Middle cerebral artery blood flow in severe fetal hemolytic disease and multiple intrauterine blood transfusions
Aim. To estimate the diagnostic performance of fetal middle cerebral artery peak systolic velocity (MCA-PSV) and ∆MCA>1.5 multiples of the median (MoM) to define indications for multiple intrauterine blood transfusions (IUT) in fetal hemolytic disease (FHD).Konoplyannikov A.G., Sichinava L.G., Panina O.B., Smirnova A.A., Naydenova I.E., Latyshkevich O.A.
Materials and methods. The study included 99 patients with moderate FHD (n = 4), FHD with severe neonatal jaundice (n = 74) and edematous FHD (n = 21). Forty, 28, and 31 patients received 3, 4, and 5-7 IUTs, respectively. The sensitivity of ∆MCA>1.5 MoM before IUT was estimated.
Results. Measuring ∆MCA>1.5 MoM allows the assessment of anemia severity in severe FHD. Blood transfusion increases hemoglobin and hematocrit levels resulting in a reduction of MCA-PSV. But 7-20 days after IUT, the hemoglobin and hematocrit levels decrease again due to hemolysis, and MCA-PSV increases, which requires subsequent IUTs. The sensitivity of determining ∆MCA> 1.5 MoM before the 2nd IUT was 90.9%. Before 3d-6th IUT, the sensitivity of sensitivity of measuring ∆MCA>1.5 MoM decreased to 79.8%; 64.4%; 51.6% and 45.5%, respectively.
Conclusion. An increase in MCA-PSV>1.5 MoM can be used as an indication for the first two IUTs. Indications for the third and subsequent IUTs should be based on calculating the expected rate of hematocrit reduction.
Keywords
In some countries, fetal hemolytic disease (FHD) is still a common cause of the fetus and newborn's morbidity and mortality. Therefore, this condition's diagnosis and treatment continue to be a relevant topic today [1–4].
Ultrasound is widely used to diagnose FHD [5–8]. Ultrasoundfindingsof FHDincludehepatosplenomegaly, placentomegaly, and polyhydramnios and signs of an edematousformofthedisease(ascites, hydropericardium, hydrothorax, and «double contour sign» of the fetal soft tissues). However, these previously recognized ultrasound markers of FHD are not specific today and are not regarded as the main ultrasound criteria for FHD severity [1, 2]. The main symptom of FHD is anemia, and due to this, the middle cerebral artery peak systolic velocity (MCA-PSV) is currently widely used for antenatal diagnosis ща FHD [2, 7, 9–13].
However, the diagnostic value of non-invasive protocol for managing Rh-sensitized pregnant women receiving multiple intrauterine blood transfusions (IUT) has not yet been studied.
The present study aimed to estimate the diagnostic performance of fetal middle cerebral artery peak systolic velocity (MCA-PSV) and ΔMCA>1.5 multiples of the median (MoM) to define indications for multiple intrauterine blood transfusions in fetal hemolytic disease.
Materials and methods
The study was conducted at the Center for Family Planning and Reproduction of the Moscow Department of Healthcare. It included 99 Rh-sensitized pregnant women, who were managed at the Center from 2014 to 2018 and received multiple IUTs between 19 and 33 weeks of gestation.
The inclusion criteria for the study patient selection were multiple intrauterine blood transfusions (three or more) in Rh-sensitized pregnant women. The exclusion criteria were the absence of FHD in Rh-sensitized pregnant women, mild FHD not requiring IUT, one or two IUTs in Rh-sensitized pregnant women, late visit of a patient with severe FHD to the Center for Family Planning and Reproduction (after 30 weeks of gestation).
The severity of anemia and the indications for cordocentesis and IUT were assessed by measuring MCA-PSV in all 99 Rh-sensitized pregnant women, followed by zonal assessment using the nomogram developed by G. Mari [14] and adopted in our clinic [15]. MCA-PSV was measured in the proximal fetal MCA 1–2 mm above its stemming from Willis's circle with an angle of insonation of 0°. The MCA-PSV was measured before and after each IUT and was expressed in cm/s; the highest value of 3 successive satisfactory quality measurements was recorded.
Identification of the MCA-PSV corresponding to zone «A» and indicating a high probability of developing severe fetal anemia and the presence of ultrasound signs of FHD and a complicated obstetric history served as an indication for cordocentesis.
Ultrasound-guided cordocentesis was performed to obtain a fetal blood sample to test for fetal blood group and the Rh factor, hemoglobin, and hematocrit using an automatic analyzer in an operating room setting.
