Molecular biological features of HPV type 16 infection and the risk of developing cervical squamous intraepithelial lesions and cervical cancer
Objective. To determine the prevalence of HPV type 16 variants in the Russian Federation (Moscow and Moscow region) and investigate the association between mutations of human papillomavirus type 16 in the long control region (LCR), E6 and E7 genes and the probability of detecting high-grade CIN and cervical cancer.Dmitryukova M.Yu., Korolenkova L.I., Romanyuk T.N., Leshkina G.V., Shipulina O.Yu., Shipulin G.A.
Material and methods. The study examined 63 cervical epithelial scrape samples positive for HPV type 16 that was confirmed by histological and cytological evidence.
Results. Sixty-two of 63 samples belonged to lineage A, and only one sample was assigned to lineage D. Six substitutions for reference sequences were detected, of which four were in the LCR (G7193T, A7316C, T7496C, C24G), and 2 in the E6 gene (T109C, T350G). C24G substitution was statistically significantly more common in samples from CIN2-3 and cervical cancer lesions (OR = 2.41, p = 0.04).
Conclusions. This study for the first time reported the prevalence of HPV type 16 variants in the Russian Federation (Moscow and the Moscow Region) and showed that the population of HPV type 16 is quite monogenic and represented by the European lineage. Six substitutions were found in the examined fragment, and one of them (C24G) was statistically significantly more common in samples from CIN+ lesions. Substitutions A7316C, T109C and T350G showed a trend towards higher detection rates in samples showing CIN2-3.
Keywords
Cervical infection with a high oncogenic risk (HR) human papillomavirus (HPV) is a primary risk factor for cervical cancer (CC) and its precursor, high-grade cervical intraepithelial neoplasia (CIN2-3). HPV is found in almost all cases of cervical cancer [1, 2]. However, less than 10% of infected women have persistent HPV infection, of which only 2-3% eventually develop clinical signs over time [3]. The need to identify women with an increased risk of cervical pathology warrants the search for additional factors of CIN progression. Among such factors are changes in micro-RNA [4] and mRNA genes expression profiles [5], as well as the determination of the most aggressive HPV variants as indicators of CIN progression. Women infected with these HPV types require more frequent follow-up to ensure appropriate detection of CIN. CIN1 in patients aged over 35 years is an indication for excisional or destructive treatment without 18-24 months of active surveillance, which may be offered to patients with other, less aggressive HPV types.
All HPV types associated with the development of cervical cancer and an obligate pre-cancer (CIN2-3) form a single genus of alpha-papillomaviruses including species α5, α6, α7, α9, which differ in prevalence among the population, the risk of persistence and the risk of developing a high-grade CIN (pathogenicity). In recent years, studies addressing this issue have been increasingly focused on sequences of the long control region and the HPV E6/E7 genes, since these regions are responsible for regulating the viral life cycle and determine HPV oncogenic potential [6, 7]. In other words, the variability of the HPV genome in these regions affects the potential of these HPV types for persistence and transformation. Investigating these issues contribute to a deeper insight into the interaction between the host and the virus and the mechanism of HPV-associated carcinogenesis.
HPV type 16 is much more often detected in samples with cancer pathology, compared with other HPV types. Studies of genetic diversity within HPV type 16, have identified several variant lineages: A (previously called Euro-Asian, including sub-lineages A1, A2, A3 (European, EUR), and A4 (Asian); sub-lineages B (African), lineages C (African 2) and D - North American, including lineages D1, D2, D3 [8]. Studies aimed at identifying the association of HPV type 16 with the risk of developing a malignancy have shown that non-European lineages are much more common in cervical samples with CIN2-3 than the European variants [9, 10]. Investigation of the HPV variants in the Russian Federation will reveal the prevalence of certain mutations by region, which is important for assessing and comparing the carcinogenic potential of HPV genotypes in a standardized manner and may also help develop an algorithm for examining and monitoring patients with questionable cytology.
