Plasma TLR2 and IGF2/H19 imprinting control region gene methylation in fetal growth restriction
Aim. To investigate the level of TLR2 and IGF2/H19 imprinting control region methylation in peripheral blood plasma in fetal growth restriction (FGR).Kan N.E., Tyutyunnik V.L., Khachatryan Z.V., Sadekova A.A., Krasnyi A.M.
Material and methods. The study included 118 pregnant women divided into those who had (group 1, n=58) and who had not had (group 2, n=60) a confirmed diagnosis of IGR. In group 1, 24 and 34 women had early and late FGR, respectively. Testing for methylation level of 22 genes was conducted using Methylation-Sensitive High-Resolution Melting (MS-HRM).
Results. TLR2 methylation level was statistically significantly reduced to 0.01 (0.0; 0.45) in early FGR. At the relative level of TLR2 methylation threshold of 0.012, the sensitivity and the specificity were 88.1% and 60.2% (AUC=0.72, 95% CI 0.56–0.88). No statistically significant differences were found in late FGR compared with a healthy pregnancy. The level of plasma IGF2/H19 imprinting control region methylation was reduced in early FGR, reaching 0.22 (0.18; 0.32); in the comparison group it was 0.49 (0.44; 0.65), (p=0.03). At the threshold of 0.2 of the relative IGF2/H19 imprinting control region methylation, the sensitivity and specificity were 80.4% and 80.1%, respectively. The relative level of ICR IGF2/H19 methylation in late FGR did not differ significantly from that in a healthy pregnancy.
Conclusion. The study findings confirm that TLR2 and IGF2/H19 imprinting control region genes methylation have a role in FGR development and can be used as non-invasive predictors. These results suggest that antenatal epigenetic changes continue in the neonatal period and are essential for fetal programming implementation.
Keywords
Fetal growth restriction (FGR) is defined as a pathological restriction of the genetically programmed fetal growth, which does not tend to decrease [1–3]. Current chronological classification categorizes the disease according to disease onset time during pregnancy into early-onset FGR and late-onset FGR [4–6]. The 32nd week of gestation was established as the cut-off point for early versus late-onset FGR [1, 7, 8].
The early onset FGR (before 32 weeks of gestation) accounts for 20–30% of all cases. Its initiation is associated with a deficient invasion of the trophoblast in the maternal spiral arteries, increasing uterine artery resistance. As a defense mechanism, the fetus exhibits a high tolerance to low levels of oxygen as well as hypoxemia [9–11]. Perinatal morbidity and mortality in early-onset FGR are extremely high, presenting the crucial clinical challenge in managing this group of pregnant women [12, 13].
Late FGR (>32 weeks) accounts for 70–80% of all FGR cases and presents with slight deficiencies in placentation, which leads to mild hypoxia and requires little adaptation of the fetal cardiovascular system. However, the degree of tolerance to hypoxia is low; in contrast to cases of early-onset FGR, the fetus cannot tolerate this low oxygen supply for long [14, 15]. Compared to early-onset FGR, the incidence and severity of placental pathology in late-onset FGR are less common. The main problem is the diagnosis since ultrasound and Doppler parameters can be normal [16, 17].
The lack of unified diagnostic criteria leads to insufficient antenatal detection of FGR [1, 2, 4, 18, 19].
Recent advances in molecular genetics have markedly improved the feasibility of assessing epigenetic regulation and helped develop new diagnostic tests based on the isolation of epigenetic marks in biological substrates [6, 10, 12, 14]. Understanding the mechanisms underlying these processes can become the basis for developing new therapeutic and diagnostic approaches in the future.
This study aimed to investigate the level of TLR2 and IGF2/H19 imprinting control region methylation in peripheral blood plasma in FGR.
Materials and methods
The study included 118 pregnant women divided into those who had (group 1, n=58) and who had not had (group 2, n=60) a confirmed diagnosis of FGR. In group 1, 24 and 34 women had early and late FGR, respectively. The local ethics committee approved the study, and all pregnant women provided signed informed consent to participate in the study. The inclusion criteria were spontaneous singleton pregnancy, absence of severe non-obstetric comorbidities, large uterine fibroids, fetal anomalies, and malformations.