The indication for IUT was hematocrit reduction in the fetal umbilical cord blood below the gestational norm by 15% at 19 to 33 weeks of pregnancy. The IUT blood volume was calculated individually using the J.M. Bowman’s formula [16] based on the estimated fetal weight, fetal and donor hematocrit, and placental blood volume corresponding to a given gestational age. Contraindications to IUT were threatened miscarriage and severe maternal blood coagulation disorders.
A total of 399 IUTs were performed in 99 Rh-sensitized pregnant women. Indications for repeating IUTs were the rate of daily decline of fetal hemoglobin at 20–24 weeks equal to 1.6–2.5%/day, 25–27 weeks – 1.2–1.5%/ day, 28–30 weeks – 0.8–1.1%/day, 31–32 weeks – 0.6–1.0%/day; the duration of the previous transfusion is 2-3 weeks; MCA-PSV values in zone A; gestation age below 33 weeks.
The daily decline of fetal hemoglobin in umbilical cord blood was considered the main indication for repeat IUTs (gold standard). It was calculated as the ratio of the difference between the hematocrit level before the next and after the previous IUT to the number of days between treatments. Out of 99 patients, 40(40.4%), 28 (28.3%), 20 (20.2%), 10 (10.1%), and 1 (1%) patients received 3, 4, 5, 6, and 7 IUTs, respectively.
Despite the ongoing therapy, there was 4 (4%) antenatal fetal death. FHD with moderate neonatal jaundice (hemoglobin level 140–100 g/l) was observed in 4 out of 95 (4.2%) surviving newborns (gestational age at delivery 32.9 (1.7) weeks). FHD with severe neonatal jaundice (hemoglobin level <100 g/l) was observed in 72 (75.8%) cases (gestational age at delivery 33.3 (1.9) weeks). Edematous form of HD (hemoglobin level <100 g/l, presence of edematous syndrome) was noted in 19 (20%) newborns (gestational age at delivery 30.6 (2.5) weeks).
Statistical analysis
Statistical analysis was performed using the IBM SPSS Statistics 23 software. The distribution of continuous variables was tested for normality using the generalized D'Agostino–Pearson test. Quantitative variables showing normal distribution were expressed as means (standard deviation) and compared by t-test. Qualitative variables were summarized as counts and percentages. Since the study compared paired samples, the t-test for paired samples was used. Differences were considered statistically significant at p<0.05. The relation between fetal MCA blood flow and parameters such as fetal weight, gestational age at the time of IUT, hematocrit, and hemoglobin levels was assessed with a Spearman correlation coefficient (rs).
To estimate the diagnostic performance of the Doppler ultrasound for the diagnosis of fetal anemia, before each IUT, the deviation of MCA-PSV (ΔMCA) from the threshold velocity value corresponding to 1.5 Multiple of Median (MoM) was determined using the nomogram developed by G. Mari [14] and adopted in our clinic [15]. All 99 enrolled pregnant women with severe FHD had MCA-PSV values in zone A, and therefore all underwent the first IUT. Since the study did not include Rh-sensitized pregnant women without FHD and patients with FHD who did not undergo multiple IUTs, before the second and subsequent blood transfusions, only the sensitivity of the Doppler study ΔMCA from 1.5 MoM was calculated as the ratio of the number of fetuses with severe anemia, identified according to the method, to the number of all fetuses that underwent Doppler measurements. A sensitivity of at least 90% was considered high.
Results
The results of measuring fetal middle cerebral artery peak systolic velocity showed that before the first intrauterine blood transfusion, the MCA-PSV values in all pregnant women were in zone A, which corresponded to severe anemia. However, we did not find statistically significant differences in MCA-PSV in fetuses with neonatal jaundice and edematous HD (p=0.78). Also, there were no statistically significant correlations between MCA-PSV and hematocrit (rs=0.13, n=99, p=0.65) and hemoglobin (rs=0.12, n=99, p=0.68). At the same time, a direct correlation was found between the MCA-PSV values before the first IUT and gestational age at the time of the IUT (rs=0.66, n=99, p=0.02) and fetal weight (rs=0.67, n=99, p=0.03).
