Злокачественные новообразования являются одной из ведущих причин заболеваемости и смертности во всем мире – в 2012 году было зарегистрировано около 14 млн новых случаев заболевания и 8,2 млн случаев смерти, связанных с раком [1]. Ожидается, что за ближайшие 20 лет заболеваемость раком возрастет примерно на 70% [1].
В Российской Федерации рак шейки матки (РШМ) занимает 5-е место после рака молочной железы, кишечника, тела матки, желудка и составляет 15 342 случаев. Таким образом, РШМ занимает 3-е место по частоте среди онкологических заболеваний органов репродуктивной системы у женщин, уступая лишь раку молочной железы и раку тела матки [1].
Известно, что вирус папилломы человека (ВПЧ) играет ключевую роль в развитии РШМ. В 2008 году немецкий медик и ученый Харальдцур Хаузен был удостоен Нобелевской премии в области физиологии и медицины за изучение ВПЧ и его роли в патогенезе РШМ.
Процесс канцерогенеза, начиная с клеточных изменений, вызванных ВПЧ-инфекцией, и заканчивая РШМ, может занимать от 10 до 40 лет, но в редких случаях РШМ может развиться и за 1–2 года [2]. Поэтому представляет интерес ранняя диагностика как РШМ, так и предраковых состояний – цервикальных интраэпителиальных неоплазий (CIN) различной степени тяжести.
В настоящее время благодаря достижениям современной медицины стала возможной диагностика заболеваний шейки матки на ранних стадиях, что позволяет врачам вести пациенток без агрессивного деструктивного воздействия на ткани, не оказывая отрицательного влияния на дальнейшую репродуктивную функцию женщины.
Современные методы диагностики CIN и РШМ
Широкое применение в выявлении раковых и предраковых заболеваний шейки матки нашли такие методы диагностики, как цитологическое исследование, расширенная кольпоскопия, молекулярно-генетические методы (генотипирование двадцати одного типа ВПЧ с определением вирусной нагрузки методом полимеразной цепной реакции в реальном времени, определение уровня экспрессии вирусных онкобелков Е6, Е7), биопсия шейки матки с гистологическим исследованием биопсийного материала, иммуноцитохимическое и иммуногистохимическое исследование (р16 и Ki67), использование опто-электронных сигналов для оценки эпителия шейки матки (аппарат TruScreen) [3–7].
Согласно рекомендациям Американской коллегии акушеров и гинекологов (ACOG) [8], скрининг РШМ следует начинать с 21 года. Для женщин в возрасте 21–29 лет предусматривается скрининг в виде мазка на онкоцитологию каждые 3 года. Женщинам 30–65 лет рекомендован скрининг в виде комбинации цитологического исследования и ВПЧ-тестирования (cotest) каждые 5 лет или в виде только цитологического исследования каждые 3 года. По мнению ACOG, жидкостная цитология и традиционные мазки с шейки матки на онкоцитологию равнозначно допустимы для проведения скрининга. Скрининг может быть прекращен после 65 лет у женщин с предшествующими негативными результатами Pap-теста и ВПЧ-теста и не имевших CIN 2 и более в анамнезе, то есть у женщин с тремя последовательными негативными результатами цитологического исследования или двумя последовательными негативными результатами цитологического исследования совместно с ВПЧ-тестированием (cotest) в течение последних 10 лет, учитывая, что с момента последнего тестирования не прошло 5 лет. Для женщин, имевших в анамнезе CIN 2,3 или рак in situ, скрининг рекомендуется в течение 20 лет после проведенного лечения, даже если возраст женщин к этому времени превысит 65 лет.
В настоящее время в клинической практике широко изучаются молекулярные маркеры пролиферации тканей. Известно, что при канцерогенезе повышается число молекулярно-генетических и метаболических повреждений эпителия шейки матки. Некоторые продукты канцерогенеза можно использовать как диагностические и прогностические маркеры опухолевой прогрессии в скрининговых программах или при разработке таргетной терапии.
Особый интерес представляют появившиеся в последние годы исследования в области протеомики, метаболомики (в том числе липидомики), геномики, транскриптомики [9]. Доказано, что злокачественная трансформация цервикального эпителия сопровождается количественными и качественными изменениями показателей уровня синтеза различных веществ, в том числе белков и низкомолекулярных соединений, в пораженных клетках [10, 11].