The levels of gene methylation in maternal blood plasma were measured in 5 ml peripheral venous blood samples collected in test tubes with K3 EDTA as an anticoagulant. Samples of EDTA-stabilized blood plasma were obtained after two-stage centrifugation. The samples were stored at -80°C. The technique of DNA isolation, bisulfite conversion, real-time polymerase chain reaction, and melting curve analysis are described in detail in previous studies [6].
Statistical analysis
Statistical analysis and plotting were performed using Attestat (Russia), Statistica 10, and OriginPro 8.5 (USA) software. Based on the normality of the data assessed by the Shapiro–Wilk test, quantitative variables were expressed as means (M) and standard deviation (SD). Data with non-normal distribution were reported as the median (Me) and the upper and lower quartiles (Q1; Q3); statistical significance of the differences was determined using the nonparametric Mann–Whitney test. Categorical variables were compared using Fisher’s exact test. To assess the quality of the study of the level of gene methylation using melting curve analysis, ROC curves were constructed to determine the area under the curve (AUC) with a 95% confidence interval (CI) and sensitivity and specificity at the cut-off value. Differences between the groups were considered statistically significant at p<0.05.
Results and discussion
All the study participants had comparable baseline clinical characteristics except gestational age at delivery and length and weight parameters of newborns.
There were no statistically significant differences in pregnancy complication rates (Table 1).
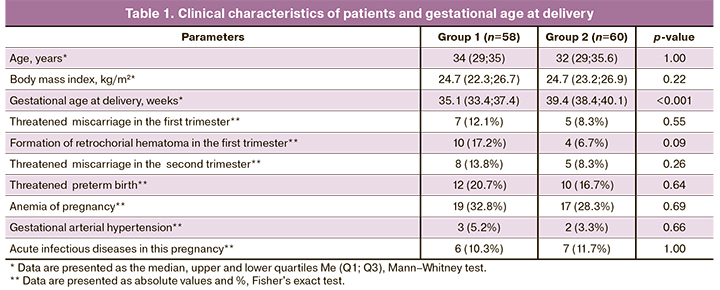
Analysis of perinatal outcomes (Table 2) showed a more unfavorable course of neonatal period among growth-restricted neonates, especially with early FGR, which was often associated with early delivery. Gestational age at delivery was 31.1 (28.1; 34, 7) and 35.9 (35.1; 38.1) weeks among early and late FGR, respectively (p <0.001). FGR was associated with more severe asphyxia (n=4, 6.9%, p=0.05), respiratory distress syndrome (n=5, 8.6%, p=0.03), congenital pneumonia (n=17, 29.3%, p <0.001), and intraventricular hemorrhage (n=10, 17.2%, p <0.001).
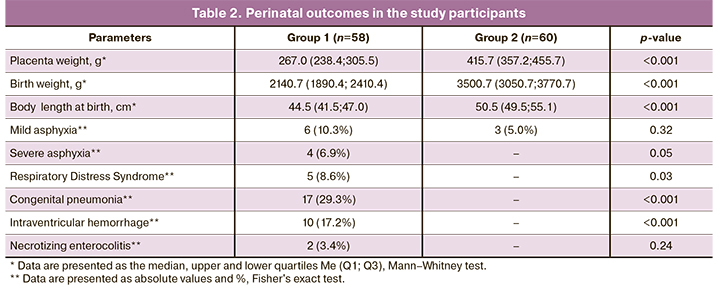
 Differences in the pathogenetic mechanisms of formation and perinatal outcomes of early and late FGR serve as the basis for studying their epigenetic regulation. Analysis of the literature [6, 8, 11, 12, 17] identified candidate genes with determining methylation levels in the blood plasma of pregnant women, including VEGF, HLAG, TIMP2, GNA12, DAPK3, MMP2, SOCS2, LEP, MEST, RASSF1, TLR2, BMP6, DKK3, CEBPA, DFNA5, CTCF, CDO1, PITX2, CDH1, SEPT9, PTEN, ICR (imprinting control region) IGF2/H19. Statistically significant differences were found in methylation levels of TLR2 and IGF2/H19 imprinting control region genes. The methylation level of the TLR2 gene in maternal blood plasma in early FGR was 0.01 (0.0; 0.45) and was statistically significantly lower than that in the control group [0.43 (0.05; 0.53], p=0.02. The relative level of TLR2 methylation in late FGR was 0.39 (0.0; 0.55) and did not differ significantly from the control group [0.43 (0.05; 0.53], p=0.87) (Fig. 1).
