WIF1 gene methylation in endometrial pathology
Relevance. Wnt signaling pathway promotes proliferation, angiogenesis, and malignant transformation to endometrial cancer (EC). However, the role of epigenetic abnormalities of its inhibitory gene WIF1 in benign endometrial pathology remains unexplored.Ashrafyan L.A., Kiselev V.I., Chernukha G.E., Ivanov I.A., Poloznikov A.A.
Aim. To investigate the WIF1 gene methylation status in different endometrial pathologies.
Materials and methods. Methylation levels of the WIF1 gene were analyzed by bisulfite sequencing in samples of endometrial polyps (EP, n=60), endometrial hyperplasia (EH, n=35), chronic endometritis (CE, n=20), and normal proliferative stage endometrium (StP, n=20).
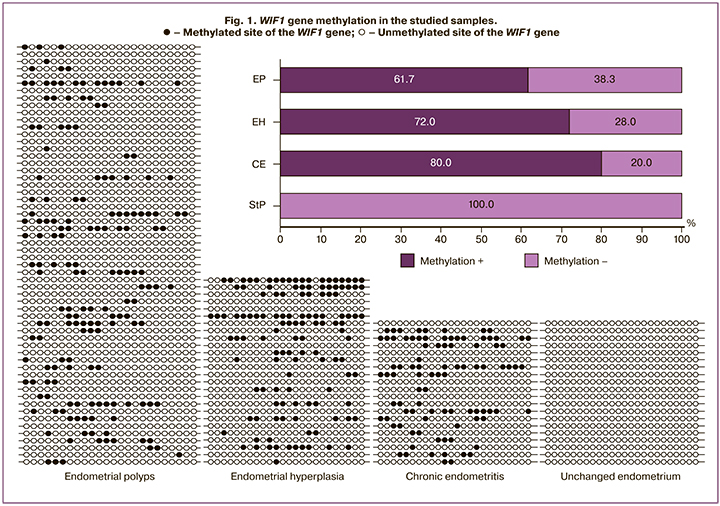
Results. WIF1 gene methylation was detected in 80%, 72%, 61.7% of CE, EH, and EP samples and not found in normal StP endometrium (p&0.001). The highest methylation level was observed in EH samples. Patients with WIF1 gene methylation were 1.6 times more likely to have a history of intrauterine interventions. The number of intrauterine interventions correlated with methylation level.
Conclusion. Epigenetic silencing of the WIF1 gene may play a significant role in forming EH and EP by activating the Wnt signaling pathway associated with an excessive proliferative and angiogenic activity. CE may be a risk factor for developing these diseases due to the induction of abnormal WIF1 gene methylation.
Keywords
Abnormal uterine bleeding is the most common indication for intrauterine interventions, both in the reproductive period and menopause [1–4]. The most common causes of abnormal uterine bleeding include polyps (EP), endometrial hyperplasia (EH), and chronic endometritis (CE) [2, 5, 6]. Hyperplastic endometrial disorders, including EP and EH, have high recurrence rates and are associated with repeat intrauterine interventions, the risk of endometrial damage, and intrauterine synechiae formation, and the development of uterine forms of infertility [1, 3, 5]. Besides, EH is considered a risk factor for endometrial cancer, which still has high incidence rates.
Despite decades of research on hyperplastic endometrial disorders, their underlying mechanisms remain unclear. A promising direction of research related to proliferative processes may be Wnt signaling pathway, a major regulator of cell proliferation, migration and differentiation, angiogenesis, and malignant transformation [7–10]. The Wnt cascade is regulated by several inhibitors, including Wnt inhibitory factor-1 (WIF1), which is a major negative regulatory factor for this pathway.
According to available research evidence, some malignancies, including endometrial cancer, are associated with the silencing of gene encoding WIF1 through its methylation [8, 9, 11–15].
DNA methylation is the most common form of epigenetic modifications involving the addition of a methyl group to the 5′-carbon of cytosine in CpG dinucleotide sequences located in the promoter region of a gene, which leads to suppression of DNA transcription [16]. Hypermethylation of tumor suppressor genes and hypomethylation of pro-oncogenes can result in excessive cell proliferation and angiogenesis, thus contributing to malignant transformation, including in the endometrium [16, 17]. However the possible role of Wnt pathway activation and WIF1 methylation in the formation of benign endometrial hyperplastic processes remains poorly understood. Although CE is not considered a proliferative disorder, there is data on the relationship between gene methylation and chronic inflammation [18–20]. However, current literature lacks studies investigating such a relationship in the female reproductive tract's inflammatory diseases.