This study aimed to investigate the prevalence of genetic variants of HPV type 16 in the Russian Federation, evaluate the association between HPV type 16 variant and the probability of detecting cervical malignancy, and determine prognostically unfavorable HPV type 16 variants.
Material and methods
Samples. The study included cervical epithelial scrape samples of 63 women residing in Moscow and the Moscow region, who participated in screening programs and were diagnosed with histologically confirmed CIN 2+ (CIN2, CIN3, cervical cancer) at N.N. Blokhin Russian Cancer Research Center of Minzdrav of Russia. All patients signed informed consent for the processing of the research results. All women with cervical lesions underwent an extended colposcopy to determine the area and the nature of the lesion. Samples were collected in the transport medium for BD SurePath liquid-based Pap test (BD Diagnostics, USA). Cytological preparations and Papanicolaou staining were carried on automatic devices BD PrepMate and BD PrepStain (Becton Dickinson, USA). Cytological diagnosis was formulated using 2014 Bethesda System terminology for reporting results of cervical cytology. The diagnosis of CIN1 was verified in an extended biopsy of the affected area. The diagnosis of CIN2 + was made based on a histological examination of tissue samples obtained by a loop excision or cervical conization. The control group of samples negative for intra-epithelial lesions and malignancy (NILM) was selected based on repeat negative cytological studies.
Identification and typing of HR HPV DNA in cervical epithelial scrape samples was performed using the AmpliSens HR HPV screen-titer-14-FL and AmpliSense HR HPV genotype-titer-FL reagent sets (CRI of Epidemiology of Rospotrebnadzor, Moscow). This study included samples of biological material containing HPV type 16.
Amplification of HPV type 16 genome fragment. Variants of HPV type 16 were determined by investigating a long coding region (LCR), the E6 gene, and the E7 gene fragment. The length of the studied fragment was about 1700 nucleotides positioned between 7050 to 780 nucleotides (indicated for the reference sequence of HPV type 16, access code GenBank K02718). The amplification of this region was carried out with two overlapping fragments using the following primers: 1: 5’-CTA CAA GCA GGA TTG AAG GC-3 straight line, reverse 1: 5’-GAT TTC GGT TAC GCC CTT AG-3 ‘, straight 2: 5’ -GGG TTA CAC ATT TAC AAG CAA C-3 ‘, reverse 2: 5’-CAA AGT ACG AAT GTC TAC GTG T-3’. Amplification program: 950 - 15 minutes; 950 - 10 seconds, 650 - 20 seconds, 720 - 20 seconds, 40 cycles; 720 - 2 minutes. Amplification was performed using a Tertsik thermal cycler (DNA-Technology, Russia).
Sequencing of LCR, E6, E7 regions was performed on an AbiPrism sequencer (Applied Biosystems, USA) using the indicated primers, with a double overlap of the sequence under study. Reference sequences were obtained from GenBank: K02718 (variant A1), AF536179 (variant A2), HQ644236 (variant A3), AF534061 (variant A4), AF536180 (variant B1), HQ644298 (variant B2), AF472509 (variant C), HQ644257 (variant D1), AY686579 (variant D2), and AF402678 (variant D3). Sequence analysis was performed using BioEdit 7 software [11].
Phylogenetic analysis was carried out with the MEGA 6 software [12] using the maximum likelihood method with 500 bootstrap replicates.
Statistical analysis was performed using the PASW Statistics18 software. The odds ratio for the presence of a histologically confirmed CIN2 + and HPV type 16 with a 95% confidence interval was calculated using Fisher’s exact test. One-sided ANOVA was used to analyze the correlation between viral load and age of the patient with the morphologically confirmed diagnosis.
Results and discussion
Samples were distributed based on the morphological diagnosis as follows: double-negative results for intraepithelial lesion and malignancy (NILM) (n = 18); low-grade squamous intraepithelial lesion (CIN1) (n = 18), high-grade squamous intraepithelial lesion (CIN2.3) (n = 15); squamous cell carcinoma (n = 11), and adenocarcinoma (n = 1). The age of women ranged from 21 to 65 years (median 34 years) and did not differ for women with normal and pathological cervical cytology (p = 0.12).