Using the table by G. Mari [14], we additionally calculated the value of ΔMCA-PSV from 1.5 MoM to the first IUT and revealed an inverse relationship between the value of ΔMCA from 1.5 MoM and hematocrit (rs = -0.67, n=99, p=0.02) and hemoglobin (rs= -0.64, n=99, p=0.02) levels before the first IUT, which undoubtedly confirms the informative value of MCA-PSV in fetal anemia. We also found that the value of ΔMCA from 1.5 MoM to the first IUT in moderate neonatal jaundice was statistically significantly (p = 0.02) lower (0.19 [0.18] MoM) than in severe neonatal jaundice (0.35 [0.29] MoM) and in the edematous form of FHD (0.45 [0.39] MoM). Additionally, determining the value of ΔMCA from 1.5 MoM, we excluded the influence of such factors as fetal weight and gestational age and more accurately assess the severity of anemia.
On days 4–7, after the first IUT, a repeat measurement of MCA blood flow was performed in all 99 fetuses (Table 1). In all cases, the MCA-PSV was statistically significantly (p<0.001) lower than before the IUT, which confirmed the therapeutic effect of intrauterine blood transfusion. The difference between the speeds before and after the first IUT was 16.9 (8.6) cm/s and was more significant in the edematous form of FHD (19.8 [11.7] cm/s) than in the severe course (16.1 [7.5] cm/s; p=0.15) and an intermediate course of the disease (10.5 [6.4] cm/s; p=0.04), which reflected a more pronounced effect after the first blood transfusion in severe forms of FHD. It is particularly noteworthy that all fetuses with edematous HD (n=21), along with a more significant difference between MCA-PSV before and after the first IUT, had lower hemoglobin (44.4 [21.7] g/L) and hematocrit (13.1 [6.2]%) levels before the first IUT.
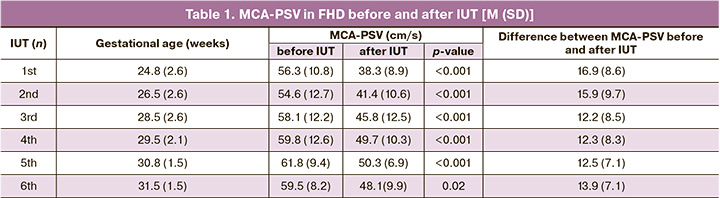
Before the second intrauterine blood transfusion, the MCA-PSV values returned to baseline and did not differ statistically significantly (p=0.34) from those before the first IUT (Table 1). This observation is probably due to the time interval between intrauterine interventions and the associated ongoing RBC hemolysis.
The ΔMCA values from 1.5 MoM before the second IUT in edematous FHD (0.34 [0.31] MoM) and severe neonatal jaundice (0.27 [0.21] MoM) were statistically significantly greater (p=0,02) than with a moderate neonatal jaundice (0.09 [0.05] MoM). The sensitivity of determining ΔMCA from 1.5 MoM before the second IUT was 90.9%.
We also calculated the daily hematocrit decline rate between the first and second IUT and found that it was higher in the edematous FHD (2.06 [1.69]%/day), compared with severe (1.67 [1.05] %/day; p=0.28) and moderate neonatal jaundice (1.38 [0.42]%/day; p=0.04). There was a correlation between the calculated daily hematocrit decline rate and the value of ΔMCA from 1.5 MoM before the second IUT (rs=0.34, n=99, p=0.03).
4–7 days after the second IUT, the MCA-PSV value, as expected, became statistically significantly lower (p<0.001) than before blood transfusion (Table 1), clearly reflecting the effect of the treatment of anemia and the high diagnostic value of this indicator.
Before the third IUT, the MCA-PSV values (Table 1) did not differ from the revealed indicators before the first (p=0.14) and second (p=0.34) IUT, which is explained by the ongoing RBC hemolysis during 7–20 days after the blood transfusion. The sensitivity of determining ΔMCA from 1.5 MoM before the third IUT was 79.8%.
Both between the first and second IUT, and between the second and third IUT, daily hematocrit decline rate was greater in the edematous FHD (1.58 [0.72]% / day), compared with a severe (1.21 [0, 49]% / day; p=0.04) and moderate (1.13 [0.53]% / day; p=0.03) neonatal jaundice.
As a result of intrauterine blood transfusions, the MCA-PSV value 5–7 days after the third IUT was lower than before the IUT (Table 1). The difference between the MCA-PSV values before and after the third IUT was 12.2 (8.5) cm/s and was lower than before and after the previous IUTs (p=0.02 and p=0.03).