The present study aimed to investigate the WIF1 gene methylation status in different endometrial pathologies.
Materials and methods
The study included 125 women aged 20 to 50 years [mean 35.6 (6.9) years] with histologically confirmed EP [n=60; 35.5 (6.7) years], EH [n=25; (37, 4 (8.1) years, and CE [n=29; (35.2 (6.6) years]. The control group consisted of 20 women [36.4 (6.1) years] without menstrual irregularities and with the proliferative phase of the endometrium (StP). The exclusion criteria were hormone therapy within three months before surgery, malignancies, and severe extragenital pathology. The obtained endometrial tissue samples were washed three times with PBS buffer and frozen at -20°C. To determine the level of WIF1 gene methylation, the obtained materials were homogenized in a FastPrep-24 homogenizer (MP Biomedicals, USA) with the addition of matrix D. DNA was isolated from the obtained samples using the ReliaPrep gDNA Tissue Miniprep System kit (Promega, USA). The DNA concentration was determined using a standard Qubit RNA HS Assay on Qubit 2.0 Fluorometer (Life Technologies).
Genomic DNA (150 ng) was bisulfite converted (conversion of unmethylated cytosine residues into thymine while keeping methylated cytosine residues unchanged) using the innuCONVERT Bisulfite Basic Kit (Analytik Jena, Germany). The concentration of the converted DNA was determined photometrically in a μ-drop plate (BMG Labtech, Germany) using a CLARIO starmultidetector(BMGLabtech, Germany). Polymerase chain reaction (PCR): 20 ng of bisulfite-converted DNA was isolated for “touchdown” PCR amplification using GoTaq Hot Start Green Master Mix polymerase mixture (Promega, USA) and primers to increase the number of copies of the promoter region of the WIF1 gene from 554 to 140 nucleotides before the start codon and containing complementary and universal sequence M13 at the 5’-end: WIF1-M13F 5’-gttttcccagtcacgacGAGTGATGTTTTAGGGGTTT -3’ WIF1-M13R5’-ggaacagctatgaccatgCCTAAATACCAAAAA ACCTAC -3’. The sequencing reaction was performed according to the standard protocol using forward primers and the ABI PRISM BigDye Terminator v. 3.1.45. Analysis of reaction products was performed on an Applied Biosystems 3730 DNA Analyzer (Applied Biosystems, USA) using universal primers M13: M13F 5’-GTTTTCCCAGTCACGAC -3’ M13R 5’-GGAAACAGCTATGA.
Statistical analysis
Statistical analysis processing was performed using the Statistica 10.0 software. Detection rates of WIF1 gene methylation were compared by the Pearson chi-square (χ2) test. The distribution of continuous variables (age, body mass index-BMI) was tested for normality using the Kolmogorov–Smirnov test. Between-group differences in age and BMI were assessed by Student's t-test for unpaired samples. Quantitative variables were expressed as means and standard deviation M (SD). Differences in WIF1 gene methylation levels, the number of past pregnancies, childbirth, and intrauterine interventions were evaluated with a nonparametric Mann–Whitney U-test. Correlation analysis was conducted by calculating Spearman's rank correlation coefficients. The strength of the relationship of R<0.3 was defined as weak, R from 0.3 to 0.6 as moderate, and R≥0.7 as strong. Differences between the groups were considered statistically significant at p<0.05.
Results
Bisulfite sequencing detected WIF1 gene methylation in 16 of 20 (80%), 16 of 25 (72%), and 37 of 60 (61.7%) samples of CE, EH, and EP. Methylation rates did not differ significantly between EP and EH (p=0.46), EP and CE (p=0.17), and EH and CE (p=0.72). Abnormal WIF1 methylation was not detected in any StP endometrial sample (Fig. 1). The WIF1 methylation level was assessed by the number of changed sites (from 1 to 24). The number of methylated sites in the EP ranged from 1 to 13, mean 4.6 (2.9); at CE – from 1 to 16, mean 5.7 (4.4). The highest methylation level was observed at EH – from 1 to 19, mean7.3 (5.2), which was statistically significantly higher compared to EP (p=0.046). There were no differences between EP and CE (p=0.08) and EH and CE (p=0.84).