Differences in the pathogenetic mechanisms of formation and perinatal outcomes of early and late FGR serve as the basis for studying their epigenetic regulation. Analysis of the literature [6, 8, 11, 12, 17] identified candidate genes with determining methylation levels in the blood plasma of pregnant women, including VEGF, HLAG, TIMP2, GNA12, DAPK3, MMP2, SOCS2, LEP, MEST, RASSF1, TLR2, BMP6, DKK3, CEBPA, DFNA5, CTCF, CDO1, PITX2, CDH1, SEPT9, PTEN, ICR (imprinting control region) IGF2/H19. Statistically significant differences were found in methylation levels of TLR2 and IGF2/H19 imprinting control region genes. The methylation level of the TLR2 gene in maternal blood plasma in early FGR was 0.01 (0.0; 0.45) and was statistically significantly lower than that in the control group [0.43 (0.05; 0.53], p=0.02. The relative level of TLR2 methylation in late FGR was 0.39 (0.0; 0.55) and did not differ significantly from the control group [0.43 (0.05; 0.53], p=0.87) (Fig. 1).
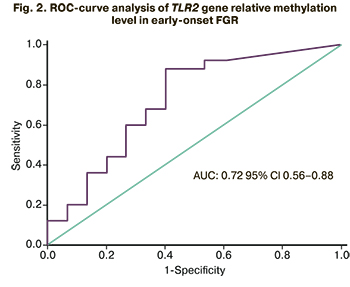 Diagnostic accuracy of testing TLR2 gene methylation was evaluated using receiver operating characteristic curve (ROC) analysis. For early-onset FGR, a good model was obtained with AUC=0.72 (95% CI 0.56–0.88), the optimal cut-off value of TLR2 gene relative methylation level of 0.012, the sensitivity and specificity of 88.1% and 60.2% (Fig. 2).
Diagnostic accuracy of testing TLR2 gene methylation was evaluated using receiver operating characteristic curve (ROC) analysis. For early-onset FGR, a good model was obtained with AUC=0.72 (95% CI 0.56–0.88), the optimal cut-off value of TLR2 gene relative methylation level of 0.012, the sensitivity and specificity of 88.1% and 60.2% (Fig. 2).
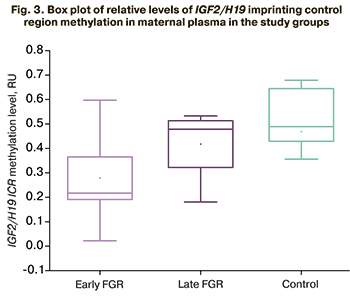
In patients with early FGR, relative levels of IGF2/H19 imprinting control region methylation [0.22 (0.18; 0.32)] was statistically significantly lower than that in the control group [0.49 (0.44; 0.65], p=0.03. In patients with late FGR, relative levels of IGF2/H19 imprinting control region methylation did not differ statistically significantly and was 0.48 (0.32.0.52, p=0.53) (Fig. 3).
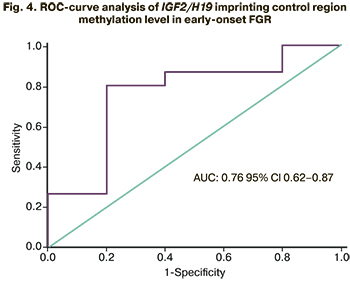
 Diagnostic accuracy of testing IGF2/H19 imprinting control region methylation for diagnosing early FGR was evaluated using receiver operating characteristic curve (ROC) analysis. A good model was obtained with AUC=0.79 (95% CI 0.62–0.87). An optimal cut-off value of IGF2/H19 imprinting control region methylation level was 0.2, and the sensitivity and specificity were 80.4% and 80.1% (Fig. 4).
Diagnostic accuracy of testing IGF2/H19 imprinting control region methylation for diagnosing early FGR was evaluated using receiver operating characteristic curve (ROC) analysis. A good model was obtained with AUC=0.79 (95% CI 0.62–0.87). An optimal cut-off value of IGF2/H19 imprinting control region methylation level was 0.2, and the sensitivity and specificity were 80.4% and 80.1% (Fig. 4).