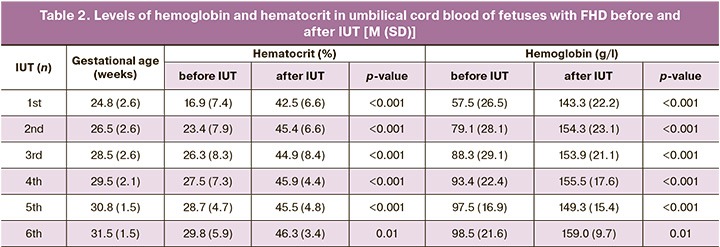
The hematocrit and hemoglobin levels immediately after the third blood transfusion (Table 2) were 44.9 (8.4)%, and 153.9 (21.1) g/L, did not differ statistically significantly from those after the second IUT (p=0.67 and p=0.78).
Forty out of 99 (40.4%) pregnant women received three IUTs and had a delivery at 23–36 weeks of gestation. Of 38 live babies, 5 (13.2%), 20 (52.6%), and 13 (34.2%), and were born at 23–30, 30–34, and 34–36 weeks, respectively. Four (10.5%) babies were born with HD and moderate jaundice, 25 (65.8%) with severe jaundice, and 9 (23.7%) with an edematous form of HD. Two fetuses (5%) died antenatally: one at 25 weeks of gestation due to chorioamnionitis, the other at 33 weeks due to acute hypoxia.
Before the fourth intrauterine blood transfusion in the remaining 59 pregnant women, the MCA-PSV value did not differ from the baseline values before the previous intrauterine transfusions (Table 1). The sensitivity of determining ΔMCA from 1.5 MoM before the fourth IUT was 64.4%. The daily decline of fetal hematocrit between the third and fourth IUT did not differ in the edematous form of FHD and severe of the disease with neonatal jaundice (p=0.67), in contrast to previous IUTs.
On days 5–7 after the fourth IUT, the MCA-PSV value was statistically significantly lower (p<0.001) than the values before intrauterine transfusion, reflecting the therapeutic effect of the IUT. The difference between the MCA-PSV values before and after the fourth IUT was 12.3 (8.3) cm/s and was lower than before and after the second (p=0.02), as well as before and after the first IUT (p=0.03). Twenty-eight out of 59 (47.5%) pregnant women received 4 IUTs and had a delivery at 26–37 weeks of gestation. Of 26 live babies, 1 (3.8%), 15 (57.7%), and 10 (38.5%) were born at 26–30, 30–34, 34–37 weeks gestation, respectively.
Twenty-two (84.6%) infants were born with HD and severe jaundice, and 4 (15.4%) with edematous HD. Two fetuses (7.1%) with edematous HD died antenatally due to the disease severity at 26 and 28 weeks of gestation.
Before the fifth and sixth intrauterine blood transfusions, the absolute MCA-PSV values and the ΔMCA values from 1.5 MoM were not statistically significantly different between patients with severe jaundice and edematous FHD (p=0.56 and p=0.68). The daily decline of fetal hematocrit before the fifth and sixth IUT was the same for these forms of FHD (p=0.67 and p=0.59). The sensitivity of determining ΔMCA from 1.5 MoM before the fifth IUT was 51.6%, before the sixth – 45.5%. On days 5–7 after the fifth and sixth IUT, the MCA-PSV value in all patients was statistically significantly lower than before the transfusion (Table 1).
Twenty and ten pregnant women received 5 and 6 IUTs, respectively. All of them had a delivery at 31–36 weeks of gestation. Twenty and 10 living infants were born, respectively: within 31–34 weeks – 12 (60%) and 6 (60%); 34–36 weeks – 8 (40%) and 4 (40%). A severe HD jaundice was detected in 17 (85%) and 7 (70%) newborns, the edematous HD was found in 3 (15%) and 3 (3%). There were no antenatal losses in patients after five and six IUTs.
One 34-year-old Rh-sensitized pregnant women with complicated obstetric history (intrapartum fetal death, two postnatal deaths of a newborn), who underwent seven IUTs, deserves special attention. When a pregnant woman was admitted to the hospital at 22 weeks of gestation, an edematous FHD (ascites, hydropericardium) was diagnosed, paracentesis was performed (with effect). MCA-PSV values were highest before the first and second blood transfusions (54 and 78 cm/s). After that, pre-IUT MCA-PSV before each subsequent IUT decreased and remained relatively stable throughout the IUT. At the same time, the hemoglobin level gradually increased from 37 g/L (before the first IUT) to 85 and 126 g/L (before and after the seventh IUT), which confirms the pronounced therapeutic effect of multiple blood transfusions. The patient had a delivery at 32 weeks gestation. The newborn was diagnosed with HD (hemoglobin level – 98 g/l, hematocrit – 28.9%) and underwent one exchange blood transfusion. On the 13th day, he was transferred to the second stage of nursing.