To identify possible risk factors for epigenetic silencing of WIF1, clinical and anamnestic data of patients with and without gene methylation were compared (Table). Patients with epigenetic silencing of the WIF1 gene were 1.6 times more likely to have a history of intrauterine interventions (p=0.02). The number of hysteroscopies was also higher among patients with WIF1 methylation (p=0.04).
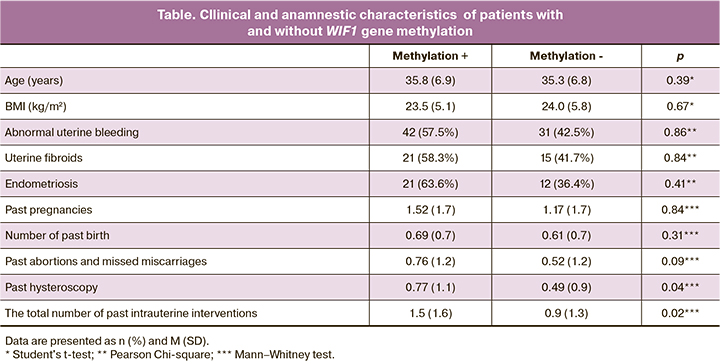
The number of surgical interventions performed for abortions and missed miscarriage did not differ significantly (p=0.09). There was a direct correlation between the number of intrauterine interventions and the number of methylated sites (R=0.197; p=0.03). No statistically significant differences were found regarding age, BMI, parity, and the presence of gynecological comorbidities.
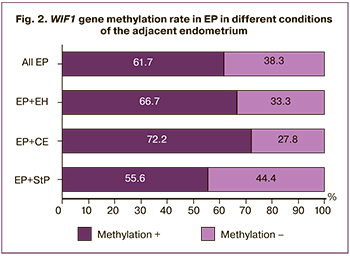 Analysis of the methylation status of the adjacent endometrium showed that 18, 6, and 36 EPs were detected in the setting of StP endometrium, EH, and CE, respectively. In samples with concomitant CE in EP, there was a tendency to a higher proportion of methylated samples (72.2%), compared with EP in the setting of StP endometrium (55.6%). However, there were no statistically significant differences (p=0.45). With EP in combination with EH, the methylation rate was 66.7%, which did not differ significantly from the EP detected against the background of StP (p=0.69) (Fig. 2).
Analysis of the methylation status of the adjacent endometrium showed that 18, 6, and 36 EPs were detected in the setting of StP endometrium, EH, and CE, respectively. In samples with concomitant CE in EP, there was a tendency to a higher proportion of methylated samples (72.2%), compared with EP in the setting of StP endometrium (55.6%). However, there were no statistically significant differences (p=0.45). With EP in combination with EH, the methylation rate was 66.7%, which did not differ significantly from the EP detected against the background of StP (p=0.69) (Fig. 2).
Discussion
EH and EP are the most common intrauterine pathologies, the leading causes of abnormal uterine bleeding, and risk factors for endometrial cancer. Investigation of molecular genetic fundamentals of hyperplastic endometrial disorders remains a challenging issue in gynecology, presenting the basis for developing new, pathogenetically oriented approaches to therapy. Current literature is lacking studies investigating epigenetic conditions of the Wnt pathway in EP. Domenico et al. analyzed a small sample of EP (8 samples) and in every third case found methylation of genes of the SFRP group, important suppressors of the Wnt pathway [11]. A recently published article by Feng et al. reported that compared with the normal endometrium, EP samples had higher expression of the Wnt1 protein, one of this signaling pathway's activators. This, according to the authors' conclusion, indicates the activity of the Wnt cascade in EP [21]. However, in the literature addressing the Wnt pathway's role in the development of benign endometrial pathology, there is more evidence regarding EH. Many studies have reported an association between EH and the methylation of WNT pathway inhibitors, particularly SFRP, PRICKLE1, CSNK1E, SKP1, NFATC2 [11, 16, 22]. In one of the experimental studies, long-term activation of the Wnt pathway in knockout mice resulted in overexpression of Ki-67, a proliferation marker, and the development of EH [23].