Доказано, что более 90% злокачественных новообразований возникает в результате повреждения структуры генома – мутаций, причем, как правило, множественных, происходящих в соматических клетках отдельных органов и тканей. Сегодня, благодаря усилиям молекулярных генетиков, известен определенный спектр генов, мутации в которых имеют прямое отношение к злокачественному перерождению клеток. «Классическими» стали онкогены, получившие название RAS, MYC, BCL [12–14].
Кроме того, имеются и антионкогены, которые препятствуют развитию рака (например, BRCA1 и BRCA2) [15].
Однако в последнее время в рамках изучения патогенеза и методов ранней диагностики рака большое внимание уделяется не только геномике и протеомике, но и метаболомике (в частности, липидомике).
Известно, что метаболические изменения в клетке играют важную роль в развитии и прогрессировании рака. Метаболизм злокачественных клеток перепрограммирован на поддержание их неконтролируемой пролиферации. Одна из важнейших особенностей метаболизма раковых клеток – повышение синтеза жирных кислот, необходимых как для самого процесса бесконтрольного деления злокачественных клеток, так и для их дальнейшего «выживания». Ученые пришли к выводу, что снижение активности основных липогенных ферментов замедляет рост опухолевых клеток и снижает их выживаемость [16].
Липидомика в онкологии
Весьма актуальны немногочисленные зарубежные исследования в области липидомики, цель которых заключается в обнаружении новых липидных маркеров злокачественных заболеваний.
Липидомика – это системный анализ липидов и взаимодействующих с ними молекул (белки, нуклеиновые кислоты, глутатион и др.). Возросший интерес к липидомике связан с накоплением данных о нарушении метаболизма липидов при различных заболеваниях: при патологии сердечно-сосудистой системы – простагландинов и эйкозаноидов [17]; при нейродегенеративных заболеваниях, шизофрении, депрессии – полиненасыщенных жирных кислот [18]; при астме – лейкотриенов [19].
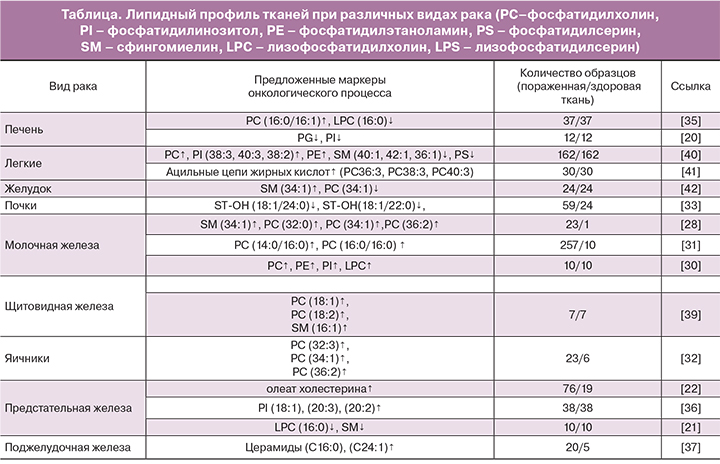
На липидомику возлагаются большие надежды с позиций поиска новых маркеров онкологических заболеваний. Имеющиеся на сегодняшний день исследования уже продемонстрировали потенциальную перспективу липидомики во многих областях медицины, в том числе в онкологии. Так, в зарубежной литературе имеется описание ряда исследований липидного профиля тканей при раке легких, щитовидной железы, молочной железы, желудка, поджелудочной железы, колоректальном раке, а также раке печени, почек, предстательной железы, яичников с помощью метода масс-спектрометрии (таблица).
Анализ липидного профиля проводят как в сыворотке крови, так и в пораженных тканях. Доказано, что синтаза жирных кислот (онкоантиген 519) повышается в сыворотке крови пациентов с некоторыми видами рака и может использоваться в качестве маркера неоплазии [16].
Метаболизм липидов при канцерогенезе
Липиды играют важную роль в клеточном метаболизме. Жирные кислоты (в свободной форме или в форме триглицеридов) являются источником энергии для метаболических процессов, а холестерол и фосфолипиды – важнейшими компонентами клеточных мембран. Кроме того, холестерол является предшественником витамина D и стероидных гормонов. В настоящее время особый интерес представляет исследование метаболизма липидов при канцерогенезе, а именно в малигнизированных тканях.