Discussion
Currently, most authors support the diagnostic value of MCA-PSV in FHD for the diagnosis of fetal anemia. An increase in MCA-PSV >1.5 MoM is diagnostic for severe anemia and suggests the need for active intervention [8, 9, 11, 13, 17].
Given the increasing diagnostic role of fetal MCA-PSV in the diagnosis of anemia in HD, many clinicians evaluated the effectiveness of intrauterine treatment by changing this indicator [7, 12, 17, 18]. As well as the authors listed above, we performed repeated MCA-PSV studies to evaluate the efficacy of multiple IUTs after 4–7 days.
The difference between the MCA-PSV values before and after the first two blood transfusions was the most pronounced, as evidenced by an increase in hemoglobin and hematocrit levels. On subsequent blood transfusions, the difference between the MCA-PSV values before and after the IUT decreased; at the same time, no statistically significant differences in this indicator were found for different number of transfusions.
Current literature is controversial regarding the role of MCA-PSV in predicting the severity of anemia to determine the optimal timing of repeat IUT. I. Babovic et al. [19] concluded that fetal MCA-PSV measurements are necessary to predict the best timing of the first and subsequent IUTs.
S. Friszer et al. [20] reported high sensitivity of MCA-PSV when performing the first, second, and third IUT (96.2%, 87.5%, and 91.3%, respectively). However, with each subsequent blood transfusion, the authors noted a significant decrease in the positive predictive value (75.3%, 46.7%, and 48.8%), which was explained by the reduction in the number of fetuses with anemia as the number of IUTs increased, as a result of effective treatment of FHD.
L. Ghesquière et al. [7] suggested that after the second IUT, the fetal IUT interval calculation should be based on the estimated fetal hemoglobin decline, not on the threshold values of MCA-PSV. According to the authors [7], after the first IUT, both methods for determining the interval between blood transfusions (MCA-PSV and daily hematocrit decline rate) are equally effective. However, in their opinion, after the second blood transfusion, the daily hematocrit decline rate had higher predictive accuracy.
Our study's findings suggest that the zonal assessment of the MCA-PSV of the fetus can be used only for the first two IUTs, the indication for which, in our opinion, is the value of this indicator corresponding to zone A (>1.5 MoM).
We found an inverse relationship between the value of ΔMCA-PSV from 1.5 MoM and hematocrit and hemoglobin levels before the first IUT. Also, we proved the high sensitivity of the Doppler method (ΔMCA from 1.5 MoM) before the second blood transfusion (90.9%), which confirms the feasibility of using this additional indicator in Rh-sensitized pregnant women to diagnose fetal anemia and choose appropriate management strategy.
However, our study revealed a decrease in the sensitivity of the Doppler before the third and subsequent fourth, fifth, and sixth IUTs (79.8%; 64.4%; 51.6% and 45.5%, respectively), which can be explained by partial fetal blood replacement with donor blood, differing in physical and chemical properties [21].
Our study's limitation was that we were unable to estimate all measures of diagnostic accuracy of the Doppler (ΔMCA from 1.5 MoM) since the study did not include Rh-sensitized pregnant women without FHD, as well as patients with FHD who did not undergo multiple IUTs. Nevertheless, the study of the Doppler method's sensitivity for diagnosing fetal anemia in our work was sufficient to conclude that the measurement of ΔMCA from 1.5 MoM cannot be considered predictive for the need for the third and subsequent IUTs.
What should be the indications for the third and subsequent IUTs? Considering the low diagnostic value of MCA-PSV in the diagnosis of anemia of fetuses subjected to repeat IUTs, some authors [20] proposed to increase the MCA-PSV threshold to 1.73 MoM, others [7, 22] to use for this purpose the calculation of the expected daily fetal hemoglobin decline rate (0.3 g/dL per day). Some authors repeated fetal IUT at a daily fetal hemoglobin decline rate of 1–2% per day [23, 24].
However, based on our many years' experience in the intrauterine treatment of FHD, we suggest that indications for the third and subsequent IUTs should be based on the expected fetal hematocrit decline rate depending on the gestational age and not on the fetal MCA-PSV measurement.
Conclusion
Antenatal diagnosis of fetal hemolytic anemia should be based on measurements of Doppler MCA-PSV using nomograms previously established for various gestational ages.