These findings are consistent with our results showing WIF1 gene methylation in most cases of EH and EP. This observation suggests the Wnt cascade's excessive activity and subsequent induction of proliferative and angiogenic activity [7, 8, 10]. It should be noted that EH samples had higher WIF1 gene methylation levels compared to EP and CE. It cannot be ruled out that this may contribute to more significant proliferative activity and an increased risk of progression from non-atypical EH to atypical. This assumption is partially supported by the literature reporting the frequent observation of WIF1 gene methylation in endometrial cancer and atypical EH [9–11]. Therefore, it can be concluded that WIF1 gene methylation may have a significant role in the formation of EH and EP through activation of the Wnt signaling pathway.
Endometrial hyperplastic processes' pathogenesis is associated with impaired proliferation and apoptosis, which is believed to be related to the endometrium's impaired hormonal regulation [5, 23, 24]. Considering the information that excessive exposure to estrogens leads to activation and progestogens to suppression of the Wnt cascade, it can be assumed that an imbalance of receptors for sex steroids can contribute to the formation of hyperplastic endometrial processes by increasing the action of estrogens and activating the Wnt signaling pathway [10, 21].
Not being a proliferative endometrial disorder, CE has a different origin, mediated by a microbial factor and endometrial trauma. Our findings suggest the relationship between endometrial hyperplastic processes and CE, mediated by epigenetic disorders leading to the Wnt signaling pathway's activation. Chronic inflammation and oxidative stress predispose malignant transformation. This susceptibility may be associated with the methylation of various genes by activating the DNA methyltransferase enzyme, which induces a methyl group's attachment to CpG islands [19, 20]. We have not found any similar studies on epigenetic disorders in CE in the available literature. However, some studies showed the pathological methylation of tumor-suppressive genes in the gastrointestinal tract's inflammatory diseases, particularly in ulcerative colitis, Barrett's esophagus, hepatitis B and C, pancreatitis, and gastritis [19, 20]. The inflammatory genesis of DNA methylation was shown in a study of Japanese researchers on rodents. The infection of the gastric mucosa with H. pylori bacteria led to chronic gastritis and DNA methylation. Some of the rodents underwent immunosuppressive therapy, which helps suppress inflammation and inhibit DNA methylation, even with the preservation of H. pylori, which may indicate the inflammatory genesis of methylation. Moreover, after H. pylori eradication, the authors note that methylation persisted even after discontinuing therapy due to the persistence of chronic gastritis [20].
Our findings showed that patients with WIF1 methylation were more likely to have a history of intrauterine interventions, the number of which correlated with methylation level. Intrauterine interventions are a risk factor for CE, often associated with the formation of EP [24, 25]. The role of CE in the development of EP is confirmed by the data on the high expression of proinflammatory cytokines in the EP, compared with the normal endometrium StP [24, 26], as well as the relatively high rates of EP formation in the setting of CE, which varies from 19 to 61.7% [2, 5, 25, 27]. There is limited evidence on the possible relationship between CE and EH. Thus, some studies have described an increase in COX-2 expression in EH and endometrial cancer, as compared to StP endometrium [28, 29]. According to Sheshukova N.A. et al., samples of EH with concurrent CE had a higher expression of the proliferation marker Ki-67 and growth factors than with EH without CE. The authors hypothesized that chronic inflammation activates proliferation and angiogenesis, resulting in hyperplastic endometrial processes [30]. Based on our results, it can also be assumed that CE is a risk factor for hyperplastic endometrial processes due to the induction of abnormal WIF1 gene methylation.
Conclusion
Summing up our findings on methylation of the WIF1 gene promoter region, detected in most EH and EP samples but not in the endometrium StP, it can be concluded that epigenetic disorders are associated with hyperplastic endometrial processes. The high detection rates of the WIF1 gene methylation in CE may indicate the involvement of chronic inflammation in the development of hyperplastic endometrial processes. Our results suggest the feasibility of using demethylating agents to treat these diseases and lend support for more extensive clinical trials aimed to explore this hypothesis further and increase the effectiveness of therapy and secondary prevention of hyperplastic endometrial processes.
