Morphological abnormalities in oocytes in patients with HIV infection receiving antiretroviral therapy
Aim: To study the prevalence of oocyte dysmorphisms in HIV-infected women receiving antiretroviral therapy (ART). Materials and methods: The retrospective study included 210 married couples who underwent IVF. The main group included 113 HIV-infected patients, and the control group included 97 women without HIV infection, who underwent 163 and 123 ovarian stimulation cycles, respectively. Morphological assessment of 2321 mature MII oocytes was performed, of them 1228 oocytes were obtained in the main group and 1093 in the control group.. Results: The median age of patients with HIV infection was 34 (31;37) years; duration of the diseases and Art WAS 8 (5;11) and 3.6 (2;6) years, respectively. 65 of 113 women (57.5%; 95% CI: 47.8–66.4) had subclinical stage 3 of the disease, 43 women (38.1%; 95% CI: 29.2–47.8) had stage 4A, 2 women (1.8%; 95% CI: 0–4.4) had stage 4B, and 3 women (2.7%; 95% CI: 0–6.2) had stage 3C of HIV infection (Russian HIV classification). The average CD4+ lymphocytes count was 582 (432;807) cells/µl, and immunoregulatory index (IRI) was 0.87. All patients received ART and had had an undetectable viral load in the blood beforee IVF treatment. The prevalence of various types of oocyte dysmorphisms in HIV-infected women was significantly higher versus the women in the control group and was 29.5% (362/1228) and 14.1% (154/1093), respectively (р<0.001, RR 2.0; 95% CI: 1.765–2.480). Among ocyte cytoplasmic abnormalities in both groups, centrally located cytoplasmic granulation (CLCG) prevailed. Detection rate of this type of dysmorphisms was 17.3% (213/1229) in the main group, and 10% (109/1093) in the control group. The difference was statistically significant (р<0.001; RR 2.26; 95% CI: 1.8–2.7). The prevalence of extracytoplasmic abnormalities of oocytes, as well as combination of their types in HIV-infected patients was comparable with HIV-seronegative control. Conclusion: The higher prevalence of oocyte dysmorphisms in HIV-infected patients who received ART versus healthy women, indicates deterioration of the morphological features of oocytes in this group of patients.Mityurina E.V., Perminova S.G., Kravchenko A.V., Kozyrina N.V., Veyukova M.A., Gaponenko A.A.
Keywords
A key factor for success in assisted reproductive technology cycles is functional and morphological maturity of gametes. It has been shown in animal experiments, that oocyte quality is the most important factor than sperm characteristics [1, 2]. Oocyte competence, the ability to fertilize and further development of the early embryo is determined by synchronization between nuclear and cytoplasmic maturation. When this process is impaired or asynchronous, it can lead to certain morphological abnormalities in the structure of oocytes, and in vitro fertilization (IVF) cycles their prevalence reaches 50% [3]. It is commonly accepted that there are differences between extracytoplasmic and cytoplasmic abnormalities in the structure of oocytes, and they are called dysmorphisms. Cytoplasmic dysmorphisms of oocytes include centrally located cytoplasmic granularity (CLCG), vacuoles, smooth endoplasmic reticulum aggregates (SERa), as well as dark or non-homogenous cytoplasm. A number of researchers assume that oocytes with dysmorphic features have a low fertilization rate, and embryos derived from such oocytes have a low potential for implantation and further development [4–6]. Extracytoplasmic dysmorphisms include changes in oocyte shape, granularity in the perivitelline space, shape or thickness change in zona pellucida, as well as abnormal polar body. The experts of the Istanbul consensus workshop on embryo assessment reported that extracytoplasmic abnormalities should be considered as phenotypic abnormalities (variations), and some of them as indicators of oocyte aging [7]. The issue of a negative impact of dysmorphisms on fertilization rate and embryonic development remains controversial [4, 5, 8, 9].
