Использование высокоэффективных и безопасных методов контрацепции позволяет сохранить репродуктивное здоровье женщины [1]. Среди методов контроля рождаемости и планирования семьи применение пероральных препаратов для контрацепции занимает одну из лидирующих позиций во всем мире. По данным ООН, в 2019 г. 151 млн женщин во всем мире использовали противозачаточные таблетки, причем в последние 30 лет количество принимающих эти препараты возрастает с каждым годом [2]. Большинство комбинированных оральных контрацептивов (КОК) содержит этинилэстрадиол (ЭЭ) в комбинации с прогестагеном. Именно прогестагенный компонент в первую очередь отвечает за противозачаточный эффект. Основная функция компонента с эстрогеном заключается в уравновешивании воздействия прогестина на эндометрий, тем самым обеспечивается приемлемый характер кровотечения. Первые гормональные КОК содержали высокие дозы как синтетического эстрогена (местранол 150 мкг), так и прогестагена (норэтинодрел 10 мг), обладали высокой контрацептивной эффективностью, но их использование сопровождалось серьезными побочными эффектами [3]. КОК, содержащие высокие дозы ЭЭ, были связаны с такими медицинскими рисками, как увеличение частоты венозной тромбоэмболии и сердечно-сосудистых заболеваний.
Пути снижения побочных эффектов комбинированных оральных контрацептивов
В настоящее время в состав современных КОК традиционно входят как эстрогенный, так и прогестагенный компоненты, различающиеся по эффективности, сродству к стероидным рецепторам и физиологическим эффектам [4]. С целью улучшения переносимости и безопасности КОК при одновременном сохранении высокой контрацептивной эффективности исследователи долгое время работали в трех направлениях: модификация гестагенного компонента, снижение дозы ЭЭ, замена ЭЭ на его природные аналоги.
Прогестины первых поколений, производные тестостерона (например, норэтиндрон, левоноргестрел), обычно связаны с появлением андрогензависимых побочных эффектов, включая акне, изменение углеводного и липидного обменов. Для более селективного связывания с рецепторами прогестерона были разработаны прогестины, производные прогестерона и спиронолактона (например, хлормадинона ацетат, дроспиренон), что привело к минимизации побочных эффектов и более нейтральному влиянию на метаболические параметры [5]. Кроме того, некоторые новые прогестины, такие как дроспиренон (ДРСП), обладают антиандрогенным и антиминералокортикоидным действием [6]. Поиск наиболее эффективной и безопасной комбинации эстрогена с прогестином является актуальной задачей современной медицины и фармакологии на протяжении уже многих лет.
Эстрогены традиционно считаются половыми гормонами, ответственными за развитие и регуляцию женской репродуктивной системы и вторичных половых признаков, хотя на самом деле они вызывают изменения практически во всех клетках позвоночных [7]. Эстрогеновые рецепторы присутствуют повсеместно, что обуславливает воздействие эстрогенов практически на все физиологические системы и ткани, включая мочевыводящие пути и сердечно-сосудистую систему, а также на головной мозг, кости, молочную железу, кожу, волосы, слизистую оболочку, мышцы таза и пищевое поведение [8]. Эстрогены воздействуют на функцию печени, таким образом влияя на толерантность к глюкозе, липидный гомеостаз, выработку в печени белков, ответственных за активацию свертывающей системы крови. Кроме того, эстрогены также играют определенную роль в развитии таких заболеваний, как рак эндометрия, молочной железы, яичников, предстательной железы, колоректальный рак, наряду с эндометриозом, тромбофлебитом глубоких вен нижних конечностей, тромбоэмболией легочной артерии. Хотя с точки зрения любого увеличения риска рака молочной железы более значим именно прогестагенный компонент КОК и менопаузальной гормональной терапии, а не эстроген [9]. С другой стороны, эстрогены обладают профилактическим эффектом при остеопорозе, ожирении, резистентности к инсулину, сердечно-сосудистых и нейродегенеративных заболеваниях [10]. Снижение дозы ЭЭ ≤20 мкг позволило сократить побочные эстрогензависимые эффекты, но стало сопровождаться менее благоприятным профилем кровотечения, что уменьшило комплаентность у пациенток и приверженность к приему КОК [11].