In recent years, several processes have been described that, by influencing the regulation of pathogenetic mechanisms of FGR formation, contribute to a decrease in the prognostic and diagnostic performance of its potential markers [1, 4, 9, 13]. The most studied are epigenetic modifications, which are gene expressions outside of the encoding in the DNA sequence [6, 11, 16]. The study of the epigenome, located at the junction between genetic and external factors, is of great importance in expanding knowledge about FGR origin and its long-term consequences. As is known, TLR2 is a class of toll-like receptors that play a crucial role in the innate immune system and pathogenesis of apoptosis induced by bacterial peptidoglycan [5].
 It has been shown that activation of TLR2 in trophoblast cells in the first and third trimesters leads to the pronounced secretion of cytokines and chemokines by trophoblasts and can induce increased apoptosis of trophoblast cells and, accordingly, cause pregnancy complications [5, 6, 8, 12]. Our findings suggest that FGR is associated with a decrease in TLR2 gene methylation level, which can increase the expression of this protein and, consequently, to increased apoptosis in the placenta. Pathological intensification of apoptosis in the early stages of pregnancy can lead to disruption of placenta formation and the development of FGR.
It has been shown that activation of TLR2 in trophoblast cells in the first and third trimesters leads to the pronounced secretion of cytokines and chemokines by trophoblasts and can induce increased apoptosis of trophoblast cells and, accordingly, cause pregnancy complications [5, 6, 8, 12]. Our findings suggest that FGR is associated with a decrease in TLR2 gene methylation level, which can increase the expression of this protein and, consequently, to increased apoptosis in the placenta. Pathological intensification of apoptosis in the early stages of pregnancy can lead to disruption of placenta formation and the development of FGR.
In recent years, studies have been increasingly focused on the growth hormone/insulin-like growth factor-1axis. At the same time, epigenetic modifications that regulate its functioning are of great importance. IGF 1 and 2 are regulatory peptides that, being closely related to each other, are involved in the processes of placenta formation and early fetal growth. IGF1 is involved in the regulation of anabolic, antioxidant, and anti-inflammatory processes [15]. The role of IGF1 is mainly associated with the regulation of placental transport of nutrients [13, 18, 19]. At the same time, IGF2 is a regulatory protein of proliferation and differentiation and trophoblast invasion of the myometrial spiral arteries [6, 8, 11, 15]. Changes in IGF2 concentration at any stage of pregnancy can significantly contribute to the disruption of the normal placenta formation and, consequently, the development of FGR. As one of the epigenetic processes, genomic imprinting is the expression of one of two allelic genes located on the paternal or maternal chromosome. The molecular basis for such monoallelic gene expression is the methylation of their regulatory regions. One of the most studied mechanisms underlying fetal programming is the methylation of the IGF2/H19 locus. The IGF2 gene and the adjacent noncoding RNA H19 are located on the short arm of chromosome 11. The reduced methylation level of the IGF2/H19 imprinting control region in early FGR, found in our study, may cause a corresponding decrease in IGF2 expression, which is consistent with the results of other studies [8, 16, 17]. J. St-Pierre et al. [11] reported a negative correlation between IGF2 methylation level and the weight of newborns. Also, there is evidence of a relationship between epigenetic changes, namely hypomethylation of the IGF2/H19 imprinting control region on the paternal allele of chromosome 11, with the development of hereditary diseases characterized by pronounced postnatal growth restriction. The preceding suggests the potential of studying the methylation of these genes to clarify their role in developing FGR and identifying new diagnostic and prognostic markers.
Conclusion
The study findings confirm that methylation of TLR2 and IGF2/H19 imprinting control region genes have a role in FGR development and can be used as non-invasive predictors. These results suggest that antenatal epigenetic changes continue in the neonatal period and are essential for fetal programming implementation.
References
- Игнатко И.В., Денисова Ю.В., Филиппова Ю.А., Дубинин А.О. Дифференциальная диагностика ранней и поздней форм синдрома задержки развития плода. Уральский медицинский журнал. 2020; 12: 91-7. [Ignatko I.V., Denisova Yu.V., Filippova Yu.A., Dubinin A.O. Differential diagnosis of early and late forms of fetal retardation syndrome. Ural Medical Journal. 2020; 12: 91-7. (in Russian)].
- Ярыгина Т.А., Батаева Р.С. Задержка (замедление) роста плода: современные принципы диагностики, классификации и динамического наблюдения. Ультразвуковая и функциональная диагностика. 2019; 2: 33-44. [Yarygina T.A., Bataeva R.S. Fetal growth delay (deceleration): modern principles of diagnosis, classification and dynamic observation. Ultrasound and functional diagnostics. 2019; 2: 33-44. (in Russian)].