The role of a woman's age, as well as some gynecological diseases and metabolic disorders in occurrence of oocyte morphological abnormalities is discussed in literature. [10]. Moreover, some researchers consider the duration of ovarian stimulation and total dose of gonadotropins to be a risk factor for dysmorphisms [4, 11–13]. However, there is no a common point of view regarding the influence of the above factors on morphology of oocytes. It should be mentioned, that morphological characteristics of oocytes in patients receiving gonadotropins are not well studied yet. So, HIV-infection takes a special place among chronic infectious diseases. According to the Joint United Nations Programme on HIV/AIDS (UNAIDS), the number of people living with HIV reached 38,0 (31,6–44,5) million worldwide in 2020, of them 26,0 (25,1–26,2) million people received antiretroviral therapy (ART) at the end of June, 2020. It should be noted, that nowadays assisted reproductive technologies allow to consider this disease as a contolled chornic pathology, to preserve quality of life and extend lifetime of patients with HIV infection [14]. However, it is being discussed in literature, that both severe immunodeficiency associated with HIV infection and ART have a negative impact on the quality of oocytes. A number of studies showed mitochondrial DNA (mtDNA) depletion in oocytes and cumulus cells and high frequency of mutations in patients with HIV infection using nucleoside and nucleotide reverse transcriptase inhibitors (NRTIs) [15–17]. The authors believe that this is associated with decreased in vitro fertilizing ability of oocytes and low efficiency of IVF program among the patients of this group. However, there is no published data on the studies of morphological characteristics of oocytes in HIV-infected women receiving ART. This aspect is of scientific interest due to the increased number of women of reproductive age with HIV infection who are planning to conceive using ART.
The purpose of this research was to study the prevalence of oocyte dysmorphisms in HIV-infected women receiving antiretroviral therapy.
Materials and methods
The retrospective study included 210 married couples who underwent IVF. 113 HIV-infected patients were included in the main group and 97 non-HIV-infected women in the control group, who underwent 163 and 123 ovarian stimulation cycles, respectively. Morphological assessment of 2321 mature MII oocytes was performed, of them 1228 oocytes were obtained from women in the main group and 1093 in the control group. Inclusion criteria were: a positive HIV status of women in the main group and absence of HIV infection in the control group. Exclusion criteria in both groups were contraindications for using assisted reproductive technologies (The Order No. 803n of 31.07. 20 “On the procedure of using assisted reproductive technologies, contraindications and limitations for using them” of the Ministry of Health of the Russian Federation).
Superovulation stimulation was performed in accordance with protocols for ovarian stimulation with gonadotropin-releasing hormone antagonists (GnRH-ant) using recombinant follicle-stimulating hormone (rFSH) preparations. When the follicles reached a diameter of 14–15 mm, GnRH-ant was administered by subcutaneous injection at a dose of 0.25 mg/day to block endogenous luteinizing hormone (LH) surge before the day of ovulation after administering a trigger shot. Human chorionic gonadotropin (HCG) at a dose of 10.000 IU or 0.2 mg of GnRH-ant was used as ovulation trigger when 3 or more follicles of ≥17 mm in diameter were visualized. Oocytes were aspirated 35–36 hours after trigger injection. Further, oocyte-cumulus complexes (OCCs) were retrieved from the obtained follicular fluid, and then placed in sterile culture medium plates for fertilization (G-IVF, VitroLife) in the incubator at T=37.2оC and 6.5% CO2. Cumulus cells were mechanically and enzymatically denuded from OCCs after 2–3 hours. Then the ICSI procedure was performed, during which the maturity degree of cells, their morphological characteristics and dysmorphisms were assessed. Сentral granularity was recognized as a dark, spongy granular area in the cytoplasm of oocytes; and vacuoles were as cytoplasmic inclusions containing fluid and surrounded by membrane; Smooth endoplasmic reticulum aggregates were a round shaped semitranslucent formations in the central part of the oocyte. Dark cytoplasm indicated a significant change in the color of the whole cytoplasm of the oocyte. Extracytoplasmic dysmorphisms included changes in the size of the perivitelline space and the presence of granularity, abnormal first polar body, as well deformation or thickening of zona pellucida of the oocyte.
Statistical analysis
IPM SРSS Statistics, Version 26 was used for statistical data processing. The results are presented as median values and inrterquartile range (Ме (Q1;Q3)). Mann–Whitney U-test was used to compare the differences between the groups. The Chi-Square Test (χ2) was used to compare categorical data and to assess significant differences between them. The odds ratio (OR) and 95% confidence interval (CI) were estimated. The differences were considered statistically significant at p<0.05.