An increase in MCA-PSV>1.5 MoM is diagnostic for severe fetal anemia and can be used as an indication for the first two IUTs. Indications for the third and subsequent IUTs should be based on the estimated daily hematocrit decline rate and not on fetal MCA-PSV measurements.
References
1. Коноплянников А.Г., Павлова Н.Г. Изосерологическая несовместимость крови матери и плода. Гемолитическая болезнь плода и новорожденного. В кн.: Айламазян Э.К., Серов В.Н., Радзинский В.Е., Савельева Г.М., ред. Акушерство. Национальное руководство. M.: ГЭОТАР-Медиа; 2015: 324-34. [Konoplyannikov A.G., Pavlova N.G. Isoserological incompatibility of maternal and fetal blood. Hemolytic disease of the fetus and newborns. In: Aylamazyan E.K., Serov V.N., Radzinsky V.E., Savelyeva G.M., ed. Obstetrics: National Leadership. M: GEOTAR-Media Publ. 2015; 324-34. (in Russian)].
2. Кравченко Е.Н., Ожерельева М.А., Куклина Л.В., Кропмаер К.П., Цыганкова О.Ю. Совершенствование алгоритма ведения беременных с резус иммунизацией: диагностические аспекты. Мать и дитя в Кузбассе. 2017; 4: 43-7. [Kravchenko E.N., Ozherelyeva M.A., Kuklina L.V., Kropmaer K.P., Tsygankova O.Yu. Improving the management of pregnant women with Rh immunization: diagnostic aspects. Mat' i ditya v Kuzbasse / Mother and child in Kuzbass. 2017; 4 (71): 43-7. (in Russian)].
3. Савельева Г.М., Курцер М.А., Сичинава Л.Г., Коноплянников А.Г., Латышкевич О.А., Сонголова Е.Н. 50 лет иммунопрофилактике резусиммунизации: на страже перинатальной заболеваемости и младенческой смертности (исторический экскурс). Акушерство и гинекология. 2018; 12: 177-83. [Savelyeva G.M., Kurtser M.A., Sichinava L.G., Konoplyannikov A.G., Latyshkevich O.A., Songolova E.N. 50 years of immunization with Rh immunization: on guard of perinatal morbidity and infant mortality (historical excursus). Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2018; 12: 177-83. (in Russian)]. https://dx.doi.org/10.18565/ aig.2018.12.177-183.
4. Hassan M.Z., Iberahim S., Abdul Rahman W.S.W., Zulkafli Z., Bahar R., Ramli M. et al. Severe anti-D haemolytic disease of fetal and newborn in rhesus D negative primigravida. Malays J. Pathol. 2019; 41(1): 55-8.
5. Абдрахманова Л.Р., Токтарова О.А., Ситарская М.В., Мусина Д.М. Анализ результатов ультразвукового допплерометрического исследования кровотока в средней мозговой артерии у плода при резус-иммунизации. Практическая медицина. 2015; 4-2: 7-9. [Abdrakhmanova L.R., Toktarova O.A., Sitarskaya M.V., Mysina D.M. Analysis of the results of ultrasonic doppler study of blood flow in the middle cerebral artery in the fetus with Rh-immunization. Prakticheskaya medicina / Practical medicine. 2015; 4-2 (89): 7-9. (in Russian)].
6. Мамедалиева Н.М., Шарипбаева Н.Т., Данияров Н.Н., Джиджилава Г.М. Особенности течения беременности, родов и перинатальные исходы у пациенток с резус-сенсибилизацией. Вестник КазНМУ. 2015; 1: 18-21. [Mamedalieva N.M., Sharipbaeva N.T., Daniyarov N.N., Dzhidzhilava G.M. Features of the course of pregnancy, childbirth and perinatal outcomes in patients with Rh sensitization. Vestnik KazNMU / Bulletin of KazSMU. 2015; 1: 18-21. (in Russian)].
7. Ghesquière L., Houfflin-Debarge V., Behal H., Coulon C., Subtil D., Vaast P. et al. Should optimal timing between two intrauterine transfusions be based on estimated daily decrease of hemoglobin or on measurement of fetal middle cerebral artery peak systolic velocity? Transfusion. 2017; 57(4): 899-904. https:// dx.doi.org/10.1111/trf.13980. DOI: 10.1111/trf.13980.
8. American College of Obstetricians and Gynecologists' Committee on Practice Bulletins-Obstetrics. ACOG Practice Bulletin No. 192: Management of Alloimmunization During Pregnancy. Obstet. Gynecol. 2018; 131(3): e.82-90.