Доказано, что липолиз играет ключевую роль в патогенезе гепатоцеллюлярной карциномы, которая характеризуется снижением синтеза фосфатидилглицеролов и фосфатидилинозитолов [20]. Аналогичной точки зрения придерживаются и T. Goto с соавт. По данным их исследования, проведенного на 31 образце тканей рака предстательной железы методом масс-спектрометрии, в раковых тканях отмечается снижение уровней лизофосфатидилхолинов (16:0/OH)+H](+), (16:0/OH)+Na (+), (16:0/OH)+K](+), (16:0/OH)+matrix+H](+) и сфингомиелина (d18:1/16:0)+H](+). Таким образом, снижение уровня лизофосфатидилхолинов (16:0/OH) в раковых тканях предстательной железы является значимым предиктором возникновения биохимического рецидива после проведенной радикальной простатэктомии [21].
Однако группа T.C. Burch доказала обратное – по результатам их липидомного исследования, именно липогенез способствует раковой трансформации клетки и характеризует агрессивный рост метастатических клеток. Так, содержание фосфатидилхолинов, фосфатидилэтаноламинов, глицерофосфоинозитолов оказалось значительно повышенным в раковых клетках предстательной железы. Транскриптомный и биохимический анализы основных ферментов, задействованных в липидном метаболизме, продемонстрировали существенно высокий уровень холинкиназы-α в раковых клетках по сравнению с нормальными клетками [22]. По мнению J. Li и соавт., липогенез является одним из важнейших метаболических изменений в клетке, встречающихся при канцерогенезе. Липогенез способствует качественному и количественному ремоделированию фосфолипидов в раковых клетках. Так, в ходе проведенного исследования было выявлено накопление холестериновых эфиров, особенно олеата холестерина, в раковых тканях предстательной железы, по сравнению со здоровыми тканями простаты. Повышенный уровень олеата холестерина в пораженных тканях предстательной железы был связан с прогрессированием и метастазированием злокачественного процесса [23].
Отто Генрих Варбург – немецкий биохимик, доктор и физиолог, один из выдающихся ученых XX века в области цитологии, лауреат Нобелевской премии (1931 г.) – впервые предположил, что происхождение рака связано с необратимым повреждением клеточного дыхания в митохондриях, однако структурная основа такого повреждения оставалась неясной. Так как кардиолипин является основным фосфолипидом внутренней мембраны митохондрий и необходим для осуществления их функции, была предложена идея, что именно аномалии в структуре кардиолипина могут негативно сказываться на функции митохондрий и биоэнергетике [24]. Так, исследование, которое проводилось на опухолях мозга мышей, показало, что основные аномалии во всех опухолях связаны именно со структурой кардиолипина или его содержанием [25].
О наличии связи между митохондриальной дисфункцией и возникновением рака задумываются и другие исследователи. Так, S. Wang с соавт. с помощью масс-спектрометрической визуализации изучали липидомный профиль 4 линий раковых клеток молочной железы – 2 менее агрессивных линий и 2 агрессивных линий клеток (характеризующихся более злокачественным течением, быстрой инвазией). В линиях раковых клеток с более злокачественным течением были выявлены повышенные уровни фосфатидилглицерола и фосфатидных кислот и сниженный уровень сфингомиелина. Авторы делают предположение, что синтезированные de novo продукты, встраиваясь в состав фосфолипидов мембран (например, фосфатидилглицерол, содержащий олеиновую кислоту), могут участвовать в митохондриальной дисфункции и таким образом влиять на агрессивность инвазии раковых клеток молочной железы. Снижение уровня сфингомиелина в агрессивных раковых клетках авторы связывают c нарушениями процессов апоптоза в этих клетках [26].
Ученые с помощью MALDI-MS проанализировали 22 образца тканей молочной железы с HER2-позитивным метастатическим раком и здоровые ткани молочной железы в сравнительном аспекте. Результаты показали повышение уровней cфингомиелина 34:1, фосфатидилхолина 32:0, фосфатидилхолина 34:1 и фосфатидилхолина 36:2 в раковых тканях. Кроме того, были обнаружены различия липидного профиля в тканях опухолей с повышенным и пониженным уровнями экспрессии маркера Ki-67 [27].
M.L. Dória и соавт. также занимались исследованием липидома тканей молочной железы, пораженной раком. Научный интерес представляли не только различия липидома здоровой и раковой ткани, но и изменение липидного профиля в зависимости от степени агрессивности раковых клеток. Так, результаты исследования показали снижение уровня фосфатидилхолина и повышение уровня лизофосфатидилхолинов в наиболее агрессивных раковых клетках, в то время как уровень фосфатидилсерина оставался неизменным. Фосфатидилинозитолы, которые являются сигнальными молекулами и оказывают влияние на пролиферацию, миграцию и выживание клетки, характеризовались увеличением длины цепей насыщенных жирных кислот и снижением С20 жирных кислот в раковых тканях [28].