Results
The median age of patients with HIV infection was 34 years (31; 37 years). Duration of the disease and antiretroviral therapy was 8 (5; 11) and 3.6 (2; 6) years, respectively. 65 of 113 women (57.5%, 95% CI: 47.8–66.4) had subclinical stage 3 of the disease, 43 women (38.1%, 95% CI: 29.2–47.8) had stage 4А, 2 women (1.8%, 95% CI: 0–4.4) had stage 4B and 3 women (2.7%, 95% CI: 0–6.2) had stage 4C of HIV infection. At the time of inclusion in the study all women with stage 4 of HIV infection were in remission on the background of ART. Median CD4 T-cell count was 582 cells/μL. (432; 807), CD8+ lymphocytes count was 740 cells/μL (611; 982), and the value of immunoregulatory index (IRI) was 0.87. All patients received ART and had an undetectable viral load in the blood before IFV. (30.1%, 95% CI: 21.2–38.9) Every third woman had hepatitis C antibodies. Table 1 shows clinical characteristics of patients included in the study.
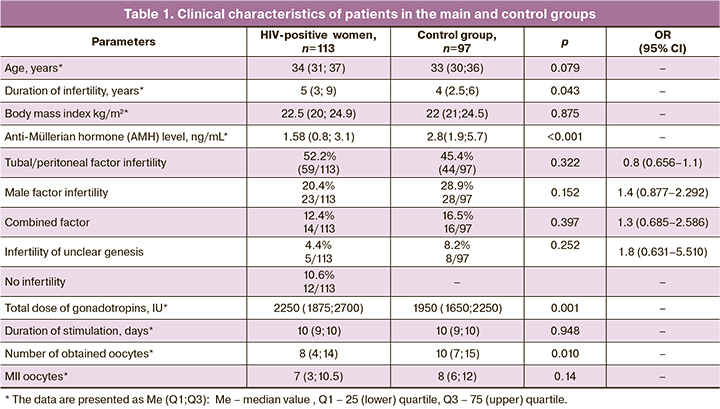
The age of patients (34 (31; 37) and 33 (30; 36) years; р=0.079), body mass index (22.5 (20; 24.9) and 22 (21; 24.5) kg/m2; р=0.875) and infertility factors mainly due to tubal/peritoneal genesis were comparable between both groups. It should be noted, that HIV-infected women had lower anti-Müllerian hormone levels (АМГ) compared to women in the control group (1.58 (0.8; 3.1) ng/mL and 2.8 (1.9; 5.7) ng/mL, respectively; р=0.001). Due to this, a high total dose of gonadotropins (2250 (1875; 2700) and 1950 (1650; 2250) IU, respectively, р=0.001) was used during ovarian stimulation in the main group of patients; and lower number of oocytes was obtained: 8 (4; 14) and 10 (7; 15), respectively, (р=0.010) (Table 1). At the same time, duration of stimulation was 10 (9; 10) and 10 (9; 10) days, respectively (р=0.948); and the number of mature MII oocytes was 7 (3; 10.5) and 8 (6; 12), respectively (р=0.14), and was comparable between the groups.
Further, the morphological characteristics of mature oocytes were assessed. The prevalence of various types of oocyte dysmorphisms in HIV-infected women was significantly higher versus women in the control group: 29.5% (362/1228) and 14.1% (154/1093), respectively (р<0.001, OR 2.092, 95% CI: 1.765–2.480).
As presented in Table 2, oocyte cytoplasmic abnormalities were significantly most common in the main group compared to the control group (26.37% and 11.7%, respectively; р<0.001, OR 2.26, 95% CI: 1.8–2.7). The most common form of cytoplasmic dysmorphisms of oocytes in the studied sample was central cytoplasmic granulation. Detection rate of the abnormal oocyte structure in the main group was 17.3% (213/1229), and 10% (109/1093) in the control group. The differences were statistically significant (р<0.001; OR 2.26, 95% CI: 1.8–2.7). Vacuoles in the oocytes of HIV-infected patients were found most often compared to the control group (1.4% and 0.3%, respectively; р=0.004; OR 5.044, 95% CI: 1.482–17.164), as well as non-homogeneous cytoplasm (7% and 1.7%, respectively; р<0.001, OR 4.029, 95% CI: 2.468–6.577); but oocytes with dark cytoplasm were found similarly often in both groups (1.1% and 0.5%, respectively; р=0.174, OR 1.9, 95% CI: 0.736–5.056). It should be noted, that smooth endoplasmic reticulum aggregates were identified only in the oocytes of HIV-infected women (1.3%, 16/1228).
