Modulation of the interaction between NK-cells and trophoblast by intravenous immunoglobulin
Intravenous immunoglobulin (IVIG) as infertility treatment is supposed to affect the cytotoxic function of NK cells, but the underlying mechanism remains unclear. Objective: To investigate the effect of IVIG on the cytotoxicity of NK cells against target cells of K-562 line and trophoblast cells of JEG-3 line. Materials and methods: This study analyzed the cytotoxicity of NK-92 cells against K-562 cells and JEG-3 trophoblast cells in the presence of IVIG and that of peripheral blood NK cells in the mononuclear fraction against JEG-3 cells in the presence of IVIG. Mononuclear cells were obtained from the peripheral blood of healthy nonpregnant donors (group 1, n=10) and healthy nonpregnant fertile women (group 2, n=12). Results: Cell death of the JEG-3 line in the presence of mononuclear cells from Group 1 and IVIG at concentrations of 12 and 6 mg/mL was lower than that without IVIG (p<0.01 and p<0.05, respectively). Cell death of the JEG-3 line in the presence of mononuclear cells from group 2 and IVIG at concentrations of 12, 6, and 1.5 mg/ml was lower than that without IVIG (p<0.001, p<0.001, and p<0.01, respectively). Conclusion: Contact interaction between trophoblast cells and IVIG is associated with lower cytotoxicity of NK cells against trophoblast cells. More studies are needed to predict the effectiveness of IVIG therapy in patients with reproductive disorders.Mikhailova V.A., Davydova A.A., Bazhenov D.O., Kovaleva A.A., Zagaynova V.A., Kogan I.Yu., Bespalova O.N., Gzgzyan A.M., Sokolov D.I., Selkov S.A.
Keywords
Maternal immune tolerance toward fetal antigens is required for successful implantation and maintenance of a pregnancy [1]. Disturbance of the mechanisms of immunological tolerance formation is associated with the pathogenesis of reproductive disorders [2]. One of the cell populations probably involved in the pathogenesis of reproductive disorders are natural killer cells (NK cells) [3]. The laboratory examination of women diagnosed with recurrent pregnancy loss (RPL) revealed high cytotoxicity of NK cells, in an in vitro model against JEG-3 trophoblast cells. It was shown that in patients with RPL this parameter was higher than in healthy nonpregnant women [4, 5].
Data on the cytotoxicity of NK cells in patients with infertility are contradictory. Several studies have described increased cytotoxicity of NK cells isolated from the peripheral blood of women with RPL and/or infertility compared to healthy women [6, 7]. However, a meta-analysis of studies evaluating the role of NK cells in in vitro fertilization (IVF) outcomes found no differences in live birth rates in women with increased NK cell cytotoxicity compared with women with normal cytotoxicity [8]. At the same time, increased cytotoxicity of peripheral blood NK cells to K-562 cells was found in infertile women compared to healthy women who had had a history of successful pregnancy [9].
One of the drugs used to overcome immune-related infertility is intravenous immunoglobulins (IVIG) [10, 11]. The use of IVIG in preparation for IVF procedure has been shown to increase the probability of successful blastocyst implantation [10–12]. However, the exact mechanism of action of IVIG remains unclear. It is assumed that it can affect the cytotoxic function of NK cells [13, 14].
One of the methods for assessing the cytotoxicity of NK cells is to evaluate the death of K-562 line cells after their coculture with NK cells. K-562 cells are standard target cells because they do not express HLA locus molecules, which makes them vulnerable to natural killer cells. However, during pregnancy, NK cells come in contact with other cells, including fetal trophoblast cells that express molecules of the HLA-C locus and nonclassical molecules of the HLA E, G loci on their surface [15] and are capable of modulating NK cell functions [16], which makes them significantly different from standard target cells. Therefore, we aimed to investigate the effect of IVIG on the cytotoxicity of NK cells against target cells of K-562 line and trophoblast cells of JEG-3 line.
Mononuclear cells were also used, which were isolated from peripheral blood by the standard method of centrifugation in the ficoll density gradient p=1.077 (Biolot, Russia).
Materials and methods
Cell lines
NK-92 cell line (ATCC, USA) was used. This cell line was isolated from human bone marrow with malignant non-Hodgkin's lymphoma. According to the main phenotypic and functional parameters NK-92 cells do not differ from activated NK cells [17]. The cells were cultured in complete α-MEM medium (Biolot, Russia) with the addition of IL-2 (500 U/ml), according to ATCC recommendations.
Human chronic myelogenous leukemia cells of K-562 line (ATCC, USA) and trophoblast cells of JEG-3 line (ATCC, USA) were used as targets. Line K-562 cells were cultured in RPMI-1640 medium (Biolot, Russia) with the addition of 10% inactivated FCS, 100 U/ml penicillin, and 2 mM L-glutamine. K-562 cells do not express molecules of the MHC-I locus [18], so these cells are used as standard target cells to evaluate the cytotoxicity of NK cells.