References
- Clark T.J., Stevenson H. Endometrial Polyps and Abnormal Uterine Bleeding (AUB-P): What is the relationship; how are they diagnosed and how are they treated? Best Pract. Res. Clin. Obstet. Gynaecol. 2017; 40: 89-104. https://dx.doi.org/10.1016/j.bpobgyn.2016.09.005.
- Чернуха Г.Е., Асатурова А.В., Иванов И.А., Думановская М.Р. Структура патологии эндометрия в различные возрастные периоды. Акушерство и гинекология. 2018; 8: 129-34. https://dx.doi.org/10.18565/aig.2018.8.129-134. [Chernukha G.E., Asaturova A.V., Ivanov I.A., Dumanovskaya M.R. Endometrial lesion’s pattern in different age groups. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2018; 8: 129-34.(in Russian)].
- Capmas P., Pourcelot A.G., Giral E., Fedida D., Fernandez H. Office hysteroscopy: A report of 2402 cases. J. Gynecol. Obstet. Biol. Reprod. (Paris). 2016; 45(5): 445-50. https://dx.doi.org/10.1016/j.jgyn.2016.02.007.
- Cooper N., Barton P., Breijer M., Caffrey O., Opmeer B.C., Timmermans A. et al. Cost-effectiveness of diagnostic strategies for the management of abnormal uterine bleeding (heavy menstrual bleeding and post-menopausal bleeding): a decision analysis. Health Technol. Assess. 2014; 18(24): 1-201, v-vi. https://dx.doi.org/10.3310/hta18240.
- Tanos V., Berry K.E., Seikkula J., Abi Raad E., Stavroulis A., Sleiman Z. et al. The management of polyps in female reproductive organs. Int. J. Surg. 2017; 43: 7-16. https://dx.doi.org/10.1016/j.ijsu.2017.05.012.
- Gon S., Kundu T., Mallick D., Ghosh G. A study on histopathological patterns of endometrium in different types of abnormal uterine bleeding among peri and postmenopausal women. J. Dent. Med. Sci. (IOSR-JDMS). 2016; 15(9): 106-11.
- Xie J., Zhang Y., Hu X., Lv R., Xiao D., Jiang L., Bao Q. Norcantharidin inhibits Wnt signal pathway via promoter demethylation of WIF 1 in human non small cell lung cancer. Med. Oncol. 2015; 32(5): 145. https://dx.doi.org/10.1007/s12032-015-0592-0.
- Есенеева Ф.М., Шалаев О.Н., Оразмурадов А.А., Радзинский В.Е., Куулар А.А., Киселев В.И., Салимова Л.Я. WNT-сигнальный путь при миоме матки. Мать и дитя в Кузбассе. 2017; 2: 33-8. [Eseneeva F.M., Shalaev O.N., Radzinskiy V.E., Kiselev V.I. et al. WNT signal way in myomautery. Mother and Child in Kuzbass. 2017; 2: 33-8.(in Russian)].
- Киселев В.И., Муйжнек Е.Л., Ашрафян Л.А., Сухих Г.Т. Эпигенетика в гинекологии и онкогинекологии: WIF реальность. Акушерство и гинекология: новости, мнения, обучение. 2018; 1: 18-26. [Kiselev V.I. Muyzhnek E.L., Ashrafyan L.A., Sukhikh G.T. Epigenetics in gynecology and oncogynecology: WIF and reality. Obstetrics and gynecology: news, opinions, education. 2018; 1: 18-26. (in Russian)].
- Kiewisz J., Wasniewski T., Kmiec Z. Participation of WNT and β-catenin in physiological and pathological endometrial changes: association with angiogenesis. Biomed. Res. Int. 2015; 2015: 854056. https://dx.doi.org/10.1155/2015/854056.
- Domenico M., Santoro A., Ricciardi C., Iaccarino M., Iaccarino S., Freda M. et al. Epigenetic fingerprint in endometrial carcinogenesis: the hypothesis of a uterine field cancerization. Cancer Biol. Ther. 2011; 12(5): 447-57. https://dx.doi.org/10.4161/cbt.12.5.15963.