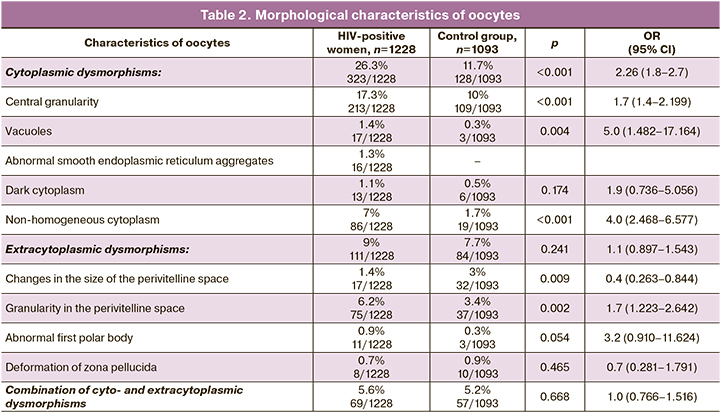
Detection rate of extracytoplasmic anomalies in the structure of oocytes, as well as in combination with other parameters in HIV-infected patients was comparable with HIV-seronegative control. At the same time, granularity in the perivitelline space was significantly more often identified in the main group – in 6.2% (75/1228) of women and in 3.4% (37/1093) of women in the control group, respectively; р=0.002, OR 1.798, 95% CI: 1.223–2.642). Changes in the size of the perivitelline space in the control group were in 1.4% (17/1228) and in 3% (32/1093) of women in the main group, respectively; (р=0.009, OR 0.471, 95% CI: 0.263–0.844). Abnormal first polar body was most often in patients with HIV infection, however, statistically significant differences were on the borderline of statistical significance – 0.9% (11/1228) and 0.3% (3/1093), respectively; (р=0.054, OR 3.2, 95% CI: 0.910–11.624). Deformation of zona pellucida was found similarly often in both groups (0.7% (8/1228) and 0.9% (10/1093), respectively; р=0.465, ОШ 0.7, 95% CI: 0.281–1.791).
Discussion
The cumulative number of people infected with HIV reached 1,492,998 in the Russian Federation (as of the end of 2020), of them 37.5% were women. 604 999 HIV-positive patients received ART [18]. The highest rate of HIV infection is among the age group of 30–44 years. Although men prevail among the Russian cohort of HIV-positive people, it should be mentioned that women have 4-7 times higher risk of infection due to their anatomical structure and prolonged presence of semen with a high proportion of viral particles in vagina. Moreover, the risk of infection increases in the presence of sexually transmitted infections and ulceration of genital mucous membranes [19]. The use of highly active antiretroviral therapy (HAART) allows HIV-infected women both to plan spontaneous pregnancy and to conceive through assisted reproductive technologies. However, there is a decline in fertility in this group of patients with any stage of HIV infection. This can be explained by high percentage of pelvic inflammatory diseases, as well as negative effect of nucleoside reverse transcriptase inhibitors (NRTIs) on lipid metabolism and insulin resistance [20, 21]. Gonadotoxic effects of antiretroviral therapy remains under dispute in the context of low effectiveness of the use of assisted reproductive programs in women with HIV infection [20, 21]. However, given that mitochondrial toxicity of NRTIs has been proved, the studies are mainly focused on assessment of mtDNA copy number in gametes. Currently, the morphological characteristics of oocytes from HIV-infected women have not been studied yet.
As already mentioned, morphological oocyte abnormalities are found in more than half of assisted reproductive technologies cycles, and when they occur, the rate of conceiving decreases [3]. In this study, the incidence rate of different dysmorphisms in patients with HIV-infection was 29.5%. This was significantly higher versus HIV-negative women (14.1%; р<0.001).
The patients with HIV-infection included in this study were of reproductive age (Ме age was 34 years), and an average duration of the disease (Me) was 8 years and mean (Me) duration of antiretroviral therapy was 3.6 years. It should be noted, that assessment of the strength of relationship between these variables showed inverse relationship between the rate of detection of oocyte morphological abnormalities and duration of ART (r=0.173; р<0.001). High incidence of dysmorphisms in HIV-infected women receiving ART versus HIV-negative patients indicates morphological feature of the oocytes in this group of patients.
Analysis of clinical and laboratory parameters showed, that that women with HIV infection had significantly lower AMH levels (1.58 and 2.8 ng/mL; р<0.001), although the age of patients in both groups was comparable (34 and 33 years; р=0.079). The obtained data correlate with the results presented by other researchers. So, Scherzer R. et al. (2015) showed that AMH levels in HIV-infected female patients was consistently lower in all age groups versus non-HIV-infected women [22]. It should be noted, that a number of studies showed that serum concentrations of AMG reflect the morphological characteristics of oocytes. The authors explain it by the fact that AMG is produced by granulosa cells and circulates in follicular fluid, thus it can influence oocyte quality due to the effects of oocyte‐derived paracrine factors. It remains unclear, whether correlation between AMH levels and the presence of oocyte dysmorphisms is associated with reduction in AMH secretion by granulosa cells in oocytes of low quality or, conversely, with a possible negative effect of low hormone levels on oocyte quality [23–25]. Based on these data, high rates of dysmorphisms among HIV-infected patients versus non-HIV-infected women can be explained by low serum AMH levels (1.58 and 2.8 ng/mL; р≤0.001).