В течение жизни в организме женщины обнаруживаются четыре природных эстрогена. Как и для всех гормонов, их названия и аббревиатуры отражают количество гидроксильных групп (ОН), присутствующих в 4-кольцевом остове (рисунок).
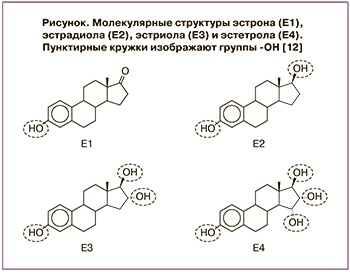
Эстрон (Е1) присутствует на протяжении всей жизни женщины и считается основным эстрогеном в период менопаузы. Эстрадиол (Е2), вырабатываемый яичниками, является основным эстрогеном репродуктивного возраста. Эстриол (Е3) естественным образом синтезируется плацентой и является основным эстрогеном во время беременности. Эстетрол (Е4) представляет собой эстроген эмбрионального периода, вырабатываемый печенью плода и присутствующий только во время беременности с относительно высокими уровнями у плода и более низкими уровнями в кровотоке матери. После родов уровень Е4 в крови быстро становится неопределяемым [13].
Эстетрол – эстроген с уникальными свойствами
Эстетрол представляет собой стероидный гормон с четырьмя группами -ОН, на две больше, чем у эстрадиола. Две дополнительные гидроксильные группы оказывают решающее влияние на фармакокинетику при пероральном приеме: период полувыведения Е4 составляет 24–28 ч по сравнению с 10–20 минутами для Е3, 1–2 ч для природного Е2 и 12 ч для микронизированного Е2. В печени плода Е4 синтезируется из Е2 и Е3 с помощью двух ферментов: 15α- и 16α-гидроксилаз, посредством гидроксилирования. Затем Е4 минимально метаболизируется и не превращается обратно в активные эстрогены – Е3 и Е2. Таким образом эстетрол является одним из наиболее стабильных представителей эстрогенов с точки зрения стероидогенеза.
Эстетрол был открыт в 1965 г. в Каролинском институте в Стокгольме [14] и изучался в течение 20 лет как биомаркер благополучного состояния плода во время беременности. В течение многих лет после открытия считалось, что Е4 может служить показателем здоровья плода, но устойчивой корреляции не было обнаружено, и даже сейчас физиологическое значение Е4 при беременности неизвестно [15]. Интерес к Е4 полностью угас в середине восьмидесятых, так как он считался очень «слабым» эстрогеном, а в качестве маркера состояния плода не подходил. Возрождение эстетрола как эффективного природного эстрогена состоялось в 2001 г., когда компания Pantarhei Bioscience провела доклиническое изучение E4, изложив результаты в статье «Эстетрол: уникальный стероид при беременности у человека», где описывались особый профиль активности E4 и его пригодность для терапевтического использования [16]. Начиная с 2009 г. Pantarhei, Mithra Pharmaceuticals и несколько академических групп объединили усилия для изучения фармакологических характеристик Е4 и его молекулярных механизмов действия. Для использования человеком Е4 синтезируют из коммерчески доступного соевого эстрона. Он имеет чистоту более 99,9% без примесей [17].