- Barker D.J. Adult consequences of fetal growth restriction. Clin. Obstet. Gynecol. 2006; 49 (2): 270-83. https://dx.doi.org/10.1097/00003081-200606000-00009.
- Figueras F., Gratacos E. Update on the diagnosis and classification of fetal growth restriction and proposal of a stage-based management protocol. Fetal Diagn. Ther. 2014; 36(2): 86-98. https://dx.doi.org/10.1159/000357592.
- Olmos-Ortiz A., Flores-Espinosa P., Mancilla-Herrera I., Vega-Sánchez R., Díaz L., Zaga-Clavellina V. Innate immune cells and Toll-like receptor-dependent responses at the maternal-fetal interface. Int. J. Mol. Sci. 2019; 20(15): 3654. https://dx.doi.org/10.3390/ijms20153654.
- Борис Д.А., Красный А.М., Куревлев С.В., Садекова А.А., Кан Н.Е., Тютюнник В.Л. Метилирование гена TLR2 и ICR IGF2/H19 в плаценте и плазме крови при преэклампсии. Акушерство и гинекология. 2020; 7: 93-8. [Boris D.A., Krasnyi A.M., Kurevlev S.V., Sadekova A.A., Kan N.E., Tyutyunnik V.L. Methylation of the TLR2 and IGF2/H19 ICR genes in the placenta and blood plasma in preeclampsia. Obstetrics and Gynecology. 2020; 7: 93-8. (in Russian)]. https://dx.doi.org/10.18565/aig.2020.7.93-98.
- Carter E.B., Conner S.N., Cahill A.G., Rampersad R., Macones G.A., Tuuli M.G. Impact of fetal growth on pregnancy outcomes in women with severe preeclampsia. Pregnancy Hypertens. 2017; 8: 21-5. https://dx.doi.org/10.1016/j.preghy.2017.02.002.
- Koukoura O., Sifakis S., Soufla G., Zaravinos A., Apostolidou S., Jones A. et al. Loss of imprinting and aberrant methylation of IGF2 in placentas from pregnancies complicated with fetal growth restriction. Int. J. Mol. Med. 2011; 28(4): 481-7. https://dx.doi.org/10.3892/ijmm.2011.754.
- Rizzo G., Mappa I., Rizzo G., D'Antonio F. International gestational age-specific centiles for umbilical artery Doppler indices: a longitudinal prospective cohort study of the INTERGROWTH-21st Project. Am. J. Obstet. Gynecol. 2021; 224(2): 248-9. https://dx.doi.org/10.1016/j.ajog.2020.08.110.
- Железова М.Е., Зефирова Т.А., Канюков С.С. Задержка роста плода: совре-менные подходы к диагностике и ведению беременности. Практическая медицина. 2019; 17(4): 8-14. [Zhelezova M.E., Zefirova T.A., Kanyukov S.S. Fetal growth restriction: modern approaches to the diagnosis and management of pregnancy. Practical medicine. 2019; 17(4): 8-14. (in Russian)].
- St-Pierre J., Hivert M.F., Perron P., Poirier P., Guay S.P., Brisson D., Bouchard L. IGF2 DNA methylation is a modulator of newborn’s fetal growth and development. Epigenetics. 2012; 7(10): 1125-32. https://dx.doi.org/10.4161/epi.21855.
- Хачатрян З.В., Кан Н.Е., Красный А.М., Садекова А.А., Куревлев С.В., Тютюнник В.Л. Метилирование генов в плаценте при задержке роста плода. Акушерство и гинекология. 2019; 12: 54-8. [Khachatryan Z.V., Kan N.E., Krasnyi A.M., Sadekova A.A., Kurevlev S.V., Tyutyunnik V.L. Gene methylation in the placenta of fetuses with fetal growth restriction. Obsterics and Gynecology. 2019; 12: 54-8. (in Russian)]. https://dx.doi.org/10.18565/aig.2019.12.54-58.
- Arroba A.I., Campos-Caro A., Aguilar-Diosdado M., Valverde Á.M. IGF-1, inflammation and retinal degeneration: A close network. Front. Aging Neurosci. 2018; 10: 203. https://dx.doi.org/10.3389/fnagi.2018.00203.