- Deng X., Hou C., Wang H., Liang T., Zhu L. Hypermethylation of WIF1 and its inhibitory role in the tumor growth of endometrial adenocarcinoma. Mol. Med. Rep. 2017; 16(5): 7497-503. https://dx.doi.org/10.3892/mmr.2017.7564.
- Cухих Г.Т., Ашрафян Л.А., Байрамова Г.Р., Бабкина И.О., Чернова В.Ф.,Осипьянц А.И., Королькова А.И., Полозников А.А., Асфарова Г.Р., Муллабаева С.М., Коган Е.А., Муйжнек Е.Л., Друх В.М., Киселев В.И. Метилирование гена WIF-1 при цервикальных плоскоклеточных интраэпителиальных поражениях. Акушерство и гинекология. 2017; 5: 114-23. https://dx.doi.org/10.18565/aig.2017.5.114-23. [Sukhikh G.T., Ashrafyan L.A., Bairamova G.R., Babkina I.O., Chernova V.F., Osipyants A.I., Korolkova A.I. et al. WIF-1 gene methylation in cervical squamous intraepithelial lesions. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2017; 5: 114-23. (in Russian)].
- Yee D., Tang Y., Li X. The Wnt inhibitory factor 1 restoration in prostate cancer cells was associated with reduced tumor growth, decreased capacity of cell migration and invasion and a reversal of epithelial to mesenchymal transition. Mol. Cancer. 2010; 9: 162. https://dx.doi.org/10.1186/1476-4598-9-162.
- van der Horst P., Wang Y., Vandenput I., Kühne L., Ewing PC, van Ijcken W.F. et al. Progesterone inhibits epithelial-to-mesenchymal transition in endometrial cancer. PLoS One. 2012; 7(1): e30840. https://dx.doi.org/10.1371/journal.pone.0030840.
- Xihai W., Jilan M., Jingyan J., Fangmei L. Analysis of methylation profiling data of hyperplasia and primary and metastatic endometrial cancers. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017; 217: 161-6. https://dx.doi.org/10.1016/j.ejogrb.2017.08.036.
- Tao M., Freudenheim L. DNA methylation in endometrial cancer. Department of Social and Preventive Medicine. Epigenetics. 2010; 5(6):491-8.
- Klutstein M., Nejman D., Greenfield R., Cedar H. DNA methylation in cancer and aging. Cancer Res. 2016; 76(12): 3446-50. https://dx.doi.org/10.1158/0008-5472.CAN-15-3278.
- O'Hagan H.M., Wang W., Sen S., Destefano Shields C., Lee S.S., Zhang Y.W. et al. Oxidative damage targets complexes containing DNA methyltransferases, SIRT1, and polycomb members to promoter CpG Islands. Cancer Cell. 2011; 20(5): 606-19. https://dx.doi.org/10.1016/j.ccr.2011.09.012.
- Niwa T., Ushijima T. Induction of epigenetic alterations by chronic inflammation and its significance on carcinogenesis. Adv. Genet. 2010; 71: 41-56. https://dx.doi.org/10.1016/B978-0-12-380864-6.00002-X.
- Feng M., Zhang T., Ma H. Progesterone ameliorates the endometrial polyp by modulating the signaling pathway of Wnt and β-catenin via regulating the expression of H19 and miR-152. J. Cell. Biochem. 2019; 120(6): 10164-74. https://dx.doi.org/10.1002/jcb.28301.
- Marichereda V.G., Bykovа N.A., Bubnov V.V., Manasova G.S., Moskalenko T.Y., Volyanska A.G. et al. The analysis of methylation of DNA promoter of SFRP2 gene in patients with hyperplastic processes of the endometrium. Exp. Oncol. 2018; 40(2): 109-13.
- Goad J., Ko Y.A., Kumar M., Jamaluddin M.F.B., Tanwar P.S. Oestrogen fuels the growth of endometrial hyperplastic lesions initiated by overactive Wnt/β-catenin signalling. Carcinogenesis. 2018; 39(9): 1105-16. https://dx.doi.org/10.1093/carcin/bgy079.
- Indraccolo U., Di Iorio R., Matteo M., Corona G., Greco P., Indraccolo S.R. The pathogenesis of endometrial polyps: a systematic semi-quantitative review. Eur. J. Gynaecol. Oncol. 2013; 34(1): 5-22.