We also used cells of the JEG-3 line, which correspond to extravasal trophoblast cells by the main phenotypic and functional parameters [19]. The cells were cultured in complete DMEM medium (Biolot, Russia) according to ATCC recommendations.
Mononuclear cells were also used, which were isolated from peripheral blood by the standard method of centrifugation in the ficoll density gradient p=1.077 (Biolot, Russia).
Patients
The study included 2 groups of women: healthy nonpregnant women of reproductive age with a regular menstrual cycle and no previous pregnancy (group 1, n=10) and healthy nonpregnant fertile women of reproductive age with a regular menstrual cycle who previously had pregnancies that ended with a full-term live births and had no history of miscarriages (group 2, n=12). Peripheral blood was collected in the secretory phase of the menstrual cycle after ovulation was monitored by ultrasound. Exclusion criteria were the same for both groups and consisted of extragenital endometriosis (stage 3–4), antiphospholipid syndrome, genital malformations, acute and exacerbation of chronic diseases, a hereditary form of high-risk thrombophilia, types 1 and 2 diabetes, grade 2–3 obesity, hormonal therapy, including combined oral contraceptives, refusal to participate in the study. The study was approved by the Research Ethics Committee of the D.O. Ott Research Institute for OG&P (Ref. No. 107 of 15.03.2021). All participants provided signed informed consent to take part in the study, including their consent for the publication of their depersonalized data.
Drugs
The IVIG used in the study had a stock concentration of 50 mg/ml (Intratect, Biotest, Germany). The peripheral blood mononuclear cells were isolated by standard Ficoll 1.077 g/ml (Biolot, Russia) density gradient centrifugation.
The toxic effect of IVIG was evaluated against NK-92, K-562, and JEG-3 cells. For this purpose, cells of NK-92 or K-562 lines were placed in round-bottomed 96-well plates for suspension cultures (Sarstedt, Germany) and precipitated by centrifugation (100g, 10 min, 22°C), then the supernatant was removed. The cells were then supplemented with IVIG preparation diluted with complete culture medium to concentrations (25 mg/ml, 12.5 mg/ml, 6.25 mg/ml, 3.175 mg/ml, 1.6 mg/ml, 0.8 mg/ml, 0.4 mg/ml, 0.2 mg/ml). A portion of the cells were cultured without drugs to determine baseline cell death. The toxicity of IVIG was then evaluated against the JEG-3 cell line.
The cells were incubated in plates for 24 hours at 37°C in a 5% CO2-humidified incubator. Then propidium iodide (PI) solution at a final concentration of 2 μg/ml (Sigma-Aldrich, USA) was added to the cells to determine the percentage of dead cells (PI+). Assessment was performed using a FACSCanto II flow cytofluorimeter (BD, USA). Propidium iodide binds irreversibly to DNA and fluoresces under the influence of the flow cytometer laser (488 nm) with an emission maximum of about 615 nm. Propidium iodide is unable to cross the cell membrane, its fluorescence is characteristic only for cells with damaged surface and nuclear membranes. Therefore, cells containing propidium iodide (PI+) were evaluated as dead cells.
Assessment of cytotoxicity of NK-92 cells towards K-562 cells in the presence of IVIG
Line K-562 cells were treated with a solution of 3.6 μM of 6-carboxyfluorescein succinimidyl ether (CFSE), which is a fluorescent dye that does not cause cell death (Sigma-Aldrich, USA). Then, 30,000 K-562 cells in 50 μl of complete α-MEM medium were added to the wells of round-bottomed 96-well plates (Sarstedt, Germany). Next, 150,000 cells of the NK-92 line were added to the same wells in 100 μl of complete alpha-MEM medium, reaching an effector-target ratio of 5:1. IVIG was then added to the cells at concentrations of 12 mg/ml, 6 mg/ml, 3 mg/ml, 1.5 mg/ml, 0.8 mg/ml, 0.4 mg/ml, and 0.2 mg/ml. Complete culture medium α-MEM without IVIG was added to a portion of the cells to determine cell death of the K-562 line in the presence of NK cells. In addition, baseline cell death of the K-562 line was assessed in each experiment.
Assessment of cytotoxicity of NK-92 line cells to JEG-3 line cells in the presence of IVIG preparation
NK-92 cells (BD, USA) were placed in the wells of round-bottomed 96-well plates with JEG-3 line cells pretreated with CFSE solution (Sigma-Aldrich, USA). An effector:target ratio of 5:1 was used. Then IVIG preparation was added to the cells at the concentrations of 6 mg/ml, 1.5 mg/ml and 0.375 mg/ml. Some cells were cultured without the drug to determine baseline trophoblast cell death in the presence of NK cells. The baseline cell death of the JEG-3 line was also assessed in each experiment.