Результаты исследования E. Cífková и соавт. продемонстрировали повышение концентрации фосфатидилинозитолов, фосфатидилэтаноламинов, лизофосфатидилхолинов более чем в 4 раза, а фосфатидилхолинов более чем в 2 раза в раковых тканях молочной железы по сравнению со здоровыми тканями. Фосфатидилэтаноламины характеризовались снижением уровней эфира и плазмалогена, чего не наблюдалось при анализе фосфатидилхолинов [29].
Исследование липидома 267 образцов раковых и здоровых тканей молочной железы, проведенное группой ученых методом HPLC-MS (высокоэффективной жидкостной хроматографии с масс-спектрометрией), показало повышение уровня пальмитат-содержащих фосфатидилхолинов в раковых тканях. Эти липиды были связаны с прогрессированием заболевания и уровнем выживаемости пациентов, так как наибольшая их концентрация определялась в эстроген-независимом раке и раке молочной железы III стадии [30].
Группе проф. S. Kang удалось зафиксировать аналогичные изменения липидного профиля тканей яичников, пораженных раком. 23 образца раковых тканей яичника и 6 здоровых образцов были исследованы с помощью MALDI-TOF-MS. Раковые ткани характеризовались повышенным уровнем фосфатидилхолинов {32:3} [M+Na]+(m/z=750.66), {34:1} [M+K]+(m/z=798.60), и {36:2} [M+K]+(m/z=824.56) [31].
I.C. Kim с соавт. проводили исследование липидного профиля 59 образцов раковых тканей почки и 24 образцов здоровой ткани в сравнительном аспекте методом MALDI-MS. Раковые ткани отличались значительным снижением уровней C24-OH сульфатида (ST-OH {18:1/24:0}[M-H]-; m/z 906.7) и C22-OH сульфатида (ST-OH {18:1/22:0}[M-H]-; m/z 878.6). Кроме того, было обнаружено, что различные виды рака почки (светлоклеточный, папиллярный, хромофобный) отличались по липидомному составу тканей [32].
Целью исследования группы проф. Y.S. Park было выявить маркеры, с помощью которых стало бы возможным разграничение не только раковых тканей и здоровых, но и разграничение между собой различных видов рака, в том числе метастатического. Так, были продемонстрированы значительные отличия липидома тканей с внутрипеченочной холангиокарциномой и здоровой ткани печени. Липидомы тканей внутрипеченочной холангиокарциномы и тканей печени, пораженных метастатическим колоректальным раком, также существенно отличались. Однако были обнаружены сходства в липидных профилях тканей внутрипеченочной холангиокарциномы и тканей печени, пораженных метастатическим раком поджелудочной железы [33].
Группой ученых было проведено масс-спектрометрическое исследование 37 образцов тканей с гепатоцеллюлярной карциномой и прилегающей здоровой тканью. Результаты показали повышение уровня фосфатидилхолина с пальмитолеиновой или олеиновой кислотами в sn-2-положении и снижение уровня лизофосфатидилхолина c пальмитиновой кислотой в sn-1-положении в тканях гепатоцеллюлярной карциномы. Кроме того, было обнаружено, что повышенная экспрессия фермента LPCAT (лизофосфатидилхолинацилтрансфераза) приводит к качественной перестройке фосфолипидных мембран раковых клеток, тем самым создавая благоприятные условия для их дальнейшей пролиферации, миграции и выживания. Таким образом, LPCAT может являться потенциальной мишенью в таргетной терапии гепатоцеллюлярной карциномы [34].
В работе других ученых основное внимание уделялось раку предстательной железы и поиску новых онкомаркеров этого заболевания с помощью метода HR-MALDI-IMS. В 14 исследованных образцах раковых тканей предстательной железы после радикальной простатэктомии был повышен уровень фосфатидилинозитолов (18:0/18:1), (18:0/20:3) и (18:0/20:2), по сравнению с нормальным эпителием [35].