- Carvalho F.M., Aguiar F.N., Tomioka R., de Oliveira R.M., Frantz N., Ueno J. Functional endometrial polyps in infertile asymptomatic patients: a possible evolution of vascular changes secondary to endometritis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013; 170(1): 152-6. https://dx.doi.org/10.1016/j.ejogrb.2013.05.012.
- Zhu Y., Du M., Yi L., Liu Z., Gong G., Tang X. CD4+ T cell imbalance is associated with recurrent endometrial polyps. Clin. Exp. Pharmacol. Physiol. 2018; 45(6): 507-13. https://dx.doi.org/10.1111/1440-1681.12913.
- Cicinelli E., Bettocchi S. Chronic endometritis: a common disease hidden behind endometrial polyps in pre-menopausal women. First evidence from a case-control study. J. Minim. Invasive Gynecol. 2019; 26(7): 1346-50. https://dx.doi.org/10.1016/j.jmig.2019.01.012.
- Sanderson P., Critchley H., Williams A., Arends M.J., Saunders P.T. New concepts for an old problem: the diagnosis of endometrial hyperplasia. Hum. Reprod. Update. 2017; 23(2): 232-54. https://dx.doi.org/10.1093/humupd/dmw042.
- Erkanli S., Bolat F., Kayaselcuk F., Demirhan B., Kuscu E. COX-2 and survivin are overexpressed and positively correlated in endometrial carcinoma. Gynecol. Oncol. 2007; 104(2): 320-5. https://dx.doi.org/10.1016/j.ygyno.2006.08.044.
- Шешукова Н.А., Макаров И.О., Овсянникова Т.В. Гиперпластические процессы эндометрия: особенности пролиферативной активности при сочетании с хроническим эндометритом. Акушерство, гинекология и репродукция. 2011; 5(3): 10-15. [Sheshukova N.A., Makarov I.O., Ovsyannikova T.V. Hyperplastic Process of Endometrium: features of proliferative activity when combined with chronic endometritis. Obstetrics, gynecology and reproduction. 2011; 5(3): 10-15. (in Russian)].
Received 30.04.2020
Accepted 22.09.2020
About the Authors
Lev A. Ashrafyan, Academician of the RAS, Dr. Med. Sci., Professor, Director of the Institute of Oncogynecology and Mammology, V.I. Kulakov NMRC for OG&P,Ministry of Health of Russia. Tel.: +7(495)531-44-44. E-mail: l_ashrafyan@oparina4.ru. 117997, Russia, Moscow, Ac. Oparina str., 4.
Vsevolod I. Kiselev, Dr. Bio. Sci., Corr. Member of the RAS, Deputy Director of the Institute of Gynecoloigy and Mammology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. Tel.: +7(495)531-44-44. E-mail: vkis10@mail.ru. https://orcid.org/0000-0002-4721-3420. 117997, Russia, Moscow, Ac. Oparina str., 4.
Galina E. Chernukha, Dr. Med. Sci., Professor at the V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, Department of Gynecological Endocrinology.
Tel.: +7(916)311-05-21. E-mail: g_chernukha@oparina4.ru. https://orcid.org/0000-0002-9065-5689. 117997, Russia, Moscow, Ac. Oparina str., 4.
Ilya A. Ivanov, Postgraduate Student at the Department of Gynecological Endocrinology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
Tel.: +7(962)980-00-18. E-mail: doctor.i.ivanov@yandex.ru. https://orcid.org/0000-0003-0751-7566. 117997, Russia, Moscow, Ac. Oparina str., 4.
Andrey A. Poloznikov, PhD. (chem. Sci.)., Deputy Director for Science, NMRRC, Ministry of Health of Russia. Tel.: +7(495)150-11-22. Е-mail: andrey.poloznikov@nmicr.ru.
125284, Russia, Moscow, 2-nd Botkinsky proezd, 3.
For citation: Ashrafyan L.A., Kiselev V.I., Chernukha G.E., Ivanov I.A., Poloznikov A.A. WIF1 gene methylation in endometrial pathology.
Akusherstvo i Ginekologiya/ Obstetrics and gynecology. 2020; 12: 122-128 (in Russian)
https://dx.doi.org/10.18565/aig.2020.12.122-128