Evaluation of cytotoxicity of peripheral blood NK cells towards JEG-3 line trophoblast cells in the presence of IVIG
Peripheral blood mononuclear cells were added to CFSE-treated JEG-3 line cells in an effector:target ratio of 10:1. IVIG was then added to the plate wells at concentrations of 12 mg/ml, 6 mg/ml, 1.5 mg/ml, and 0.375 mg/ml. Complete culture medium without the drug was added to some cells to determine the death of trophoblast cells from the JEG-3 line in the presence of mononuclear cells. In each experiment, baseline cell death of the JEG-3 line was also assessed.
Analysis of target cell death after contact interaction with NK cells
After preparation of the cell mixture, the plates were centrifuged at 100 g for 3 min, after which the plates were placed in a 5% CO2-humidified incubator at 37°C and incubated for 4 h. After incubation, cells were treated with propidium iodide solution at a concentration of 2 μg/ml at 4°C for 10 min. Fluorescence analysis was performed on a FACSCanto II Flow Cytometer (BD, USA). Cell death was assessed by the presence of the propidium iodide (PI+) label.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 8 software (GraphPad Software, California, USA). The normality of the distribution was tested by the D'Agostino–Pearson test. We also assessed the presence of outliers that were not detected. The Kruskal–Wallis test was used to compare numerical data between groups, followed by the Dunn test for multiple comparisons. The data was presented as box plots based on median, upper and lower quartiles, with minimum and maximum values shown above and below the box. Differences were considered statistically significant at p<0.05.
Results
Minimum toxic dose of IVIG against NK-92 and K-562 and JEG-3 cell lines
The number from PI+ cells of the NK-92 line and the K-562 target cells was higher in the presence of the IVIG drug at a concentration of 25 mg / ml than without IVIG drug (Fig. 1 A, B). At lower concentrations, starting at 12 mg/ml, the death of NK-92 and K-562 cells was not different from the baseline. Based on these results, IVIG concentrations of 12 mg/mL, 6 mg/mL, 3 mg/mL, 1.5 mg/mL, 0.8 mg/mL, 0.4 mg/mL, and 0.2 mg/mL were used for experiments to evaluate the effects of IVIG in co-culture of NK-92 and K-562 target cells because they were less toxic.
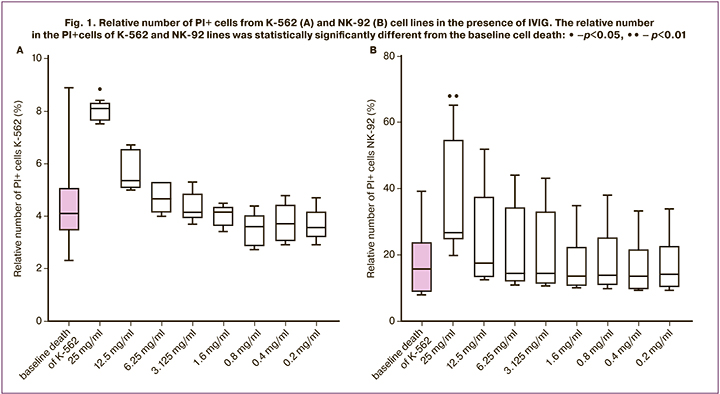
The number from PI+ cells of the JEG-3 line was also evaluated in the presence of IVIG at concentrations of 12 mg/ml, 6 mg/ml, 1.5 mg/ml, and 0.375 mg/ml. In the presence of all these concentrations, cell death from the JEG-3 line did not differ from baseline.
Cytotoxicity of NK-92 cells to K-562 cells in the presence of IVIG
In the presence of NK cells, the relative number in PI+ cells of the K-562 line was higher than in its absence. In the presence of NK cells and IVIG at a concentration of 12 mg/ml, the relative number of PI+ cells of the K-562 line was lower than in the case of incubation with NK cells but without IVIG addition, and did not differ from the baseline target level of cell death (Fig. 2A). In the presence of NK cells and IVIG at lower concentrations, the relative number of PI+ cells of the K-562 line was higher than baseline cell death (Fig. 2A).