When ovarian stimulation was carried out with a higher total dose of gonadropins in women in the main group versus the control group (Ме 2250 and 1950 МЕ; р=0.001, and lower number of oocytes was obtained (Ме 8 and 10, respectively; р=0.010). This can be explained by poor ovarian reserve. It is noteworthy, that there is a direct dependence between detection rate of oocyte morphological abnormalities and total doze of gonadotropins in HIV-infected women (r=0.222, р<0.001). Figueira et al. (2010) reported that a total dose of FSHs positively correlated with occurrence of extracytoplasmic dysmorphisms, in particlular, granularity in the perivitelline space (r=0,125; p=0.018) [26].
In both groups, central cytoplasmic granulation prevailed (17.3% and 10%) in the structure of oocyte cytoplasmic dysmorphisms. Most often it was found in HIV-infected patients (р<0.001). According to the published data, the incidence rate of this oocyte abnormality reaches 30–35% [27], and the issues related to the age of a woman as a risk factor and different protocols for ovarian stimulation are still being discussed [5]. The effect of central cytoplasmic granulation on the outcomes of IVF cycles remains disputable. P. Tulay et al. (2019) reported that there were no differences in fertilization rates between oocytes with normal cytoplasm and oocytes with central cytoplasmic granulation. Moreover, cleavage and blastulation rates and the proportion of aneuploid embryos were also comparable between the groups. However, clinical pregnancy rate in the group of patients with central cytoplasmic granulation was lower versus the control group. The authors made a conclusion that embryos derived from oocytes with central cytoplasmic granulation have a low potential for implantation [28]. On the contrary, other researchers explain low rate of pregnancy in the group of such patients by high rates of aneuploidies (52%) in blastomeres of embryos obtained after fertilization of oocytes with central granulation of the cytoplasm [27].
Vacuoles (1.4 and 0.3%; р=0.004) and dark cytoplasm (1.1 and 0.5%; р=0,174) were found not often both in the main and in the control groups. According to the data reported by other researchers, the incidence rate of oocyte vacuolization is also low and is less than 3–4% [5]. Risk factors for this dysmorphism are not clear, and fertilization rate of oocytes with vacuoles depends on their number and size. It is believed, that large vacuoles displace oocyte spindle leading to disruption of the architectonics of the cytoplasm. This prevents respective pronuclear movement, and the resulting embryos reach the blastocyst stage less often, and have a lower potential for implantation [8]. Oocytes with dark cytoplasm can be associated with oocyte cytoplasmic immaturity, and this factor also increases the likelihood of poor-quality embryos [29].
Non-homogenous oocyte cytoplasm was found most often in the main group compared to the control group (7 and 1.7%; р<0.001). Most researchers believe that biological significance of non-homogenous cytoplasm is not clear, i.e. neither genesis, nor the impact of this oocyte dysmorphism on the outcome of IVF cycles remains unclear [5, 7].
It is interesting that this study found abnormal aggregates of smooth endoplasmic reticulum only in the group of HIV-infected patients (1.3%, 16/1228). This morphological abnormality of oocytes is most important from the point of view of negative embryonic, clinical and neonatal outcomes. Accordingly, the experts of the Istanbul consensus workshop on embryo assessment do not recommend to use oocytes with abnormal SERa for fertilization. [7]. Risk factor for this dysmorphism is ovarian stimulation, duration and dose of injected gonadotropins [30]. Given the fact that in this study a high doze of gonadotropins was used for ovulation induction in women with HIV-infection versus the control group (Ме 2250 and 1950 IU; р=0.001), we believe that this is one of the factors for occurrence of abnormal SERa in this group of patients.
The rate of extracytoplasmic oocyte dysmorphisms was comparable between the groups (9 and 7.7%; р=0.241). The obtained data are confirmed by research findings reported by other researchers, who demonstrated that these morphological abnormalities are phenotypic abnormalities reflecting heterogeneity of the resulting oocytes [7].
