Our experience with multiple intrauterine fetal blood transfusions for severe hemolytic disease due to rhesus incompatibility
Objective: To investigate the feasibility and efficacy of multiple intrauterine blood transfusions (IUT) in severe Rh hemolytic disease of the fetus and newborn (HDFN). Materials and methods: The study comprised 99 pregnant women with Rh alloimmunization, including 4 (4%) patients with severe HDFN (group I), 74 (74.7%) with very severe HDFN (group II), and 21 (21.2%) with hydrops fetalis (group III). All patients received multiple (from 3 to 7) IUTs. Exchange transfusions were performed in 65 (68.4%) neonates on the 1st day of life, and 17 (17.9%) received regular blood transfusions on days 3–5. The perinatal outcomes were evaluated. Results: Multiple IUTs for severe HDFN in 72.7% of patients resulted in prolonging gestation to 32-36 weeks, 96% fetal survival, including 80.9% for hydrops fetalis. A hematocrit level <25th percentile indicates the adverse course of severe HDFN. Earlier intrauterine treatment for hydrops fetalis (at 19-23 weeks) and 4 to 6 IUTs performed resulted in fewer exchange transfusions (72.7%) and regular transfusions (18.2%) compared with intrauterine treatment started later (at 24-27 weeks) and only 3 IUTs performed (87.5% and 37.5%, respectively). Conclusion: IUT is the best available treatment for HDFN, allowing for improved perinatal outcomes in severe disease.Savelyeva G.M., Konoplyannikov A.G., Karaganova E.Ya., Smirnov A.A., Astrakhantseva M.M., Martynova N.G., Latyshkevich О.А.
Keywords
The problem of treating the hemolytic disease of the fetus and newborn (HDFN) due to Rh alloimmunization is still relevant, as it remains one of the causes of perinatal morbidity and mortality [1–5]. It is generally recognized that the most effective treatment for severe HDFN is intrauterine intravascular blood transfusion (IUT) to the fetus, which is now considered the most successful technology in the history of perinatal medicine [6–9].
However, domestic literature lacks studies investigating multiple IUT – the best available treatment for severe HDFN [10–12]. Also, to date, the results of intrauterine treatment with multiple IUT have not been compared with the features of HDFN therapy after delivery (frequency and time of initiation of exchange transfusions).
Therefore, this study aimed to investigate the feasibility and efficacy of multiple intrauterine blood transfusions in severe Rh hemolytic disease of the fetus and newborn.
Materials and methods
The present study was conducted at the tertiary care (level III) perinatal center of the Center for Family Planning and Reproduction, Moscow Healthcare Department. The study aimed to analyze the course of pregnancy and labor in 99 patients with Rh alloimmunization and severe HDFN.
The criterion for inclusion in the study was multiple IUTs (3 or more) in patients with rhesus alloimmunization in the current pregnancy. The exclusion criteria were the absence of HDFN in pregnant women with rhesus alloimmunization, mild course of HDFN not requiring IUTs, 1 or 2 IUTs in pregnant women with Rh alloimmunization, late presentation of a patient with HDFN (after 30 weeks of gestation).
Obstetric history was complicated in 56/99 (56.6%) subjects. Thirty (54.5%) patients had a history of 1 to 5 antenatal fetal deaths at 26–36 weeks of gestation from severe HDFN. In 26/55 (47.3%) of those observed, babies were born with HDFN of varying severity.
All pregnant women with Rh alloimmunization on admission and in the dynamics underwent ultrasound examination. Placenta thickening more than 0.2 cm for gestational age, an increase in liver and abdominal size by two weeks or more, and the appearance of HDFN signs such as ascites, hydrothorax, hydropericardium, and soft tissue double contours were considered to be ultrasound signs of HDFN. The amount of amniotic fluid was additionally determined by ultrasound.
Assessment of fetal condition included the peak velocity of systolic blood flow in the middle cerebral artery (MCA-PSV) with a subsequent zonal estimation of its parameters according to G. Mari [13]. If MCA-PSV was above 1.5 MoM for gestational age, indicating severe fetal anemia, cordocentesis was performed to determine the hematocrit level.