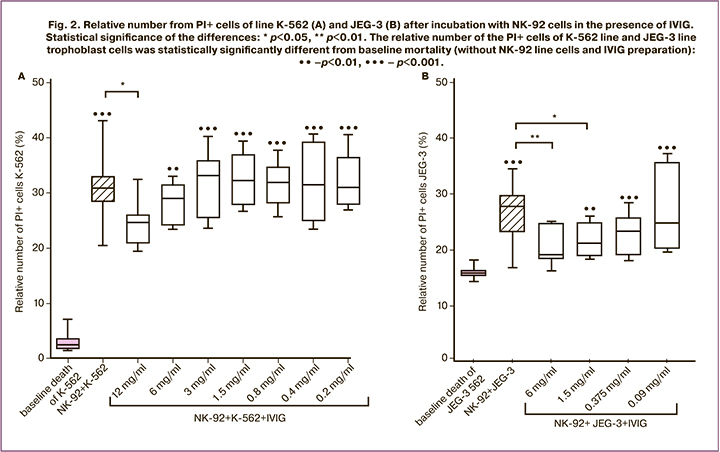
Cytotoxicity of NK-92 line cells to JEG-3 line cells in the presence of IVIG preparation
The relative number of PI+ cells of JEG-3 line was higher in the presence of NK-92 line cells than in their absence. In the presence of NK-92 line cells and IVIG at concentrations of 6 mg/ml and 1.5 mg/ml, the relative number of PI+ cells of the JEG-3 trophoblast line was lower than that without IVIG (Fig. 2B). In the presence of IVIG at concentrations of 1.5 mg/mL, 0.375 mg/mL, and 0.09 mg/mL, the number of PI+ cells from the JEG-3 line was higher than without NK cells and IVIG. Since in the presence of NK cells and IVIG at a concentration of 6 mg/ml, the number in the PI+ cells of JEG-3 line did not differ from that in the absence of NK cells, the effect of IVIG at a concentration of 12 mg/ml was not evaluated.
Cytotoxicity of peripheral blood NK cell fraction of mononuclear cells against JEG-3 line cells in the presence of IVIG preparation
Without IVIG addition, the relative number of PI+ cells of the JEG-3 line trophoblast in the presence of peripheral blood mononuclear cells in women in groups 1 and 2 was higher than without IVIG and mononuclear cells (Fig. 3A, Fig. 3B).
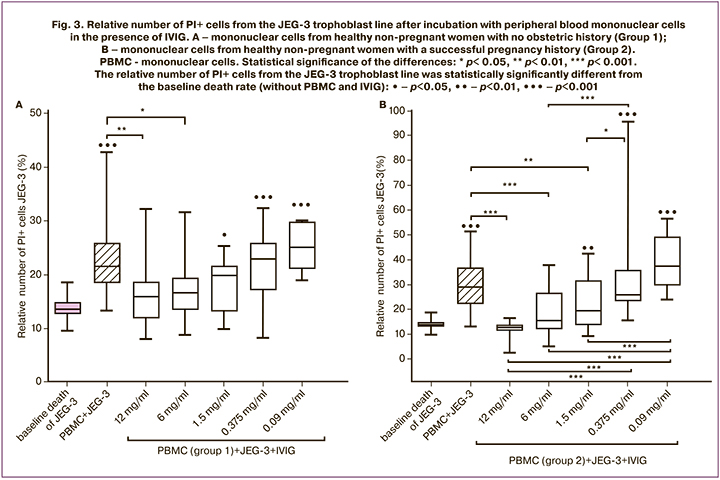
The reduced number of PI+ cells of trophoblast line JEG-3 incubated with Group 1 and IVIG mononuclear cells (12 mg/ml and 6 mg/ml) was compared with the number of PI+ trophoblast cells incubated without IVIG with mononuclear cells (Figure 3A). In the presence of IVIG at concentrations of 1.5 mg/mL, 0.375 mg/mL, and 0.09 mg/mL and Group 1 mononuclear cells, the number of PI+ trophoblast cells was higher than after incubation without mononuclear cells and IVIG (Fig. 3A).
The relative number of PI+ cells of the JEG-3 trophoblast line was lower in the presence of mononuclear cells of Group 2 and IVIG at concentrations of 12 mg/ml, 6 mg/ml, and 1.5 mg/ml compared to PI + trophoblast cells incubated with mononuclear cells but without IVIG (Fig. 3B). In the presence of mononuclear cells of Group 2 and IVIG at concentrations of 1.5 mg/ml, 0.375 mg/ml, and 0.093 mg/ml, the relative number from PI+ cells of the trophoblast line was higher than after incubation in the absence of IVIG and Group 2 (Fig. 3B). There were no differences in IVIG effects at 12 mg/ml, 6 mg/ml, and 1.5 mg/ml on trophoblast cell death in the presence of mononuclear cells, while trophoblast cell death was higher at concentrations of 0.375 mg/ml than at 1.5 mg/ml, 6 mg/ml, and 12 mg/ml. In addition, trophoblast cell death in the presence of mononuclear cells and IVIG at 0.09 mg/mL was increased compared to cell death in the presence of IVIG at concentrations of 1.5 mg/mL, 6 mg/mL and 12 mg/mL.
Discussion
In evaluating the direct effect of IVIG on target cells, we found that starting from a concentration of 12.5 mg/ml and below, the drug had no toxic effect towards the K-562 cell line. Furthermore, starting from a concentration of 12.5 mg/ml, IVIG also had no toxic effect on effector cells of the NK-92 line. When evaluating the effect of this concentration on JEG-3 line cells, no toxic effects were also detected. Based on the data obtained, we selected concentrations for further evaluation of the cytotoxicity of NK-92 line cells against K-562 line cells in the presence of IVIG. It was found that in the presence of NK cells and IVIG at a concentration of 12 mg/ml, the K-562 line cells were killed to a lesser extent than without IVIG.