Conclusion
Thus, the patients with HIV-infection using ART had reduced levels of AMH. Due to this, low number of oocytes was obtained, and their morphological characteristics were significantly poor versus women in the control group. For ethical reasons and normative standards, conduction of research related to this issue and involving HIV-infected women, who do not take ART, is not possible in Russia. Therefore, reasonable assumption of the leading role of antiretroviral therapy in the development of oocyte dysmorphisms may be premature. At the same time, the obtained data make it possible to recommend that the patients who are planning pregnancy should not postpone it, and after achieving undetectable viral load in the blood to strive for natural conception or to conceive through assisted reproductive technologies.
References
- Sirard М.-A., François R., Blondin P., Robert C. Contribution of the oocyte to embryo quality. Theriogenology. 2006; 65(1): 126-36. https://dx.doi.org/10.1016/j.theriogenology.2005.09.020.
- De Sousa P.A., Caveney A., Westhusin M.E., Watson A.J. Temporal patterns of embryonic gene expression and their dependence on oogenetic factors. Theriogenology. 1998; 49(1): 115-28. https://dx.doi.org/10.1016/s0093-691x(97)00406-8.
- Setti A.S., Figueira R.C.S., Braga D.P.A.F., Colturato S.S., Iaconelli A. Jr., Borges E. Jr. Relationship between oocyte abnormal morphology and intracytoplasmic sperm injection outcomes: a meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011; 159(2): 364-70. https://dx.doi.org/10.1016/j.ejogrb.2011.07.031.
- Горшкова А.Г., Долгушина Н.В., Макарова Н.П., Ковальская Е.В., Калинина Е.А. Факторы риска развития дисморфизмов ооцитов в программах вспомогательных репродуктивных технологий. Акушерство и гинекология. 2015; 5: 66-73. [Gorshkova A.G., Dolgushina N.V., Makarova N.P., Kovalskaya E.V., Kalinina E.A. Risk factors for the development of oocyte dysmorphisms in programs of assisted reproductive technologies. Obstetrics and gynecology. 2015; 5: 66-73. (in Russian)].
- Rienzi L., Ubaldi F.M., Iacobelli M., Minasi M.G., Romano S., Ferrero S. et al. Significance of metaphase II human oocyte morphology on ICSI outcome. Fertil. Steril. 2008; 90(5): 1692-700. https://dx.doi.org/10.1016/j.fertnstert.2007.09.024.
- Ubaldi F., Rienzi L. Morphological selection of gametes. Placenta. 2008; 29(Suppl. B): 115-20. https://dx.doi.org/10.1016/j.placenta.2008.08.009.
- Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology. The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum. Rep. 2011; 26(6): 1270-83. https://dx.doi.org/10.1093/humrep/der037.
- Wilding M., Di Matteo L., D’Andretti S., Montanaro N., Capobianco C., Dale B. An oocyte score for use in assisted reproduction. J. Assist. Reprod. Genet. 2007; 24(8): 350-8. https://dx.doi.org/10.1007/s10815-007-9143-8.
- Balakier H., Sojeck A., Motamedi G., Bashar S., Mandel R., Librach C. Is the zona pellucida thickness of human embryos influenced by women’s age and hormonal levels ? Fertil. Steril. 2012; 98(1): 77-83. https://dx.doi.org/10.1016/j.fertnstert.2012.04.015.
- Setti A.S., Braga D.P.A.F., Figueira Rde C., Vingris L., Iaconelli A., Borges E. Jr. Body mass index is negatively correlated with the response to controlled ovarian stimulation but does not influence oocyte morphology in ICSI cycles. Eur. J. Obstet. Gynecol. Reprod. Biol. 2012; 163(2): 175-9. https://dx.doi.org10.1016/j.ejogrb.2012.04.002.
- Cota A.M., Oliveira J.B., Petersen C.G., Mauri A.L., Massaro F.C., Silva L.F. et al. GnRH agonist versus GnRH antagonist in assisted reproduction cycles : oocyte morphology. Reprod. Biol. Endocrinol. 2012; 10: 33. https://dx.doi.org/10.1186/1477-7827-10-33.
- Fancsovits P., Tóthné Z.G., Murber Á., Rigó J. Jr., Urbancsek J. Importance of cytoplasmic granularity of human oocytes in in vitro fertilization treatments. Acta Biol. Hung. 2012; 63(2): 189-201. https://dx.doi.org/10.1556/ABiol.63.2012.2.3.
