly improving local immunity and favoring the growth of lactoflora.
Dienogest, as the component of the COC without anti-estrogenic activity, supports the beneficial effect of estradiol valerate on the vaginal mucosa, which together provide the support for the vaginal normocenosis, thereby contributing to a significant reduction in the recurrence of bacterial vaginosis.
References
- Дикке Г.Б. Значение оральной контрацепции в профилактике рецидивов бактериального вагиноза и снижении риска воспалительных заболеваний органов малого таза. Проблемы репродукции. 2017; 23(4): 32-36. https://doi.org/10.17116/repro201723432-36 [Dikke G.B. The importance of oral contraception in the prevention of recurrence of bacterial vaginosis and reducing the risk of inflammatory diseases of the pelvic organs. Reproduction problems. 2017; 23(4): 32-36. https://doi.org/10.17116/repro201723432-36 (in Russian)]
- Майсурадзе Л.В., Магаева Ф.Ю., Алборов Д.К. Влияние бактериального вагиноза на течение беременности, состояние плода и новорожденного. Кубанский научный медицинский вестник. 2014; 6: 8-62. https://doi.org/10.25207/1608-6228-2014-6-58-62 [Maisuradze L.V., Magaeva F.Yu., Alborov D.K. Influence of bacterial vaginosis on the course of pregnancy, the state of the fetus and newborn. Kuban scientific medical Bulletin. 2014; 6: 8-62. https://doi.org/10.25207/1608-6228-2014-6-58-62(in Russian)]
- Камалова К.А., Ящук А.Г. Комбинированные оральные контрацептивы и вагинальное здоровье. Медицинский вестник Башкортостана. 2016; 11(3): 71-74. [Kamalova K.A., Yaschuk A.G. Combined oral contraceptives and vaginal health. Medical Bulletin of Bashkortostan. 2016; 11(3): 71-74. (in Russian)]
- Радзинский В.Е., Ордиянц И.М., Побединская О.С., Буянова Н.В. Современные аспекты коррекции дисбиотических нарушений в гинекологической практике. Акушерство и гинекология: новости, мнения, обучение. 2013; 2: 72-75. [Radzinsky V.E., Ordiyants I.M., Pobedinskaya O.S., Buyanova N.V. Modern aspects of correction of dysbiotic disorders in gynecological practice. Obstetrics and gynecology: news, opinions, training. 2013; 2: 72-75. (in Russian)]
- Кира Е.Ф., Гайтукиева Р.А., Муслимова С.З., Артымук Н.В. Биоценоз и функциональная активность эпителия влагалища при местном лечении аэробного вагинита Полижинаксом и Тержинаном. Здоровье женщины. 2014; 8: 104. [Kira E.F., Gaitukiyeva R.A., Muslimov S.Z., Artymuk N.V. Ecological community and the functional activity of the vaginal epithelium in the local treatment of aerobic vaginitis Poliginaks and Argininom. Women’s health. 2014; 8: 104. (in Russian)]
- De Seta F., Restaino S., De Santo D., Stabile G., et al. Effects of hormonal contraception on vaginal flora. Contraception. 2012; 86: 526-29.
- Пустотина О.А. Бактериальный вагиноз: патогенез, диагностика, лечение и профилактика. Акушерство и гинекология. 2018; 3: 150-6. https://dx.doi.org/10.18565/aig.2018.3.150-156 [Pustotina O.A. Bacterial vaginosis: pathogenesis, diagnosis, treatment and prevention. Obstetrics and gynecology. 2018; 3: 150-6. https://dx.doi.org/10.18565/aig.2018.3.150-156.(in Russian)]
- Жаркин Н.А., Замараев В.С., Савченко Т.Н., Марушкина О.И. Бактериальный вагиноз и репродуктивное здоровье женщин Мед. альманах. 2015; 4: 84–86. [Zharkin N.A., Zamaraev V.S., Savchenko T.N., Marushkina O.I. Bacterial vaginosis and reproductive health of women Honey. almanac. 2015; 4: 84-86(in Russian)]
- Красиков Н.В., Филяева Ю.А., Тотчиев Г.Ф. Микробиоценоз влагалища: клинические аспекты, пути коррекции и профилактика нарушений. Акушерство и гинекология. 2016; 11: 57-63. http://dx.doi.org/10.18565/aig.2016.11.57-63 [Krasikov N.V., Filyaeva J.A., Totchiev G.F. Microbiocenosis of the vagina: clinical aspects, ways of correction and prevention of violations. Obstetrics and gynecology. 2016; 11: 57-63. http://dx.doi.org/10.18565/aig.2016.11.57-63 (in Russian)]
Reсeived 14.05.2019
Accepted 21.06.2019
About the Authors
Kutsenko Irina, doctor of medical Sciences, Professor, head of the Department of obstetrics, gynecology and perinatology Kuban State Medical University Ministry of Healthof Russia, Krasnodar. Tel. +7 (988)2420460, iikucenko@mail.ru https://orcid.org/0000-0003-0938-8286 350063, Russian Federation, Krasnodar Region, Krasnodar, St. Sedina, 4
Kravtsova Elena, PhD, associate Professor of obstetrics, gynecology and Perinatology Kuban State Medical University Ministry of Health of Russia, Krasnodar.
Tel. +7 (918)3915973, luzum69@mail.ru https://orcid.org/0000-0001-8987-7375
350063, Russian Federation, Krasnodar Region, Krasnodar, St. Sedina, 4 Rubinina Elita rubenovna, post-graduate student of the Department of obstetrics, gynecology and Perinatology Kuban State Medical University, Ministry of Health of Russia, Krasnodar. Tel. +7 (989)811-99-99, rubinina_edita@mail.ru, https://orcid.org/0000-0002-7599-2257
350063, Russian Federation, Krasnodar Region, Krasnodar, St. Sedina, 4
For citations: Kutsenko I.I., Kravtsova E.I., Rubinina E.R. Тon-contraceptive benefits of combined oral contraceptives: opportunities for the correction of vaginal microbiocenosis. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2019; 7: 92-7 (in Russian)
http://dx.doi.org/10.18565/aig.2019.7.92-97