Trophoblast cells of the JEG-3 line express molecules of the HLA-G locus on the surface membrane, which are ligands for the NK-cell inhibitory receptor KIR2DL4 [20]. This interaction results in a decrease in NK cell cytotoxicity to trophoblast cells as the well as secretion of IFNγ by NK cells [21], which promotes spiral artery remodeling and placental formation [22]. We found that in vitro, in the presence of IVIG at concentrations of 6 mg / ml and 1.5 mg/ml, the death of JEG-3 line cells was reduced in the incubation with NK-92 line cells. Thus, in the presence of IVIG, the cytotoxicity of NK cells was reduced both towards standard target cells, and to JEG-3 line cells reproducing the basic properties of extravillous trophoblast cells. Using a model of contact interaction between NK cells and the trophoblast allows us to approximate the events in the uterine-placental complex occurring in vivo. The mechanism of the IVIG effect on cells is currently uncertain. Previously, in women carrying antiphospholipid antibodies, IVIG has a cytoprotective effect on endothelial cells.
It is assumed that this effect is due to the adsorption of immunoglobulins on the membrane surface through the formation of covalent bonds. In addition, subsequent penetration of IVIG into the cell is possible, which causes a change in the phenotype of endotheliocytes, as well as inhibition of their procoagulant activity and inhibition of complement system activation [23]. A presumed mechanism responsible for the cytoprotective effect is the shielding of negatively charged membrane phospholipids, the so-called "umbrella barrier" [23]. It is possible that the decrease in the cytopathic effect of NK cells on trophoblast cells that we have identified is also due to the formation of an "umbrella barrier".
The next stage of the study was a model in which peripheral blood NK-cells were used as effectors in the mononuclear fraction. We established results similar to those obtained using NK-92 line cells: the cytotoxicity of NK cells was reduced in the presence of IVIG (concentrations of 12 mg/ml, 6 mg/ml and 1.5 mg/ml). Our findings are consistent with the literature on a decrease in cytotoxic activity of NK cells in RPL patients after IVIG therapy [5, 11]. The revealed changes can also be associated with the "umbrella barrier" of target cells, which prevents the formation of an immune synapse between the NK cell and the target cell.
Furthermore, the use of IVIG increases the expression of CD94 inhibitory receptors by peripheral blood NK cells, which in turn leads to a decrease in the cytotoxic activity of peripheral blood NK cells in RPL [13]. IVIGs can also affect the expression of other proteins by NK cells. NK cells have been shown to express the inhibitory co-receptor Tim-3, which is a marker of functional depletion of effector T cells [24]. There is evidence that Tim-3 is involved in suppressing the functional activity of NK cells [25]. In the presence of IVIG, the expression of Tim-3 by NK cells is increased compared to its level in the absence of the drug [26].
It is possible that IVIG also regulates the phenotype of target cells. The effect of IVIG on the expression of protein CD200 (OX-2), a member of the immunoglobulin superfamily [27], which interacts with CD200R receptor is supposed to cause the synthesis of indolamine-2,3-dioxygenase by myeloid cells. This results in a decrease in the proliferation of T and NK-cells, as well as stimulation of T-regulatory lymphocytes [29]. IVIGs may exert an indirect effect on NK cells, thus contributing to the maintenance of immunological tolerance and preservation of pregnancy [30]. More research is required on the changes in the receptor profile of NK cells and trophoblast cells under the influence of IVIG.
IVIG is used to overcome infertility associated with immune factors [11]. The high efficacy of IVIG therapy was previously established in patients with IVF failure, who have increased the number and high cytotoxicity of NK cells in peripheral blood, and high expression of CD56 and HLA-DR by NK cells and T lymphocytes. For example, women who received IVIG had twice as many successful implantations and three times as many live births compared to the control group of patients who did not receive IVIG [12]. The use of IVIG in patients with repeated implantation failures with reduced peripheral blood NK cells caused an improvement in implantation and live birth rates compared to the same group of patients who did not receive IVIG [11, 12]. Our results suggest a direct effect of IVIG on cellular interactions in the uterine decidua.
Conclusion
Contact interaction between trophoblast cells and IVIG is associated with lower cytotoxicity of NK cells against trophoblast cells. More studies are needed to predict the effectiveness of IVIG therapy in patients with reproductive disorders.
References
- Robertson S.A., Jin M., Yu D., Moldenhauer L.M., Davies M.J., Hull M.L., Norman R.J. Corticosteroid therapy in assisted reproduction – immune suppression is a faulty premise. Hum. Reprod. 2016; 31(10): 2164-73. https://dx.doi.org/10.1093/humrep/dew186.
- Makrigiannakis A., Petsas G., Toth B., Relakis K., Jeschke U. Recent advances in understanding immunology of reproductive failure. J. Reprod. Immunol. 2011; 90(1): 96-104. https://dx.doi.org/1016/j.jri.2011.03.006.