HPV type 16 was detected as a mono-infection and coinfection with other types of HPV in 52% (33 of 63) and 48% (30 of 63) of the samples, respectively. The mean viral load in NILM and LSIL samples (5.3 ± 1.3 lg of HPV DNA per 105 human cells) was statistically significantly lower than in CIN2-3 and cancer samples (6.0 ± 0.8 lg of HPV DNA per 105 human cells) (p = 0.02).
The results of the phylogenetic analysis are presented in Figure 1. The overwhelming majority (62 of 63) of samples belonged to lineage A, only one sample was isolated from a patient with a CIN3 was clustered together with the reference sequence of lineage D. At the same time, within lineage A, no separation was detected between the sequences obtained from samples with the different cytological diagnosis.

According to available literature, European variants of HPV are associated with a lower risk of developing cervical malignancy than non-European variants. In particular, next-generation sequencing of more than 3,500 samples from the USA showed a significant increase in the risk of adenocarcinoma in individuals infected with variant D2 with OR> 100 [13], compared to variants of lineage A. Also, in regions with high prevalence of non-European lineages (primarily in Asia) the presence of A4/As and D lineages have been shown associated with disease progression [14]. In Europe, where the HPV population is monogenic and is represented by European variants, no relationship has been found [15]. Thus, the presented data are completely consistent with the results of European studies.
Nucleotide sequence substitutions of the studied fragments that were found in more than three samples are shown in Figure 2. The most commonly polymorphism in the LCR was observed at position 7193 (G/T) (82.5%, 52 of 63), 7521 (G/A) (87.3%, 55 of 63), in the E6 gene at position 350 (G/T) (63.5%, 40 of 63), and at position 109 (T/C) (12.7%, 8 out of 63). Three samples failed to obtain the sequence of LCR, in one sample E6/E7 sequences were detected. Since the detected substitutions were not specific for any variant within the European lineage and are found in all HPV16 variant sub-lineages (A1, A2, A3) [6], it was not possible to divide them within the lineage to a variant.
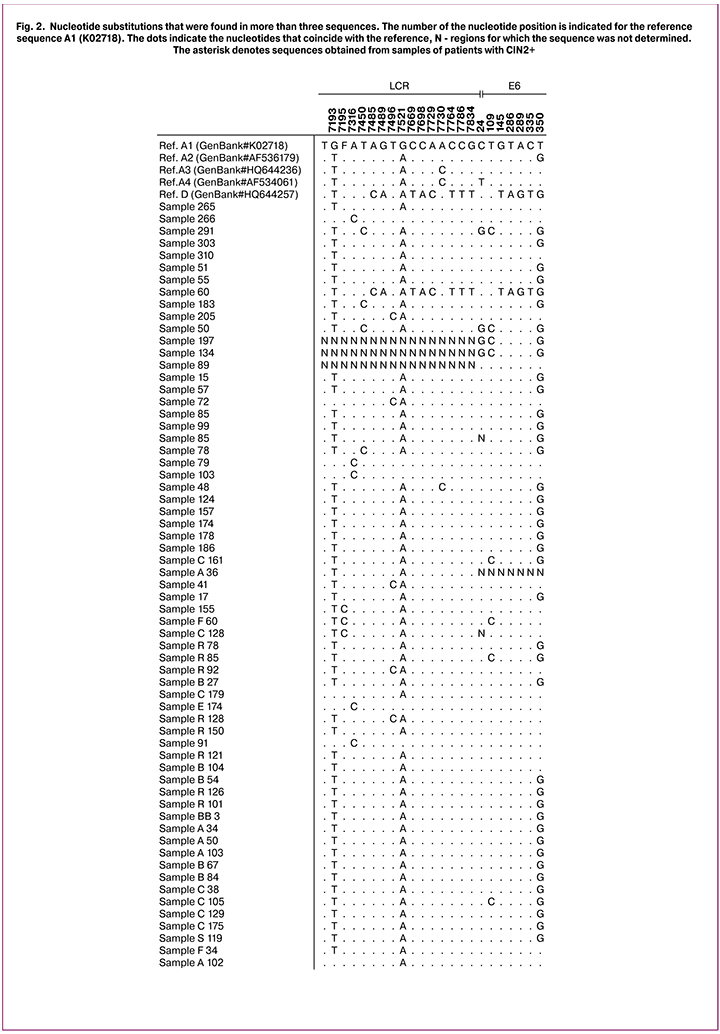
The risk of having cervical malignancy associated with the substitution in the studied fragment was assessed by calculating the odds ratios (Table 1).