Our study determined the severity of HDFN in patients with Rh conflict both prospectively and retrospectively, taking into account the evaluation of antibody titers, ultrasound, and fetal MCA-PSV values before intrauterine treatment. We conventionally identified three degrees of severity of HDFN, which formed the basis for the distribution of patients into groups. Group I consisted of 4 (4%) patients with a severe HDFN; Group II consisted of 74 (74.7%) pregnant women with a very severe course of the disease; Group III consisted of 21 (21.2%) patients who had HDFN with fetal hydrops.
The indication for the 1st and 2nd IUT was a decrease in fetal hematocrit in umbilical cord blood below the gestational norm by 15%. Indications for the 3rd and subsequent IUTs in our study were based on the calculation of the estimated rate of hematocrit decrease depending on gestational age: at 20–24 weeks – 1.6–2.5% per day, at 25–27 weeks – 1.2–1.5% per day, at 28–30 weeks – 0.8–1.1% per day, at 31–32 weeks – 0.6–1.0% per day. Forty pregnant women received 3 IUTs, 28 pregnant women received 4 IUTs, 20 pregnant women received 5 IUTs, ten pregnant women received 6 IUTs, and one pregnant woman received 7 IUTs. A total of 399 IUTs were performed.
Paracentesis was performed in 5 fetuses with edematous HDFN, in addition, to repeat IUTs to eliminate ascites.
Immediately after delivery, 65/95 (68.4%) children underwent partial transfusions by replacing 45–90 ml/kg of their blood with a similar volume of rhesus-negative packed red blood cells O (I). In 65/95 (68.4%) newborns, early exchange transfusions were performed on the first day of life to remove bilirubin and antibody-blocked red blood cells from the bloodstream and increase hemoglobin levels. In 17/95 (17.9%) children on the 3–5th day of life, standard blood transfusions were performed for anti-anemic purposes.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics 23 software. The distribution of continuous variables was tested for normality using the generalized D'Agostino–Pearson test. Quantitative variables showing normal distribution were expressed as means (M) and standard deviation (SD) and compared using the t-test. Variables not meeting normality assumptions were reported as the median (Me) and interquartile range (Q1; Q3) and were compared with a nonparametric Mann–Whitney test. Qualitative data were presented as counts (n) and %; Fisher's exact test was used to compare them. Since the study compared paired groups, statistical methods for differences in paired observations were used to determine the statistical significance of the results. Differences were considered statistically significant at p<0.05.
Results
Before the 1st IUT, fetal cord blood hematocrit levels obtained by cordocentesis ranged from 3% to 34.4% (16.9 [7.4] %) and were somewhat associated with the severity of HDFN. Hematocrit was higher (p<0.05) in severe (15.4–34.4%; 24.6 [7.7] %] and very severe (5.8–32.9%; 17.5 [7.3] %) HDFN than in hydrops fetalis (3–31.2%; 13.1 [6.2] %).
To predict adverse pregnancy outcomes in rhesus conflict, we developed a percentile scale of hematocrit values depending on the gestational age before the first IUT. At hematocrit levels <25th percentile, 3/4 (75%) of fetuses died antenatally at 25-27 weeks of gestation, and a high rate of extremely early and early preterm births (76.8%) was observed. Therefore, the 25th percentile can be considered as a threshold for predicting adverse outcomes in pregnant women with Rh conflict and severe HDFN who underwent multiple IUTs (Fig. 1).
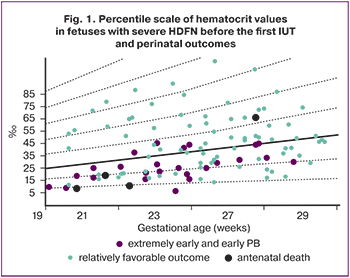
The first IUT was performed between 19 and 29 weeks (24.8 [2.6] weeks). The first IUT was performed later (p<0.05) for fetuses with severe (27.4 [0.3] weeks) and very severe (25.0 [2.4] weeks) HDFN compared to fetuses with edematous HDFN (23.4 [2.8] weeks).
We evaluated the efficacy of multiple IUTs by the dynamics of hematocrit levels before and after IUT (Fig. 2).