- Агнаева А.О., Беспалова О.Н., Соколов Д.И., Сельков С.А., Коган И.Ю. Роль естественных киллеров (NK-клеток) в репродуктивных потерях. Журнал акушерства и женских болезней. 2017; 66(3): 143-156. [Agnaeva A.O., Bespalova O.N., Sokolov D.I., Selkov S.A., Kogan I.Yu. Role of natural killer cells in reproductive failure. Journal of obstetrics and women's diseases. 2017; 66(3): 143-156. (in Russian)]. https://dx.doi.org/10.17816/jowd663143-156.
- Lee S.K., Na B.J., Kim J.Y., Hur S.E., Lee M., Gilman-Sachs A. et. al. Determination of clinical cellular immune markers in women with recurrent pregnancy loss. Am. J. Reprod. Immunol. 2013; 70(5): 398-411. https://dx.doi.org/10.1111/aji.12137.
- Ahmadi M., Ghaebi M., Abdolmohammadi-Vahid S., Abbaspour-Aghdam S., Hamdi K., Abdollahi-Fard S. et al. NK cell frequency and cytotoxicity in correlation to pregnancy outcome and response to IVIG therapy among women with recurrent pregnancy loss. J. Cell Physiol. 2019; 234(6): 9428-37. https://dx.doi.org/10.1002/jcp.27627.
- Sacks G., Yang Y., Gowen E., Smith S., Fay L., Chapman M. Detailed analysis of peripheral blood natural killer cells in women with repeated IVF failure. Am. J. Reprod. Immunol. 2012; 67(5): 434-42. https://dx.doi.org/10.1111/j.1600-0897.2012.01105.x.
- Salazar M.D., Wang W.J., Skariah A., He Q., Field K., Nixon M. et al. Post-hoc evaluation of peripheral blood natural killer cell cytotoxicity in predicting the risk of recurrent pregnancy losses and repeated implantation failures. J. Reprod. Immunol. 2022; 150: 103487. https://dx.doi.org/10.1016/j.jri.2022.103487.
- Seshadri S., Sunkara S.K. Natural killer cells in female infertility and recurrent miscarriage: a systematic review and meta-analysis. Hum. Reprod. Update. 2014; 20(3): 429-38. https://dx.doi.org/10.1093/humupd/dmt056.
- Karami N., Boroujerdnia M.G., Nikbakht R., Khodadadi A. Enhancement of peripheral blood CD56(dim) cell and NK cell cytotoxicity in women with recurrent spontaneous abortion or in vitro fertilization failure. J. Reprod. Immunol. 2012; 95(1-2): 87-92. https://dx.doi.org/10.1016/j.jri.2012.06.005.
- Polanski L.T., Barbosa M.A., Martins W.P., Baumgarten M.N., Campbell B., Brosens J. et al. Interventions to improve reproductive outcomes in women with elevated natural killer cells undergoing assisted reproduction techniques: a systematic review of literature. Hum. Reprod. 2014; 29(1): 65-75. https://dx.doi.org/10.1093/humrep/det414.
- Ho Y.K., Chen H.H., Huang C.C., Lee C.I., Lin P.Y., Lee M.S. et al. Peripheral CD56(+)CD16(+) NK cell populations in the early follicular phase are associated with successful clinical outcomes of intravenous immunoglobulin treatment in women with repeated implantation railure. Front. Endocrinol. (Lausanne). 2019; 10: 937. https://dx.doi.org/10.3389/fendo.2019.00937.
- Chernyshov V.P., Dons'koi B.V., Sudoma I.O., Goncharova Y.O. Multiple immune deviations predictive for IVF failure as possible markers for IVIG therapy. Immunol. Lett. 2016; 176: 44-50. https://dx.doi.org/10.1016/j.imlet.2015.12.010
- Shimada S., Takeda M., Nishihira J., Kaneuchi M., Sakuragi N., Minakami H. et al. A high dose of intravenous immunoglobulin increases CD94 expression on natural killer cells in women with recurrent spontaneous abortion. Am. J. Reprod. Immunol. 2009; 62(5): 301-7. https://dx.doi.org/10.1111/j.1600-0897.2009.00739.x.
- Han A.R., Lee S.K. Immune modulation of i.v. immunoglobulin in women with reproductive failure. Reprod. Med. Biol. 2018; 17(2): 115-24. https://dx.doi.org/10.1002/rmb2.12078.
- Apps R., Murphy S.P., Fernando R., Gardner L., Ahad T., Moffett A. Human leucocyte antigen (HLA) expression of primary trophoblast cells and placental cell lines, determined using single antigen beads to characterize allotype specificities of anti-HLA antibodies. Immunology. 2009; 127(1): 26-39. https://dx.doi.org/10.1111/j.1365-2567.2008.03019.x.