9. Dodd J.M., Andersen C., Dickinson J.E., Louise J., Deussen A., Grivell R.M. et al. Fetal middle cerebral artery Doppler to time intrauterine transfusion in red-cell alloimmunization: a randomized trial. Ultrasound Obstet. Gynecol. 2018; 51(3): 306-12. https://dx.doi.org/10.1002/uog.18807.
10. Morales Roselló J., Scarinci E., Sánchez Ajenjo C., Santolaria Baig A., Gonzalez Martínez I.M., Cañada Martinez A.J. et al. Unexpected middle cerebral artery peak systolic velocity values in the normal fetal population. Are they a matter of concern? J. Matern. Fetal Neonatal Med. 2020; 33(8): 1282-7. https:// dx.doi.org/10.1080/14767058.2018.1517322.
11. Argoti P.S., Mari G. Fetal anemia. Minerva Ginecol. 2019; 71(2): 97-112. https://dx.doi.org/10.23736/S0026-4784.18.04334-4.
12. Kennedy A.M., Woodward P.J. A radiologist's guide to the performance and interpretation of obstetric Doppler US. Radiographics. 2019; 39(3): 893-910. https://dx.doi.org/10.1148/rg.2019180152.
13. Prefumo F., Fichera A., Fratelli N., Sartori E. Fetal anemia: Diagnosis and management. Best Pract. Res. Clin. Obstet. Gynaecol. 2019; 58: 2-14. https:// dx.doi.org/10.1016/j.bpobgyn.2019.01.001.
14. Mari G., Deter R.L., Carpenter R.L., Rahman F., Zimmerman R., Moise K.J.Jr. et al. Noninvasive diagnosis by Doppler ultrasonography of fetal anemia due to maternal red-cell alloimmunization. Collaborative Group for Doppler Assessment of the Blood Velocity in Anemic Fetuses. N. Engl. J. Med. 2000; 342(1): 9-14. https://dx.doi.org/10.1056/NEJM200001063420102.
15. Клинический протокол Министерства здравоохранения РФ от 18 мая 2017 г. N 15-4/10/2-330. О направлении клинических рекомендаций (протокола) «Резус-сенсибилизация. Гемолитическая болезнь плода». Available at: http://docs.cntd.ru/document/456085287 [The clinical Protocol of the Ministry of health of the Russian Federation from 18 may 2017 N 15-4/10/2-330. About the direction of clinical recommendations (Protocol) "RH-sensitization. Hemolytic disease of the fetus" (in Russian)].
16. Bowman J.M. The management of Rh immunization. Obstet. Gynecol. 1978; 52(1): 1-16.
17. Керимова Э.А., Путилова Н.В., Кинжалова С.В., Чистякова Г.Н. Динамика показателей врожденного и адаптивного иммунитета у плодов с гемолитической болезнью, обусловленной резус-конфликтом, перенесших однократное внутриутробное внутрисосудистое переливание крови. Российский вестник акушера-гинеколога. 2018; 18(1): 15-8. [Kerimova E.A. Putilova N.V., Kinzhalova S.V., Chistyakova G.N. Dynamics of indicators of innate and adaptive immunity in fetuses with hemolytic disease due to Rhesus conflict after a single intrauterine intravascular blood transfusion. Rossiiskii vestnik akushera-ginekologa/Russian Bulletin of obstetrician-gynecologist. 2018; 18 (1): 15-8. (in Russian)]. https://doi.org/10.17116/ rosakush201818115-18.
18. Babović I., Plešinac S., Sparić R., Dotlić J., Pilić I., Nejković L., Plećaš D. Fetal hydrops and middle cerebral artery Doppler in prediction degree of fetal anemia and the best timing for therapy. Clin. Exp. Obstet. Gynecol. 2017; 44(3): 423-8.
19. Babović I., Plešinac S., Radojičić Z., Antonović O., Sparić R., Plećaš D., Radunović N. Middle cerebral artery Doppler in prediction degree of fetal anemia and the best timing for the second intrauterine intravascular transfusion in red cell alloimmune disease. Clin. Exp. Obstet. Gynecol. 2015; 42(6): 792-6.
20. Friszer S., Maisonneuve E., Macé G., Castaigne V., Cortey A., Mailloux A. et al. Determination of optimal timing of serial in-utero transfusions in red-cell alloimmunization. Ultrasound Obstet. Gynecol. 2015; 46(5): 600-5. https:// dx.doi.org/10.1002/uog.14772.
