Ежегодно в мире регистрируется более 165 тыс. новых случаев злокачественных новообразований яичников. В России ежегодно опухоли яичников выявляют более чем у 12,3 тыс. женщин (16,5 на 100 тыс.), они занимают 7-е место (7%) в структуре общей онкологической заболеваемости и 3-е среди гинекологических новообразований, уступая раку эндометрия и шейки матки [1, 2].
Злокачественные новообразования яичников – гистологически гетерогенная группа, при этом опухоли эпителиального происхождения составляют 85–90% [2, 3]. Около 75–80% злокачественных опухолей яичника выявляются на III–IV стадиях, а 5-летняя выживаемость по всем стадиям при всех гистологических формах не превышает 25%. Признание рака яичников, рака фаллопиевых труб и первичного рака брюшины разными стадиями единого патологического процесса повлекло за собой изменения в классификации рака яичников в соответствии с классификацией FIGO [4]. В частности, теперь наличие метастазирования в ретроперитонеальные лимфатические узлы является достаточным для отнесения опухоли к стадии IIIA, а метастазирование в паховые, кардио-диафрагмальные и умбиликальные лимфоузлы считается дистантным (IVB) [4–6]. Предоперационная дифференциальная диагностика и адекватное стадирование злокачественных опухолей является определяющим звеном в выборе адекватного объема оперативного вмешательства, необходимости сочетанной лучевой и/или химиотерапии. Однако, несмотря на активное развитие визуализационных технологий, ее эффективность остается невысокой [3, 7].
Ультразвуковое исследование обладает высокой чувствительностью и является методом выбора на первом этапе диагностического поиска опухолевых образований яичников, а использование магнитно-резонансной томографии (МРТ), согласно рекомендациям ESUR и ACR, является золотым стандартом уточняющей дифференциальной диагностики опухолей яичника [6]. Несмотря на многолетний опыт использования МРТ в диагностике патологических состояний органов малого таза, как за рубежом, так и в России не существует единой концепции относительно стандартов ее проведения, оптимальных протоколов исследования [5, 6].
В связи с активным внедрением в практику таких современных методик, как диффузионная и перфузионная МРТ, появились новые возможности, дающие представление не только о структуре опухоли, но и о функциональном состоянии тканей, включая определение скорости диффузии молекул воды, скорости «накопления» и «вымывания» контрастных агентов. Изменения данных показателей обусловлены повышенной проницаемостью сосудистой стенки в опухоли и уменьшением межклеточных пространств вследствие патологического деления клеток. Возможность изучения коэффициентов диффузии и уровней перфузии открывают кардинально новые аспекты МР-диагностики, позволяющие определять изменения, происходящие в опухоли под воздействием консервативных методов лечения in vivo [3, 6].
В связи с усовершенствованием технических возможностей МРТ и внедрением новых современных методик изложенная проблема представляет научный и практический интерес, требует углубленного исследования ряда аспектов, связанных с изучением роли данного метода медицинской визуализации в дифференциальной диагностике образований яичника [1, 4, 7].
Цель исследования: оценить возможности комплексной МРТ в дифференциальной предоперационной диагностике опухолей яичников.
Материал и методы исследования
Проведен анализ МР-исследований с возможностью мультипараметрической оценки, выполненных с 2011 по 2015 гг. (256 больным с 284 образованиями яичника) в рамках первичной предоперационной диагностики.
МРТ выполнялась на сверхпроводящем МР-томографе Vintage Atlas (Тoshiba Medical System) с напряженностью магнитного поля 1.5Т, обладающей полем обзора 55 см, размером туннеля 71 см и диапазоном движения стола 205 см. Для исследования органов малого таза использовалась гибкая 32-канальная катушка для тела (Atlas body coil).
Подготовка пациенток перед исследованием:
Для уменьшения перистальтики кишечника пациентка соблюдала бесшлаковую диету в течение 2 дней, 2–3 часа голодания перед исследованием, использовали антиперистальтический препарат (Hyoscini butylbromidum – «Бускопан»).