- Huhn O., Zhao X., Esposito L., Moffett A., Colucci F., Sharkey A.M. How do uterine natural Killer and innate lymphoid cells contribute to successful pregnancy? Front. Immunol. 2021; 12: 607669. https://dx.doi.org/10.3389/fimmu.2021.607669.
- Gong J.H., Maki G., Klingemann H.G. Characterization of a human cell line (NK-92) with phenotypical and functional characteristics of activated natural killer cells. Leukemia. 1994; 8(4): 652-8.
- Drew S.I., Terasaki P.I., Billing R.J., Bergh O.J., Minowada J., Klein E. Group-specific human granulocyte antigens on a chronic myelogenous leukemia cell line with a Philadelphia chromosome marker. Blood. 1977; 49(5): 715-8.
- Kohler P.O., Bridson W.E. Isolation of hormone-producing clonal lines of human choriocarcinoma. J. Clin. Endocrinol. Metab. 1971; 32(5): 683-7. https://dx.doi.org/10.1210/jcem-32-5-683.
- Manaster I., Goldman-Wohl D., Greenfield C., Nachmani D., Tsukerman P., Hamani Y. et al. MiRNA-mediated control of HLA-G expression and function. PLoS One. 2012; 7(3): e33395. https://dx.doi.org/10.1371/journal.pone.0033395.
- Bespalova O., Bakleicheva M., Ivashchenko T., Tral T., Tolibova G., Kogan I. Expression of HLA-G and KIR2DL4 receptor in chorionic villous in missed abortion. Gynecol. Endocrinol. 2020; 36(Suppl. 1): 43-7. https://dx.doi.org/10.1080/09513590.2020.1816716.
- Robson A., Lash G.E., Innes B.A., Zhang J.Y., Robson S.C., Bulmer J.N. Uterine spiral artery muscle dedifferentiation. Hum. Reprod. 2019; 34(8): 1428-38. https://dx.doi.org/10.1093/humrep/dez124.
- Arumugam T.V., Tang S.C., Lathia J.D., Cheng A., Mughal M.R., Chigurupati S. et al. Intravenous immunoglobulin (IVIG) protects the brain against experimental stroke by preventing complement-mediated neuronal cell death. Proc. Natl. Acad. Sci. USA. 2007; 104(35): 14104-9. https://dx.doi.org/10.1073/pnas.0700506104.
- Zhu C., Anderson A.C., Schubart A., Xiong H., Imitola J., Khoury S.J. et al. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat. Immunol. 2005; 6(12): 1245-52. https://dx.doi.org/10.1038/ni1271.
- Wang F., Hou H., Wu S., Tang Q., Huang M., Yin B. et al. Tim-3 pathway affects NK cell impairment in patients with active tuberculosis. Cytokine. 2015; 76(2): 270-9. https://dx.doi.org/10.1016/j.cyto.2015.05.012.
- So E.C., Khaladj-Ghom A., Ji Y., Amin J., Song Y., Burch E. et al. NK cell expression of Tim-3: First impressions matter. Immunobiology. 2019; 224(3): 362-70. https://dx.doi.org/10.1016/j.imbio.2019.03.001.
- Holmannová D., Kolackova M., Kondelkova K., Kunes P., Krejsek J., Andrys C. CD200/CD200R paired potent inhibitory molecules regulating immune and inflammatory responses; Part I: CD200/CD200R structure, activation, and function. Acta Medica (Hradec Kralove). 2012; 55(1): 12-7. https://dx.doi.org/10.14712/18059694.2015.68.
- Barclay A.N., Wright G.J., Brooke G., Brown M.H. CD200 and membrane protein interactions in the control of myeloid cells. Trends Immunol. 2002; 23(6): 285-90. https://dx.doi.org/10.1016/s1471-4906(02)02223-8.
- Clark D.A., Wong K., Banwatt D., Chen Z., Liu J., Lee L. et al. CD200-dependent and nonCD200-dependent pathways of NK cell suppression by human IVIG. J. Assist. Reprod. Genet. 2008; 25(2-3): 67-72. https://dx.doi.org/10.1007/s10815-008-9202-9.
- Сельков С.А., Соколов Д.И., Чепанов С.В. Иммунорегуляторные эффекты иммуноглобулинов для внутривенного введения. Медицинская иммунология. 2013; 15(1): 5-12. [Selkov S.A., Sokolov D.I., Chepanov S.V. Immunoregulatory effects of intravenous immunoglobulins. Medical Immunology. 2013; 15(1): 5-12. (in Russian)].
Received 19.05.2022
Accepted 03.06.2022
About the Authors
Valentina A. Mikhailova, Senior Researcher at the Laboratory of Intercellular Interactions, Department of Immunology and Intercellular Interactions, D.O. Ott Research Institute for Obstetrics, Gynecology and Reproductology, +7(812)328-98-50, https://orcid.org/0000-0003-1328-8157, 199034, Russia, St. Petersburg, Mendeleevskaya line, 3.Alina A. Davydova, Junior Researcher at the Laboratory of Intercellular Interactions, Department of Immunology and Intercellular Interactions, D.O. Ott Research Institute for Obstetrics, Gynecology and Reproductology, +7(812)328-98-50, https://orcid.org/0000-0001-5313-2910, 199034, Russia, St. Petersburg, Mendeleevskaya line, 3.