The findings showed that C24TG substitution was significantly more common in samples from CIN2+ lesions (p = 0.04). Similar results for this substitution were found in a study conducted in Taiwan [9].
The T109C substitution in this study was also more often found in samples showing CIN2+ (OR = 2.91). However, the differences were not statistically significant (p = 0.22), which is probably due to a small number of samples. This substitution is rarely reported, but, in the cases described, it is more often detected in samples showing HSIL [16, 17].
In the literature, the most frequently reported mutation is at nucleotide position 350 within the E6 gene resulting in an amino acid change from leucine to valine at position 83 in the E6 protein. In this study, the prevalence of 350G variant was 63.5%, the mutation was somewhat more common in samples from CIN2+ lesions (OR = 1.89), but the differences were not statistically significant (p = 0.29), which may also be due to a small number of observations.
The in-vitro study of primary human keratinocytes conducted by Sichero et al. in 2012, 350G variant showed a higher oncogenic potential than 350T variant [18]. However, findings of in vivo studies are not so unambiguous. For example, in some studies, the 350G variant was much more common in samples showing CIN2 + [19], while some other studies have not identified such a relationship [17, 20]. Also, Cornet et al., 2013 [21] reported that variants containing the 350T variant persisted more often than those containing 350G, although no differences were found in the risk of progression.
Conclusion
This study for the first time in the Russian Federation reported the intra-genotype variability of HPV type 16. Several detected mutations (C24G, T109C, and T350G) are more frequently found in samples from CIN2-3 and cervical cancer lesions. The differences for C24G substitution are statistically significant, indicating a more aggressive effect of this variant on the neoplastic process. For clinicians, information about the presence or absence of risk factors for the occurrence and progression of CIN will help select an appropriate management strategy, determine optimal follow-up intervals for patients with persistent HPV type 16 infection and LSIL, and avoid unnecessary treatment.
The study of the prevalence of HPV type 16 variants showed that the population of HPV type 16 is quite monogenic and is represented by the European lineage.
The findings of this study need to be validated in a larger study with a greater number of samples, including those from the regions neighboring the Russian Federation and full-genomic studies. Understanding the genetic abnormality that drives the oncogenic potential of HPV type 16 variants, including other regions of the viral genome, will help reveal important biological and/or immunological mechanisms of interaction between the virus and the host, which will contribute to the creation of effective tools for controlling HPV infection and the malignant transformation of HPV-related lesions.
References
- Роговская С.И., Михеева И.В., Шипулина О.Ю., Минкина Г.Н., Подзолкова Н.М., Радзинский В.Е., Шипулин Г.А. Распространенность папилломавирусной инфекции в России. Эпидемиология и вакцинопрофилактика. 2012; 1: 25-33. [Rogovskaya S.I., Mikheeva I.V., Shipulina O.Yu., Minkina G.N., Podzolkova N.M., Radzinsky V.E., Shipulin G.A. The prevalence of human papillomavirus infection in Russia. Epidemiology and vaccine prevention. 2012; 1: 25-33. (in Russian)]
- Минкина Г.Н., Савичева А.М., Холл К., де Соза С.К., Шипицына Е.В., Колимиец Л.А. Распространенность различных типов вируса папилломы человека у женщин с цервикальной интраэпителиальной неоплазией тяжелой степени. Вопросы гинекологии, акушерства и перинатологии. 2013; 12(3): 32-7. [Minkina G.N., Savicheva A.M., Hall K., de Soza S.K., Shipitsyna E.V., Kolimiets L.A. The prevalence of various types of human papillomavirus in women with severe cervical intraepithelial neoplasia. Issues of gynecology, obstetrics and perinatology. 2013; 12 (3): 32-7. (in Russian)]
- Gravitt P. The known unknowns of HPV natural history J. Clin. Invest. 2011; 121(12): 4593-9. doi: 10.1172/JCI57149.