Уникальные свойства Е4 обусловлены его селективностью. Аналогично другим эстрогенам он активирует ядерные эстрогеновые рецепторы (ER). Однако в отличие от своих предшественников Е4 противодействует активности мембранных рецепторов. Поэтому в различных тканях организма он может проявлять себя и как агонист ядерных эстрогеновых рецепторов, и как антагонист мембранных рецепторов и таким образом оказывать эстрогенный эффект на влагалище, эндометрий, костную ткань, головной мозг и сердечно- сосудистую систему, при этом подавляя избыточную пролиферацию тканей молочной железы. Кроме того, он практически не влияет на метаболизм в печени, углеводный, липидный обмен и суррогатные маркеры активации гемостаза. Е4 демонстрирует высокоселективное связывание со своими основными мишенями ER-α и ER-β, причем селективность к ER-α в 4–5 раз выше, чем к ER-β. В последние годы Е4 изучался в прогностических, валидированных моделях на крысах. In vivo наблюдались дозозависимые фармакодинамические эффекты на ткани влагалища и матки, массу и прочность костей, приливы и подавление овуляции, причем потентность (молярная активность лиганда) у Е4 в 10–20 раз ниже, чем у ЭЭ, что гарантирует более низкий риск появления неспецифических побочных эффектов при его применении у человека. Также оказалось, что на моделях клеток молочной железы и опухолей Е4 действовал как антагонист эстрогена (в присутствии Е2) с эффективностью, сравнимой с тамоксифеном и овариэктомией [17].
Также уникальные свойства Е4 создают предпосылки к изучению его противоопухолевой активности, обусловленной выраженным снижением пролиферативного действия Е2 [18]. На основании наблюдаемого в доклинических исследованиях противоопухолевого потенциала Е4 были проведены исследования, изучающие эффективность E4 для лечения инвазивного рака молочной железы и рака предстательной железы, демонстрирующие противоопухолевый эффект высоких доз E4 (20–40 мг) при обоих видах рака [19, 20]. В целом результаты как доклинических, так и первоначальных клинических исследований предполагают, что Е4 оказывает антиэстрогенное действие на молочные железы и не оказывает негативного влияния на нормальные или злокачественные ткани молочной железы при использовании в терапевтических концентрациях. Это свойство Е4 связано с антагонистическим действием на пролиферацию, миграцию и инвазию клеток молочной железы в присутствии Е2.
Клинические исследования по оценке эффективности и безопасности нового комбинированного орального контрацептива с эстетролом
Первоначальные клинические исследования I и II фазы были сосредоточены на использовании Е4 в составе КОК. В исследовании 2015 г. Е4 оценивался в комбинации с ДРСП и ЛНГ. Участницы получали 5 или 10 мг эстетрола/3 мг дроспиренона; 5, 10 или 20 мг ЭЭ/150 мкг левоноргестрела; 20 мкг ЭЭ/3 мг дроспиренона в течение трех последовательных циклов по схеме 24/4 дня. Оценивались характер менструальноподобной реакции, степень подавления овуляции, изменения биохимических показателей функции печени, углеводного и липидного обмена, костных маркеров и гормонов роста до и в процессе использования КОК в сравнительном аспекте. Все препараты продемонстрировали достаточную безопасность и эффективность. Однако в группах E4/ДРСП и E4/ЛНГ влияние препаратов на транспортные белки было менее выражено по сравнению с группой ЭЭ/ДРСП, где наблюдалось значительное увеличение в крови концентрации глобулина, связывающего половые гормоны (ГСПГ). Также в группах с Е4/ДРСП наблюдалось менее выраженное влияние на уровень липопротеинов и триглицеридов, чем в группе ЭЭ/ДРСП, изменений инсулиноподобного фактора роста также не отмечалось. Это можно объяснить тем, что Е4 не взаимодействует с ферментами семейства цитохрома Р450 и практически не влияет на метаболизм в печени. Поэтому на фоне использования препаратов, содержащих Е4, отмечается снижение уровня D-димера, отсутствуют изменения в количестве и активности антитромбина и протеина S [21].