В результате исследования липидома раковых тканей поджелудочной железы, проведенного под руководством Y. Jiang, было обнаружено, что у пациентов с раком поджелудочной железы на стадии метастатического поражения лимфоузлов был повышен уровень церамидов (C16:0 и C24:1), по сравнению с пациентами с раком поджелудочной железы без метастатического поражения лимфоузлов и пациентами с панкреатитом. Также у пациентов, больных раком поджелудочной железы с метастатическим поражением лимфоузлов, в сыворотке крови отмечалось повышение метаболитов церамидов (сфингозин- и сфингагин-1 фосфат, цереброзид) [36].
Цель исследования J. Ryu и соавт. заключалась в поиске биомаркеров, с помощью которых возможно бы было выявлять злокачественные изменения в тканях и, соответственно, разграничивать эти ткани со здоровыми. Методом MALDI-MS были проанализированы раковые ткани щитовидной железы. Результаты исследования продемонстрировали повышение уровней фосфатидилхолинов 32:0 и 34:1, сфингомиелина 34:1 и нескольких фосфатидилинозитолов, а также снижение уровней лизофосфатидилхолина 18:3 и лизофосфатидилсерина 18:1 в раковых тканях щитовидной железы по сравнению со здоровыми тканями [37].
S. Ishikawa с соавт. также изучали липидный профиль тканей при раке щитовидной железы методом масс-спектрометрии. В результате исследования 7 образцов раковых тканей было выявлено повышение уровней фосфатидилхолинов (16:0/18:1) и (16:0/18:2) и сфингомиелина (d18:0/16:1) в раковых тканях щитовидной железы по сравнению со здоровыми [38].
Результаты исследования под руководством проф. E. Marien показали, что в раковых тканях легких отмечалось значительное снижение уровней сфингомиелина и фосфатидилсерина и повышение уровней фосфатидилинозитола, фосфатидилэтаноламина и фосфатидилхолина [39]. Фосфолипиды раковых тканей характеризовались удлинением ацильных цепей жирных кислот (более 36 атомов углерода). В ходе исследования был выявлен фермент, отвечающий за элонгацию ацильных цепей – ELOVL6. Ожидается, что полученные результаты в дальнейшем будут использованы при разработке таргетной терапии рака легких [40].
Группой S.Y. Kwon в 2015 году было продемонстрировано значительное повышение уровня сфингомиелина и снижение уровня фосфатидилхолина в раковых тканях желудка [41].
Ассоциация вирусной персистенции и злокачественной трансформации
Известно, что цирроз и первичные злокачественные новообразования печени в большинстве случаев являются следствием длительной персистенции вирусной инфекции – вирусного гепатита В или С [42]. Хронические вирусные гепатиты протекают длительно, постоянно оказывая повреждающее действие на ткани печени. В результате этого происходит трансформация клеток печени с формированием цирроза. При этом многократно повышается риск развития рака печени. Многие исследователи считают цепочку «хронический гепатит В, С – вирусный цирроз печени – рак печени» последовательными ступенями одного процесса, перетекающими один в другой [43].
Результаты проведенного липидомного анализа сывороток крови пациентов с гепатитами, циррозами и раком печени показали, что пациенты с циррозом и раком (то есть с более тяжелыми поражениями эпителия) имели схожие между собой липиды, отличающиеся от липидов, выявленных у пациентов с гепатитами (глицерофосфохолины, глицерофосфосерины, глицерофосфоинозитолы) [44, 45]. Таким образом, можно предположить, что вирус-ассоциированные заболевания имеют схожий патогенез, и, возможно, сыворотки крови таких пациентов имеют схожий липидный состав в зависимости от степени поражения эпителия. Ожидается, что липидомный анализ пораженных тканей шейки матки может являться прогностически значимым методом диагностики ВПЧ-ассоциированных заболеваний шейки матки.
При анализе отечественных и зарубежных литературных источников оказалось, что данные об изменении липидного профиля тканей шейки матки при развитии неоплазий отсутствуют.
Заключение
Липиды играют одну из ключевых ролей в процессе канцерогенеза. Доказано, что именно липидный метаболизм клетки одним из первых претерпевает изменения в ходе трансформации здоровой клетки в раковую. В связи с вышеперечисленным, исследование диагностического и прогностического потенциала липидома тканей при развитии неопластических процессов шейки матки представляется новым, актуальным и перспективным.
Липидомный анализ тканей шейки матки при ВПЧ-ассоциированных заболеваниях поможет в дальнейшем раскрыть патогенетические механизмы трансформации эпителия шейки матки в CIN и РШМ, а также открывает перспективы для разработки таргетной терапии РШМ.