- Прилепская В.Н., Назарова Н.М., Мзарелуа Г.М., Фазуллин Л.З., Трофимов Д.Ю. ВПЧ-ассоциированные заболевания шейки матки - новое в диагностике. Акушерство и гинекология. 2015; 9: 20-6. [Prilepskaya V.N., Nazarova N.M., Mzarelua G.M., Fazullin L.Z., Trofimov D.Yu. HPV-associated cervical disease - a new diagnosis. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2015; 9: 20-6. (in Russian)]
- Бурменская О.В., Назарова Н.М., Прилепская В.Н., Мзарелуа Г.М., Бестаева Н.В., Трофимов Д.Ю., Сухих Г.Т. Прогнозирование риска развития и прогрессирования цервикальных интраэпителиальных неоплазий, ассоциированных с папилломавирусной инфекцией. Акушерство и гинекология. 2016; 2: 92-8. [Burmenskaya O.V., Nazarova N.M., Prilepskaya V.N., Mzarelua G.M., Bestaeva N.V., Trofimov D.Yu., Sukhikh G.T. Prediction of the risk of development and progression of cervical intraepithelial neoplasias associated with human papillomavirus infection. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2016; 2: 92-8. (in Russian)]
- Cornet I., Gheit T., Franceschi S., Vignat J., Burk R.D., Sylla B.S. et al. Human papillomavirus type 16 genetic variants: phylogeny and classification based on E6 and LCR. J. Virol. 2012; 86(12): 6855-61. doi: 10.1128/JVI.00483-12.
- Cornet I., Gheit T., Iannacone M.R., Vignat J., Burk R.D., Sylla B.S. et al. HPV16 genetic variation and the development of cervical cancer worldwide. Br. J. Cancer. 2013; 108(1): 240-4. doi: 10.1038/bjc.2012.508.
- Bernard H.U. Taxonomy and phylogeny of papillomaviruses: An overview and recent developments. Infect. Genet. Evol. 2013; 18: 357-61.doi: 10.1016/j.meegid.2013.03.011.
- Chang Y.J., Chen H.C., Pan M.H., Lee B.H., You S.L., Lin C.Y. et al. Intratypic variants of human papillomavirus type 16 and risk of cervical neoplasia in Taiwan. J. Med. Virol. 2013; 85(9): 1567-76. doi: 10.1002/jmv.23651.
- Sun Z., Lu Z., Liu J., Wang G., Zhou W., Yang L. et al. Genetic variations of E6 and long control region of human papillomavirus type 16 from patients with cervical lesion in Liaoning, China. BMC Cancer. 2013; 13: 459. doi: 10.1186/1471-2407-13-459.
- Hall T.A. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl. Acids Symp. Ser. 1999; 41: 95-8.
- Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013; 30(12): 2725-9. doi: 10.1093/molbev/mst197.
- Mirabello L., Yeager M., Cullen M., Boland J.F., Chen Z., Wentzensen N. et al. HPV16 sublineage associations with histology-specific cancer risk using HPV whole-genome sequences in 3200 women. J. Natl. Cancer Inst. 2017; 108(9).pii: djw100. doi: 10.1093/jnci/djw100.
- Park J.S., Shin S., Kim E.C., Kim J.E., Kim Y.B., Oh S. et al. Association of human papillomavirus type 16 and its genetic variants with cervical lesion in Korea. APMIS. 2016; 124(11): 950-7. doi: 10.1111/apm.12592.
- Marongiu L., Godi A., Parry J.V., Beddows S. Human papillomavirus type 16 long control region and E6 variants stratified by cervical disease stage. Infect. Genet. Evol. 2014; 26: 8-13. doi: 10.1016/j.meegid.2014.05.009.