Оценка эффективности и безопасности нового КОК, состоящего из 15 мг Е4 в сочетании с 3 мг дроспиренона (прогестагена, производного спиронолактона), у сексуально активных женщин в возрасте от 18 до 50 лет с регулярными менструальными циклами и индексом массы тела ≤35 кг/м2 проводилась в двух открытых многоцентровых исследованиях III фазы в США/Канаде и в Европейском Союзе и России [22–27]. Всего под наблюдением находились 3417 женщин в возрасте от 16 до 50 лет, из них 3027 в возрасте от 16 до 35 лет включительно, включенных в каждое исследование в течение 1 года. В США и Канаде исследуемый препарат получали 1864 женщины в возрасте от 16 до 50 лет, из них 1674 были в возрасте от 16 до 35 лет включительно. В Евросоюзе и России участвовало 69 центров, исследуемый препарат получали 1553 женщины в возрасте от 18 до 50 лет включительно, в том числе 1353 женщины в возрасте от 18 до 35 лет. Исследуемый препарат представлял собой таблетку, принимаемую перорально 1 раз в сутки по схеме 24/4 дня (т.е. 24 дня приема таблеток, содержащих активный препарат, с последующим приемом таблеток плацебо в течение 4 дней). Общая продолжительность применения препарата составляла тринадцать 28-дневных циклов, т.е. 12 месяцев. Визиты к врачу проводились во время второго, четвертого, седьмого, десятого цикла приема препарата и в течение 3 недель после прекращения приема препарата, в это время оценивались возможные нежелательные явления. Женщины, участвующие в исследовании, в специальном ежедневном дневнике отмечали прием препарата, регистрировали дни и интенсивность вагинального кровотечения или кровянистых выделений, использование других методов контрацепции и половые контакты. При анализе контрацептивной эффективности в исследовании у женщин в Евросоюзе и России в возрасте от 18 до 35 лет включительно индекс Перля (PI) составил 0,47 беременности на 100 женщин (95% ДИ 0,15–1,11), в исследовании США/Канада у женщин в возрасте от 16 до 35 лет включительно PI составил 2,65 (95% ДИ 1,73–3,88). Эти значения PI согласуются с другими исследованиями по изучению эффективности КОК и показывают, что E4/ДРСП обеспечивает высокий уровень контрацептивной защиты, высокую степень удовлетворенности пользователей, а также хорошую переносимость и профиль безопасности. Кроме того, препарат практически не оказывает влияния на функцию печени, систему гемостаза и липидный спектр крови у женщин в возрасте 16–50 лет с ИМТ ≤35,0 кг/м2.
В этих исследованиях также был отмечен хороший контроль цикла на фоне приема нового КОК. О регулярных менструальноподобных кровянистых выделениях длительностью в среднем в 4,5 дня сообщили 82,9–87% пациенток из США и Канады и 91,9–94,4% женщин из Евросоюза и России. В течение всего исследования было зафиксировано снижение частоты межменструальных кровотечений в течение 6 циклов: с 23,5% в 1-м цикле до <16 с 6-го цикла в исследовании, проведенном в Евросоюзе и России, и с 30,3% в 1-м цикле до 15,5–19,2% через 4 цикла приема препарата в США и Канаде. Чем дольше пациентка принимала препарат, тем реже встречались межменструальные кровотечения. Суммарное число дней с незапланированными кровянистыми выделениями на фоне приема Е4/ДРСП составило 1,3 дня. В Евросоюзе и России наиболее распространенными нежелательными явлениями были головная боль (7,7%), метроррагия (5,5%), вагинальное кровотечение (4,8%) и акне (4,2%), и было сообщение об одном нежелательном явлении в виде венозного тромбоза нижних конечностей. В США и Канаде наиболее распространенными нежелательными явлениями были головная боль (5%) и метроррагия (4,6%). В Евросоюзе и России 141 женщина (9,1%) досрочно прекратила участие в исследовании из-за побочных эффектов, связанных с лечением. В США и Канаде 132 женщины (7,1%) досрочно прекратили участие в исследовании, чаще всего причиной были метроррагии (0,9%), меноррагии (0,8%) и увеличение веса (0,5%). Таким образом, контрацептивные таблетки, содержащие 15 мг E4 и 3 мг ДРСП, продемонстрировали переносимость и безопасность в диапазоне других зарегистрированных и доступных КОК.