Пациентке было рекомендовано опорожнить мочевой пузырь за 1 час до исследования, далее не мочиться.
Далее инструктировали в отношении правильной техники дыхания преимущественно грудной клеткой с целью снижения количества артефактов от движения передней брюшной стенки. На область малого таза накладывали широкий пояс толщиной
25–30 см – для фиксации передней брюшной стенки с целью снижения артефактов от движения пациентки. Перед исследованием проводили катетеризацию вены с последующей установкой катетера и подсоединением инжектора.

Для первичной оценки анатомических соотношений органов малого таза выполняли Т2-взвешенные последовательности без подавления сигнала от жира, в сагиттальной плоскости между головками бедренных костей, в аксиальной плоскости от ворот почек до лобкового симфиза, при необходимости в корональной плоскости и с использованием подавления сигнала от жировой ткани (FS) в третьей плоскости, для оценки наличия крови/жирового компонента опухоли – Т1-В.И. Дополнительно для опухолей с высокой интенсивностью сигнала (ИС) на Т1-В.И., Т1-В.И. GE с подавлением сигнала от жира (FatSat) – для дифференциальной диагностики жирового компонента, муцинозного компонента, реже меланина в других опухолях. На серии изображений в сагиттальной плоскости выделяли срез с наилучшей визуализацией образования, который является основным ориентиром для определения выделения области использования функциональных последовательностей.
Диффузионно-взвешенные изображения в аксиальной плоскости с толщиной срезов, соответствующей аксиальным Т2-В.И. Для повышения воспроизводимости (снижение влияния других параметров) в автоматическом режиме определяли измеряемый коэффициент диффузии (ИКД) – количественный параметр диффузии, не зависящий от времен спин-спиновой и спин-решетчатой релаксации.
С помощью автоматического инжектора внутривенно, болюсно вводили контрастное вещество из расчета 0,1 ммоль/кг со скоростью 2 мл/сек (20 мл физиологического раствора) и выполняли постконтрастные серии с аналогичными техническими параметрами – Dynamic 3D FatSat (Phase encode Spider 2.0) последовательность в аксиальной плоскости с толщиной среза 3 мм (межсрезовый интервал 0,3 мм), с высокой разрешающей способностью. Общее время комплексной МРТ малого таза и диффузионно-взвешенной МРТ брюшной полости составляло в среднем 31 мин (22–35 мин).
Затем с использованием специализированной программы Mirian XP-female pelvis (Instrasence, Франция) оценивали изменение МР-сигнала в выбранных областях во всех сериях динамического исследования в виде кривых «интенсивность сигнала – время», в которых за точку отсчета принимали интенсивность сигнала от зоны интереса до введения контрастного препарата.
Оценка распространенности злокачественных новообразований яичников осуществлялась в соответствии с МР-классификацией, разработанной на основе системы стадирования FIGO (2013).
Результаты исследования
Структура выявленных патологий: истинные опухоли яичника – 71%, эндометриомы – 16%, кисты – 11%, тубовариальные абсцессы – 2%. Среди истинных опухолей по гистологическому типу наибольшую группу составили серозные эпителиальные опухоли (51%), муцинозные эпителиальные опухоли (26%), эндометриоидные (2%), дермоидные кисты (6%), светлоклеточные карциномы (2%), гранулезоклеточные опухоли (6%), фибромы (4%), опухоли Бреннера (1%), метастатические опухоли (3%).
Распределение выявленных опухолей по степени злокачественности: доброкачественные – 49%, пограничные – 12% – (встречались только в группе эпителиальных опухолей), злокачественные – 39%. При стадировании пограничных опухолей яичника согласно FIGO 66% были классифицированы как IA стадия, 34% – IC. Среди злокачественных опухолей: 7,3% – IА, 17% – IIA, 12,2% – IIB, 17% – IIC, 21,9% – IIIB, 14,6% – IIIC, 9,7% – IV.