- Zuna R.E., Moore W.E., Shanesmith R.P., Dunn S.T., Wang S.S., Schiffman M. et al. Association of HPV16 E6 variants with diagnostic severity in cervical cytology samples of 354 women in a US population. Int. J. Cancer. 2009; 125(11): 2609-13. doi: 10.1002/ijc.24706.
- Szostek S., Zawilinska B., Klimek M., Kosz-Vnenchak M. HPV16 E6 polymorphism and physical state of viral genome in relation to the risk of cervical cancer in women from the south of Poland. Acta Biochim. Pol. 2017; 64(1): 143-9.doi: 10.18388/abp.2016_1364.
- Sichero L., Sobrinho J.S., Villa L.L. Oncogenic potential diverge among human papillomavirus type 16 natural variants. Virology. 2012; 432(1): 127-32.doi: 10.1016/j.virol.2012.06.011.
- Ortiz-Ortiz J., Alarcón-Romero Ldel C., Jiménez-López M.A., Garzón-Barrientos V.H., Calleja-Macías I., Barrera-Saldaña H.A. et al. Association of human papillomavirus 16 E6 variants with cervical carcinoma and precursor lesions in women from Southern Mexico. Virol. J. 2015; 12: 29. doi: 10.1186/s12985-015-0242-3.
- Gudlevičienė Ž., Stumbrytė A., Juknė G., Simanavičienė V., Žvirblienė A. Distribution of human papillomavirus type 16 variants in Lithuanian women with cervical cancer. Medicina (Kaunas). 2015; 51(6): 328-35.doi: 10.1016/j.medici.2015.11.005.
- Cornet I., Gheit T., Clifford G.M., Combes J.D., Dalstein V., Franceschi S. et al. Human papillomavirus type 16 E6 variants in France and risk of viral persistence. Infect. Agents Cancer. 2013; 8: 4. doi: 10.1186/1750-9378-8-4.
Received 22.05.2018
Accepted 22.06.2018
About the Authors
Dmitryukova, Marina Yu., PhD (bio.sci.), Researcher at the CRI of Epidemiology of Rospotrebnadzor.111123, Russia, Moscow, Novogireevskaya str. 3a. Tel.: +74959749646 ext. 2601. E-mail: mdmitryukova@cmd.su
Korolenkova, Lyubov’ I., MD, professor, senior researcher, Blokhin Russian Cancer Research Center of Minzdrav of Russia.
115478 Russia, Moscow, Kashirskoye sh. 23. Tel.: +74953244406. E-mail: l.korolenkova@mail.ru
Romanyuk, Tat’yana N., junior researcher, CRI of Epidemiology of Rospotrebnadzor. 111123, Russia, Moscow, Novogireevskaya str. 3a. E-mail: tatiana.romaniuk@pcr.ms
Leshkina, Gul’nara V., biologist, CRI of Epidemiology of Rospotrebnadzor. 111123, Russia, Moscow, Novogireevskaya str. 3a. E-mail: glyoshkina@yandex.ru
Shipulina, Ol’ga Yu., PhD, research team leader, CRI of Epidemiology of Rospotrebnadzor.
111123, Russia, Moscow, Novogireevskaya str. 3a. Tel.: +74959749646 ext. 1131. E-mail: olga.shipulina@pcr.ms
Shipulin, German A., Ph.D., Head of the department of Molecular Diagnostics and Epidemiology, CRI of Epidemiology of Rospotrebnadzor.
Address: 111123, Russia, Moscow, Novogireevskaya str. 3a. E-mail: german@pcr.ru
For citations: Dmitryukova M.Yu., Korolenkova L.I., Romanyuk T.N., Leshkina G.V., Shipulina O.Yu., Shipulin G.A. Molecular biological features of HPV type 16 infection and the risk of developing cervical squamous intraepithelial lesions and cervical cancer. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2019; (2): 113-19. (in Russian)
http://dx.doi.org/10.18565/aig.2019.2.113-119