В России данное открытое многоцентровое исследование III фазы для оценки эффективности и безопасности КОК, содержащего 15 мг эстетрола и 3 мг дроспиренона, проводилось с 28 марта 2016 г. по 31 мая 2018 г. Набор и обследование пациентов выполнялись в 10 центрах: ФГБУ «Национальный медицинский исследовательский центр акушерства, гинекологии и перинатологии им. академика В.И. Кулакова» МЗ РФ (главный исследователь – И.А. Аполихина, соисследователи – А.Е. Бычкова, Е.А. Горбунова, Е.Г. Додова, Е.А. Межевитинова); ФГБУ «Научный центр проблем здоровья семьи и репродукции человека» (главный исследователь – Л.В. Сутурина, соисследователи – Т.А. Базарова, И.Н. Данусевич, И.И. Коваленко, Л.М. Лазарева, А.В. Левина, О.Я. Лещенко, Я.Г. Наделяева, А.Г. Хомякова); ФГБУ «Научно-исследовательский институт акушерства, гинекологии и репродуктологии им. Д.О. Отта» (главный исследователь – М.А. Тарасова, соисследователи – Е.В. Мусина, М.А. Шалина); ФГБУ «Ивановский научно-исследовательский институт материнства и детства имени В.Н. Городкова» (главный исследователь – И.А. Панова, соисследователи – Е.Л. Бойков, А.К. Красильникова, Л.М. Салахова); ГБУЗ Московской области «Московский областной научно-исследовательский институт акушерства и гинекологии» (МОНИИАГ) (главный исследователь – Е.С. Булычева, соисследователи – Л.К. Джиджихия, Н.В. Зароченцева); ФГБОУ ВО «Казанский государственный медицинский университет» МЗ РФ (главный исследователь – А.А. Хасанов, соисследователи – Ю.В. Орлов, Л.В. Щеголихина); ГБУЗ г. Москвы «Городская клиническая больница № 13 Департамента здравоохранения г. Москвы» (главный исследователь – Ф.М. Григорян, соисследователи – С.Р. Османова, А.А. Плотников); Санкт-Петербургской ГБУЗ «Родильный дом № 17» (главный исследователь – Н.А. Татарова, соисследователи – Е.В. Жигалова, С.В. Петрова); ООО «Гранти-мед» (главный исследователь – А.М. Маржевская, соисследователи – В.А. Линде, В.В. Маржевская); ООО «Медицинский центр женского здоровья» (главный исследователь – И.М. Ордиянц, соисследователь – Е.Г. Ордиянц). Общая продолжительность исследования составила 13 28-дневных циклов, т.е. 12 месяцев, полностью завершили участие в исследование 343 пациентки. Проведение данного исследования в Российской Федерации позволило завершить регистрацию лекарственного препарата «Эстеретта» для пероральной гормональной контрацепции у женщин уже в 2022 г. и получить для женщин в России самый современный на сегодняшний день контрацептив.