- Yoldemir T. Does the duration of gonadotropin stımulation affect embryo quality on post-retrieval day 3? Gynecol. Endocrinol. 2011; 27(5): 324-30. https://dx.doi.org/10.3109/09513590.2010.491571.
- UNAIDS. Global HIV and AIDS statistics - Fact sheet. Global HIV statistics. 2020. Available at: www.unaids.org
- López S., Coll O., Durban M., Hernàndez S., Vidal R., Suy A. et al. Mitochondrial DNA depletion in oocytes of HIV-infected antiretroviral-treated infertile women. Antiv. Ther. 2008; 13(6): 833-8.
- Bostan A., Demeestere I., Vanderwinden J.-M., Devreker F., Englert Y. Nucleoside analog stavudine depletes mitochondrial DNA with no organelle loss in mouse oocytes. Curr. HIV Res. 2010; 8(2): 127-33. https://dx.doi.org/10.2174/157016210790442678.
- Митюрина Е.В., Перминова С.Г., Селимова Ф.Н., Бурменская О.В., Козырина Н.В., Кравченко А.В. Гонадотоксичные эффекты антиретровирусной терапии у ВИЧ-инфицированных женщин. Акушерство и гинекология. 2020; 4: 111-9. [Mityurina E.V., Perminova S.G., Selimova F.N., Burmenskaya O.V., Kozyrina N.V., Kravchenko A.V. Gonadotoxic effects of antiretroviral therapy in HIV-infected women. Obstetrics and Gynecology. 2020; 4: 111-9 (in Russian)]. https://dx.doi.org/10.18565/aig.2020.4.111-119.
- Федеральный научно-методический центр по профилактике и борьбе со СПИДом. Справка «ВИЧ-инфекция в Российской Федерации» на 31 декабря 2020 г. Доступно по VICH-infektsiya-v-Rossijskoj-Federatsii-na-31.12.2020-..pdf (www.hivrussia.info). [Federal Centre for AIDS Prevention and Control. Reference "HIV infection in the Russian Federation" as of December 31, 2020. (in Russian)]. Available at: VICH-infektsiya-v-Rossijskoj-Federatsii-na-31.12.2020-..pdf (www.hivrussia.info).
- Simon V., Ho D.D., Karim Q.A. HIV/AIDS epidemiology, pathogenesis, prevention, and treatment. Lancet. 2006; 368(9534): 489-504. https://dx.doi.org/10.1016/S0140-6736(06)69157-5.
- Savasi V., Ferrazzi E., Lanzani C., Oneta M., Parrilla B., Persico T. Safety of sperm washing and ART outcome in 741 HIV-1-serodiscordant couples. Hum. Reprod. 2007; 22(3): 772-7. https://dx.doi.org/10.1093/humrep/del422.
- Marques C., Guerreiro C., Soares S.R. Lights and shadows about the effectiveness of IVF in HIV infected women: a systematic review. Infect. Dis. Obstet. Gynecol. 2015; 2015: 517208. https://dx.doi.org/10.1155/2015/517208.
- Scherzer R., Bacchetti P., Messerlian G., Goderre J., Maki P.M., Seifer D.B. et al. Impact of CD4+ lymphocytes and HIV infection on Anti-Müllerian Hormone levels in a large cohort of HIV-infected and HIV-uninfected women. Am. J. Reprod. Immunol. 2015; 73(3): 273-84. https://dx.doi.org/10.1111/aji.12332.
- Ebner T., Sommergruber M., Moser M., Shebl O., Schreier-Lechner E., Tews G. Basal level of anti-Müllerian hormone is associated with oocyte quality in stimulated cycles. Hum. Reprod. 2006; 21(8): 2022-6. https://dx.doi.org/10.1093/humrep/del127.
- Azizi E., Naji M., Nazari L., Salehpour S., Karimi M., Borumandnia N. et al. Serum anti‐Müllerian hormone is associated with oocyte dysmorphisms and ICSI outcomes. Int. J. Gynaecol. Obstet. 2019; 147(2): 179-86. https://dx.doi.org/10.1002/ijgo.12941.
- Borges E., Braga D.P.A.F., Setti A., Figueira R.C., Iaconelli A. Jr. The predictive value of serum concentrations of anti‐Müllerian hormone for oocyte quality, fertilization, and implantation. JBRA Assist. Reprod. 2017; 21(3): 176-82. https://dx.doi.org/10.5935/1518-0557.20170035.