21. Welch R., Rampling M.W., Anwar A., Talbert D.G., Rodeck C.H. Changes in hemorheology with fetal intravascular transfusion. Am. J. Obstet. Gynecol. 1994; 170(3): 726-32. 10.1016/s0002-9378(94)70271-3.
22. Scheier M., Hernandez-Andrade E., Fonseca E.B., Nicolaides K.H. Prediction of severe fetal anemia in red blood cell alloimmunization after previous intrauterine transfusions. Am. J. Obstet. Gynecol. 2006; 195(6): 1550-6. https:// dx.doi.org/10.1016/j.ajog.2006.03.060.
23. Керимова Э.А., Путилова Н.В., Чистякова Г.Н., Пестряева Л.А., Устьянцева Н.Ю. Клинико-иммунологическое обоснование внутриутробных гемотрансфузий при гемолитической болезни плода по системе резус. Акушерство и гинекология. 2016; 12: 24-7. https://doi.org/10.18565/ aig.2016.12.24-7. [Kerimova E.A. Putilova N.V., Chistyakova G.N., Pestryaeva L.A., Ustyantseva N.Yu. Clinical and immunological substantiation of intrauterine blood transfusions with hemolytic disease of the fetus according to the rhesus system. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2016; 12: 24-7. (in Russian)]. https://doi.org/10.18565/aig.2016.12.24-7.
24. Макогон А.В., Андрюшина И.В. Диагностика и лечение гемолитической болезни плода. Акушерство и гинекология. 2012; 1: 43-8. [Makagon A.V., andriushina I.V. Diagnostics and treatment of fetal hemolytic disease. Akusherstvo i Ginekologiya / Obstetrics and Gynecology. 2012; 1: 43-8 (in Russian)].
Received 29.05.2020
Accepted 09.07.2020
About the Authors
Alexander G. Konoplyannikov, Dr. Med. Sci., Professor at the Department of Obstetrics and Gynecology, Pediatric faculty, N.I. Pirogov RNRMU. Tel.: +7(499)723-04-20. E-mail: npo.med@gmail.com. ORCID: 0000-0001-9923-8833. 117997, Russian Federation, Moscow, Ostrovityanova str., 1.Lali G. Sichinava, Dr. Med. Sci., Professor at the Department of Obstetrics and Gynecology, Pediatric faculty, N.I. Pirogov RNRMU. Tel.: +7(495)718-34-72. E-mail: lalisichinava@gmail.com. ORCID: 0000-0003-0820-4772. 117997, Russian Federation, Moscow, Ostrovityanova str., 1.
Olga B. Panina, Dr. Med. Sci., Professor, Head of the Department of Obstetrics and Gynecology, Faculty of Fundamental Medicine, M.V. Lomonosov Moscow State University. Tel.: +7(499)268-55-33. E-mail: olgapanina@yandex.ru. ORCID: 0000-0003-1397-6208. 119991, Russian Federation, Moscow, Leninskie gory, 1, p. 51.
Aleksandra A. Smirnova, MD, Head of the Consulting and Diagnostic Department, Centre for Family Planning and Reproduction. Tel.: +7(916)189-40-72. E-mail: salexandra2006_0@mail.ru. ORCID: 0000-0002-8852-1980. 117209, Russian Federation, Moscow, Sevastopolsky Avenue, 24A.
Irina E. Naydenova, Obstetrician-Gynaecologist at the Centre for Family Planning and Reproduction. Tel.: +7(495)718-24-78.
E-mail: 4143428@gmail.com. ORCID: 0000-0002-7366-7303. 117209, Russian Federation, Moscow, Sevastopolsky Avenue, 24A.
Оleg А. Latyshkevich, Ph.D., Teaching Assistant at the Department of Obstetrics and Gynecology, Pediatric faculty, N.I. Pirogov RNRMU;
Chief physician of the Centre for Family Planning and Reproduction. Tel.: +7(495)718-20-70. E-mail: latishkevich@mail.ru. ORCID: 0000-0002-3467-4236. 117997, Russian Federation, Moscow, Ostrovityanova str., 1; 117209, Russian Federation, Moscow, Sevastopolsky Avenue, 24A.
For citation: Konoplyannikov A.G., Sichinava L.G., Panina O.B., Smirnova A.A., Naydenova I.E., Latyshkevich O.A. Middle cerebral artery blood flow in severe fetal hemolytic disease and multiple intrauterine blood transfusions. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2020; 10: 48-54 (in Russian)
https://dx.doi.org/10.18565/aig.2020.10.48-54