В исследовании 2021 г. было показано, что сочетание 15 мг эстетрола с 3 мг дроспиренона не влияет на образование тромбина по сравнению с препаратами, содержащими ЭЭ, которые в сочетании с левоноргестрелом или дроспиреноном могут повышать выработку прокоагулянтных факторов и снижать выработку антикоагулянтных, тем самым приводить к активации свертывающей системы крови. Эксперты Европейского агентства по лекарственным средствам по надлежащей оценке тромбогенного профиля новых препаратов КОК (EMEA) рассматривают приобретенную резистентность к активированному протеину С (ACP) в качестве суррогатного маркера риска тромбоза [24]. Прием препарата, содержащего Е4/ДРСП, оказывает менее выраженное влияние на нормализованный коэффициент чувствительности к активированному протеину С (nAPCsr) по сравнению с КОК, содержащим ЭЭ/ЛНГ и ЭЭ/ДРСП [24, 25]. К суррогатным биомаркерам влияния КОК на систему гемостаза так же относится и ГСПГ, белок-носитель эстрогена и тестостерона, вырабатываемый печенью. Доказано, что Е4 не взаимодействует с ГСПГ и оказывает минимальное влияние на его синтез и свертывающую систему крови, а следовательно, КОК, содержащий Е4/ДРСП, обладает высокой гемостазиологической безопасностью. Таким образом, в результате анализа вышеперечисленных исследований было сделано заключение, что комбинации, содержащие Е4/ДРСП, оказывают минимальное влияние на функцию печени, липидный обмен, костную ткань и эндокринные параметры роста. Известно, что изменения в системе гемостаза во время приема любого КОК в основном обусловлены действием эстрогенов на синтез белков печени, влияющих на свертывающую систему крови. Комбинация Е4/ДРСП обладает существенно меньшей эстрогенной активностью в отношении гепатоцитов, гемостатических биомаркеров и клеток эндотелия сосудов по сравнению с препаратами, включающими ЭЭ/ДРСП. На основании полученных данных ДРСП был выбран оптимальным прогестином для комбинации с Е4 [18, 19, 26]. Терапевтическая доза Е4 для КОК в сочетании с дроспиреноном может обеспечить лучший профиль польза/риск в отношении возникновения рака молочной железы по сравнению с гормональной контрацепцией, доступной в настоящее время [27]. Кроме того, как описано выше, положительный эффект E4 в исследовании 2-й фазы для лечения женщин с поздней стадией эндокринно-резистентного рака молочной железы является многообещающим [19].
Использование КОК с Е4/ДРСП в 2021 г. было одобрено Управлением по санитарному надзору за качеством пищевых продуктов и медикаментов США (FDA), Европейским агентством по лекарственным средствам (EMA), Министерством здравоохранения Канады и Управлением терапевтических товаров (TGA) в Австралии. С 2022 г. данный контрацептив доступен для использования в РФ. КОК в комбинации эстетрол/дроспиренон хорошо переносится и расширяет возможности доступной гормональной контрацепции с потенциально сниженным риском тромботических событий [28]. Новый гормональный КОК с эстетролом «Эстеретта» может быть рекомендован как для широкого применения в рутинной практике, так и для дальнейшего изучения в ходе клинических исследований и наблюдательных программ. Проведение новых сравнительных и наблюдательных исследований КОК и других контрацептивов поможет врачам-гинекологам в их повседневной практике в формировании персонализированного подхода в подборе контрацептивных средств [29].
Заключение
Таким образом, эстетрол, обладая уникальным профилем активности, представляет собой химическое соединение, отличное от всех других эстрогенов. Этот эстроген, впервые описанный более 80 лет назад, может удовлетворить потребность в более безопасной гормональной контрацепции женщин репродуктивного возраста. Благодаря минимальному влиянию на метаболизм белков в печени, гемостаз, липиды и молочную железу, а также благоприятному профилю, связанному с тромбозами, Е4 потенциально является более безопасным эстрогеном. На основании приведенных данных можно сделать вывод, что комбинация эстетрола и дроспиренона не только обладает доказанной контрацептивной эффективностью и отличным профилем безопасности/переносимости, но и представляется перспективной с точки зрения комплаентности пациенток, поскольку встречаемость побочных эффектов, традиционно приписываемых КОК, у данной комбинации минимальна или отсутствует. Использование высокоэффективной, безопасной и рациональной контрацепции способствует борьбе с абортами и их осложнениями, сохранению репродуктивной функции женщины, рождению желанного здорового ребенка и сохранению будущего нации.