- Figueira R.C., Braga D.P.A.F., Semião-Francisco L., Madaschi C., Iaconelli A. Jr., Borges E. Jr. Metaphase II human oocyte morphology: contributing factors and effects on fertilization potential and embryo developmental ability in ICSI cycles. Fertil. Steril. 2010; 94(3): 1115-7. https://dx.doi.org/10.1016/j.fertnstert.2009.11.039.
- Kahraman S., Yakin K., Dönmez E., Samli H., Bahçe M., Cengiz G. et al. Relationship between granular cytoplasm of oocytes and pregnancy outcome following intracytoplasmic sperm injection. Hum. Reprod. 2000; 15(11): 2390-3. https://dx.doi.org/10.1093/humrep/15.11.2390.
- Tulay P., Arslan H., Buran A., Koprulu Y. Assessment of successful pregnancy using granular oocytes in ICSI treatments. Zygote. 2019; 27(2): 97-100. https://dx.doi.org/10.1017/S096719941900008X.
- Ten J., Mendiola J., Vioque J., De Juan J., Bernabeu R. Donor oocyte dysmorphisms and their influence on fertilization and embryo quality. Reprod. Biomed. Online. 2007; 14(1): 40-8. https://dx.doi.org/10.1016/S1472-6483(10)60762-6.
- Shaw-Jackson C., Van Beirs N., Thomas A.-L., Rozenberg S., Autin C. Can healthy babies originate from oocytes with smooth endoplasmic reticulum aggregates? A systematic mini-review. Hum. Reprod. 2014; 29(7): 1380-6. https://dx.doi.org/10.1093/humrep/deu101.
Received 28.05.2021
Accepted 15.06.2021
About the Authors
Elena V. Mityurina, PhD, Senior Researcher, F. Paulsen Research and Clinical Department for Assisted Reproductive Technologies, V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Healthcare of the Russian Federation, +7(964)796-74-65, mity-elena@yandex.ru,117997, Russia, Moscow, Academica Oparina str., 4.
Svetlana S. Perminova, Dr. Med. Sci., Leading Researcher, F. Paulsen Research and Clinical Department for Assisted Reproductive Technologies, V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Healthcare of the Russian Federation, +7(916)202-16-87, perisvet@list.ru,
117997, Russia, Moscow, Academica Oparina str., 4.
Alexey V. Kravchenko, Dr. Med. Sci., Professor, Leading Researcher, Central Research Institute of Epidemiology of Federal Service for Surveillance on Consumer Rights Protection and Human Wellbeing (Rospotrebnadzor), +7(495)366-05-18, alexey-kravtchenko@yandex.ru, 111123, Russia, Moscow, Novogireevskaya str., 3a.
Nadezhda V. Kozyrina, PhD, Researcher, Central Research Institute of Epidemiology of Federal Service for Surveillance on Consumer Rights Protection
and Human Wellbeing (Rospotrebnadzor), +7(495)366-05-18, nad-kozyrina@yandex.ru, 111123, Russia, Moscow, Novogireevskaya str., 3a.
Maria A. Veyukova, PhD (Bio), Researcher of the F. Paulsen Research and Clinical Department for Assisted Reproductive Technologies, V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Healthcare of the Russian Federation, +7(977)053-44-44, veymary@gmail.com,
117997, Russia, Moscow, Academica Oparina str., 4.
Alexandra A. Gaponenko, post-graduate student, F. Paulsen Research and Clinical Department for Assisted Reproductive Technologies, V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Healthcare of the Russian Federation, +7(926)700-44-14, sasha.gap@mail.ru,
117997, Russia, Moscow, Academica Oparina str., 4.
Authors’ contribution: Mityurina E.V., Perminova S.G. – the concept and design of the study, writing the text of the article; Mityurina E.V., Gaponenko A.A., Veyukova M.A. – material collection and processing; Mityurina E.V. – statistical data processing; Kozyrina N.V., Kravchenko A.V. – editing the text of the article.
Conflicts of interest: The authors declare that they have no conflicts of interest.
Funding: The study was carried out without any sponsorship.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Mityurina E.V., Perminova S.G., Kravchenko A.V., Kozyrina N.V.,
Veyukova M.A., Gaponenko A.A. Morphological abnormalities in oocytes
in patients with HIV infection receiving antiretroviral therapy.
Akusherstvo i Gynecologia/Obstetrics and Gynecology. 2021; 11: 135-142 (in Russian)
https://dx.doi.org/10.18565/aig.2021.11.135-142