Согласно результатам исследования, наиболее информативными критериями злокачественности при оценке нативных изображений были следующие:
- Первичные: размер образования (более 4 см) в сочетании со сложной структурой, наличие двух и более камер, септы – утолщенные (более 3 мм), наличие вегетаций, наличие зон некроза.
- Вторичные: наличие имплантов (перитонеальных, мезентериальных, сальниковых), инвазия передней брюшной стенки, поражение лимфатических узлов, асцит.
Количественная оценка параметров перфузионных изображений показала, что амплитуда накопления контрастного препарата была достоверно выше у злокачественных опухолей – 167% (115,2–212,5%), чем у доброкачественных – 61,2% (41,2–99,0%) (Р<0,001) и пограничных опухолей – 85,7% (58,3–138,2%) (P<0,01); период полуподъема ИС достоверно больше у доброкачественных опухолей – 35,1 сек (30,8–42,5 сек), чем у пограничных – 27,9 сек (23,5–29,8 сек) (P<0,05), и у злокачественных – 23,1 сек (20,5–30,9 сек) (Р=0,01). Максимальная кривизна (изгиб) кривой (%/сек) составила 1,78 (1,0–2,6); 2,86 (2,01–3,95) и 6,1 (4,19–9,46) для доброкачественных, пограничных и злокачественных опухолей соответственно, и была достоверно выше у злокачественных инвазивных новообразований (P<0,01).
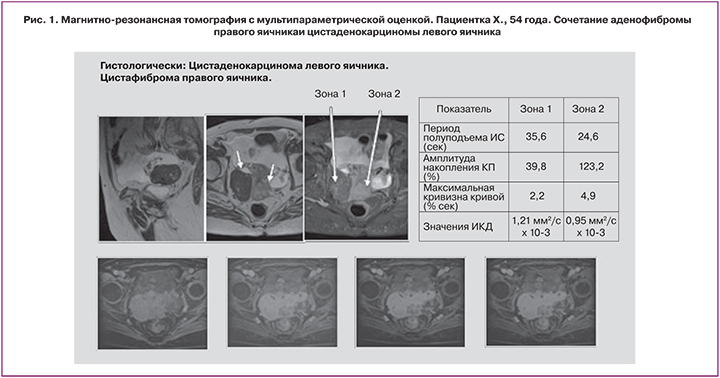
Пороговые значения параметров количественной оценки перфузионных кривых составляют: период полуподьема ИС – менее 29,7 сек, амплитуда накопления контрастного препарата – более 116%, максимальная кривизна кривой – более 4,6% (рис. 1).
Средние значения ИКД злокачественных образований были достоверно ниже соответствующих значений у доброкачественных (1,012±0,18 мм2/с × 10-3 и 1,54±0,25 мм2/с × 10-3 соответственно), интервалы значений не пересекались. Пороговые значение ИКД для злокачественных опухолей яичника: менее 1,139 мм2/с × 10-3 (рис. 2).
Показатели информативности при применении усовершенствованной методики МРТ составили: точность 92,1%, чувствительность 93,6% и специфичность 91,2%.
Выводы
- Использование МРТ с количественной оценкой параметров перфузионных кривых и ИКД позволяет с высокой точностью дифференцировать доброкачественные и злокачественные опухоли яичников (чувствительность – 93,6%, специфичность – 91,2%, точность – 92,1%).
- Пороговые значения параметров количественной оценки перфузионных кривых составляют: период полуподьема ИС – менее 29,7 сек, амплитуда накопления контрастного препарата – более 116%, максимальная кривизна кривой – более 4,6%. Пороговые значение ИКД для злокачественных опухолей яичника составляют 1,139 мм2/с × 10-3.
- Включение магнитно-резонансного исследования с количественной оценкой перфузионных параметров и диффузионно-взвешенных изображений в комплексный алгоритм обследования позволяет c высокой достоверностью дифференцировать степень злокачественности опухолей яичника, определяя возможности оптимизации тактики ведения больных.