- Zhu Z., Cao F., Li X. Epigenetic programming and fetal metabolic programming. Front. Endocrinol. (Lausanne). 2019; 10: 764. https://dx.doi.org/10.3389/fendo.2019.00764.
- Harris L.K., Crocker I.P., Baker P.N., Aplin J.D., Westwood M. IGF2 actions on trophoblast in human placenta are regulated by the insulin-like growth factor 2 receptor, which can function as both a signaling. Biol. Reprod. 2011; 84(3): 440-6. https://dx.doi.org/10.1095/biolreprod.110.088195.
- Llères D., Moindrot B., Pathak R., Piras V., Matelot M., Pignard B. et al. CTCF modulates allele-specific sub-TAD organization and imprinted gene activity at the mouse Dlk1-Dio3 and IGF2-H19 domains. Genome Biol. 2019; 20(1): 272. https://dx.doi.org/10.1186/s13059-019-1896-8.
- Tabano S., Colapietro P., Cetin I., Grati F.R., Zanutto S., Mandò C. et al. Epi-genetic modulation of the IGF2/H19 imprinted domain in human embryonic and extra-embryonic compartments and its possible role in fetal growth restriction. Epigenetics. 2010; 5(4): 313-24. https://dx.doi.org/10.4161/epi.5.4.11637.
- Martín-Estal I., de la Garza R.G., Castilla-Cortázar I. Intrauterine growth re-tardation (IUGR) as a novel condition of insulin-like growth factor-1 (IGF-1) deficiency. Rev. Physiol. Biochem. Pharmacol. 2016; 170: 1-35. https://dx.doi.org/10.1007/112_2015_5001.
- Aouache R., Biquard L., Vaiman D., Miralles F. Oxidative stress in preeclampsia and placental diseases. Int. J. Mol. Sci. 2018; 19(5): 1496. https://dx.doi.org/10.3390/ijms19051496.
Received 04.02.2021
Accepted 09.04.2021
About the Authors
Natalia E. Kan, Dr. Med. Sci., Professor, Deputy Director for Research, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russian Federation. Tel.: +7(926)220-86-55. E-mail: kan-med@mail.ru. Researcher ID: B-2370-2015, SPIN-код: 5378-8437,Authors ID: 624900, Scopus Author ID: 57008835600, ORCID ID: 0000-0001-5087-5946. 117997, Russia, Moscow, Ac. Oparina str., 4.
Victor L. Tyutyunnik, Dr. Med. Sci., Professor, Leading Researcher at the Department of Research Administration, Academician V.I. Kulakov National Medical Research
Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russian Federation; Head Physician of the Perinatal Center, European Medical Center.
Tel.: +7(903)969-50-41. E-mail: tioutiounnik@mail.ru. Researcher ID: B-2364-2015, SPIN-код: 1963-1359, Authors ID: 213217, Scopus Author ID: 56190621500,
ORCID ID: 0000-0002-5830-5099. 117997, Russia, Moscow, Ac. Oparina str., 4; 125040, Russia, Moscow, Pravda str., 15/1.
Zarine V. Khachatryan, Ph.D. Student, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology,
Ministry of Health of Russian Federation. Tel.: +7(909)656-24-56. E-mail: z.v.khachatryan@gmail.com. 117997, Russia, Moscow, Ac. Oparina str., 4.
Alsu A. Sadekova, Ph.D., Senior Researcher at the Cytology Laboratory, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russian Federation. Tel.: +7(495)438-22-72. E-mail: a_sadekova@oparina4.ru. 117997, Russia, Moscow, Ac. Oparina str., 4.
Aleksey M. Krasnyi, Ph.D. (bio.sci.), Head of the Cytology Laboratory, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russian Federation. Tel.: +7(495)438-22-72. E-mail: alexred@list.ru. 117997, Russia, Moscow, Ac. Oparina str., 4.
For citation: Kan N.E., Tyutyunnik V.L., Khachatryan Z.V., Sadekova A.A., Krasnyi A.M. Plasma TLR2 and IGF2/H19 imprinting control region gene methylation in fetal growth restriction.
Akusherstvo i Ginekologiya / Obstetrics and Gynecology. 2021; 5: 79-84 (in Russian)
http://dx.doi.org/10.18565/aig.2021.5.79-84