Dmitriy O. Bazhenov, Junior Researcher at the Laboratory of Intercellular Interactions, Department of Immunology and Intercellular Interactions, D.O. Ott Research Institute for Obstetrics, Gynecology and Reproductology, +7(812)328-98-50, https://orcid.org/0000-0003-4532-7720, 199034, Russia, St. Petersburg, Mendeleevskaya line, 3.
Anastasia A. Kovaleva, Laboratory Technician at the Laboratory of Intercellular Interactions, Department of Immunology and Intercellular Interactions, D.O. Ott Research Institute for Obstetrics, Gynecology and Reproductology, +7(812)328-98-50, https://orcid.org/0000-0002-8024-3461, 199034, Russia, St. Petersburg, Mendeleevskaya line, 3.
Valeriya A. Zagaynova, Junior Researcher, obstetrician-gynecologist at the Department of Assisted Reproductive Technologies, D.O. Ott Research Institute for Obstetrics, Gynecology and Reproductology, +7(812)328-98-33, https://orcid.org/0000-0001-6971-7024, 199034, Russia, St. Petersburg, Mendeleevskaya line, 3.
Igor Yu. Kogan, Corresponding Member of RAS, Dr. Med. Sci., Professor, Director, D.O. Ott Research Institute for Obstetrics, Gynecology and Reproductology,
+7(812)328-98-33, https://orcid.org/0000-0001-6971-7024, 199034, Russia, St. Petersburg, Mendeleevskaya line, 3.
Olesya N. Bespalova, Dr. Med. Sci., Deputy Director, D.O. Ott Research Institute for Obstetrics, Gynecology and Reproductology, +7(812)679-55-51,
https://orcid.org/0000-0002-6542-5953, 199034, Russia, St. Petersburg, Mendeleevskaya line, 3.
Alexandr M. Gzgzyan, Dr. Med. Sci, Head of the Department of Assisted Reproductive Technologies, D.O. Ott Research Institute for Obstetrics, Gynecology and Reproductology, +7(812)328-98-22, https://orcid.org/0000-0003-3917-9493, 199034, Russia, St. Petersburg, Mendeleevskaya line, 3.
Sergey A. Selkov, Merited Scolar of the Russian Federation, Professor, Head of the Department of Immunology and Intercellular Interactions, D.O. Ott Research Institute
for Obstetrics, Gynecology and Reproductology, +7(812)328-98-50, https://orcid.org/0000-0003-1560-7529, 199034, Russia, St. Petersburg, Mendeleevskaya line, 3.
Dmitry I. Sokolov, Dr. Bio. Sci., Head of the Laboratory of Intercellular Interactions, Department of Immunology and Intercellular Interactions, D.O. Ott Research Institute
for Obstetrics, Gynecology and Reproductology, +7(812)328-98-50, http://orcid.org/0000-0002-5749-2531, 199034, Russia, St. Petersburg, Mendeleevskaya line, 3.
Corresponding author: Valentina A. Mikhailova, mva_spb@mail.ru
Authors' contributions: Selkov S.A., Sokolov D.I. – conception and design of the study; Davydova A.A., Bazhenov D.O., Kovaleva A.A., Mikhailova V.A., Zagaynova V.A., Bespalova O.N., Gzgzyan A.M., Kogan I.Yu. – data collection and analysis; Davydova A.A., Kovaleva A.A., Bazhenov D.O. – statistical analysis; Davydova A.A., Mikhailova V.A. - manuscript drafting; Mikhailova V.A., Sokolov D.I., Selkov S.A. – manuscript editing.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: RSF grant No. 21-15-00021 – assessment of cytotoxicity of NK-92 cells to K-562 trophoblast cells in the presence of IVIG, PNI No. АААА-А20-120041390033-4 – assessment of cytotoxicity of NK-92 cells and peripheral NK cells blood to JEG-3 trophoblast cells in the presence of IVIG.
Ethical Approval: The study was approved by the Research Ethics Committee of the D.O. Ott Research Institute for Obstetrics, Gynecology and Reproductology (Ref. No. 107 of 15.03.2021).
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Mikhailova V.A., Davydova A.A., Bazhenov D.O., Kovaleva A.A.,
Zagaynova V.A., Kogan I.Yu., Bespalova O.N., Gzgzyan A.M., Sokolov D.I., Selkov S.A.
Modulation of the interaction between NK-cells and trophoblast by intravenous immunoglobulin.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2022; 6: 105-113 (in Russian)
https://dx.doi.org/10.18565/aig.2022.6.105-113