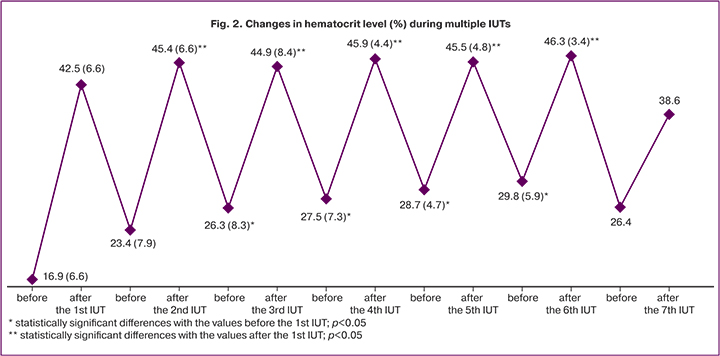
We found that umbilical cord blood hematocrit in fetuses immediately after the 1st IUT increased 2.5 times; after the 2nd IUT, 1.9 times; after the 3rd and 4th, 1.7 times; after the 5th and 6th, 1.6 times; after the 7th IUT, 1.5 times (p<0.001). After the 2nd and subsequent IUTs, hematocrit levels were higher than after the first IUT (42.5 [6.6] %; p<0.05). However, because of continued erythrocyte hemolysis before each subsequent IUT, the fetal blood hematocrit decreased again, but not to baseline values. Moreover, before the 3rd and subsequent IUTs, the hematocrit level was statistically significantly higher than before the first IUT (16.9 [7.4] %; p<0.05), which reflected the effectiveness of intrauterine transfusions.
The overall survival rate of fetuses with severe HDFN after multiple IUTs was 96%, higher than the 79.2% rate in the Centre for Family Planning and Reproduction in earlier years (1995 to 2006) [14].
In our study, four fetuses died antenatally (4%). The causes of death were chorioamnionitis in 1 (at 25 weeks gestation; 3 IUTs were performed), acute hypoxia in 1 (33 weeks; 3 IUTs), and severe condition due to fetal hydrops in 2 (at 26 and 28 weeks; 4 IUTs). Two (of 4) of the deceased were diagnosed with fetal hydrops, and 2 had a very severe course of the disease. Antibody titers were 1:28, 1:4096; 1:4096 and 1:2048. The four fetuses that died antenatally had hematocrit levels of 5.8, 6.4; 9.7; and 28.2%; the timing of the 1st IUT were: 20, 22, 21, and 27 weeks, respectively.
All 99 patients were delivered between 24 and 36 weeks of gestation. All of them underwent cesarean section. Extremely preterm labor (at 22 to 27 weeks) was in 3 patients (2 fetuses died antenatally, one baby survived), early labor (at 28–31 weeks) in 24 (all babies survived), preterm labor (at 32–36 weeks) in 72 (1 baby died antenatally).
Thanks to the intrauterine treatment, we were able to prolong pregnancy up to 32–36 weeks of gestation in 72/99 (72.7%) patients: in 75% (3 of 4) – with a severe course of HDFN, in 83.8% (62 of 74) – with a very severe course and in 33.3% (7 of 21) – with fetal hydrops.
The Apgar score of 95 newborns with HDFN was 5.2 (2; 7) at minute 1 and 6.3 (3; 8) at minute 5. Differences in Apgar scores between groups and with different forms of HDFN were statistically insignificant (p>0.05). Only 15 of 95 children (15.8%) were born in a satisfactory condition (score 8–10), 70 (73.7%) were born in moderate asphyxia (score 4–7), and ten children (10.5%) were born in severe asphyxia (score 1–3).
Four of 95 (4.2%) newborns with HDFN had moderate neonatal jaundice, 72 (75.8%) had HDFN with severe neonatal jaundice, and 19 (20%) had the HDFN with fetal hydrops in the intrauterine period (in 2 ascites persisted). Consequently, we noted the effect of multiple IUTs in most observations. The efficacy of intrauterine treatment for HDFN with fetal hydrops was 80.9% (17 of 21).
In cases of very severe disease, partial exchange transfusions, exchange transfusions, and standard blood transfusions were performed in 69.4%, 69.4%, and 16.7% of the case, respectively; for fetal hydrops in 78.9%, 78.9%, and 26.3%, respectively.
In recent years in our clinic, due to the success of intrauterine treatment of HDFN, the rate of partial exchange transfusions in newborns has decreased to 72.6%, exchange transfusions to 68.4%, and standard blood transfusions to 17.9%, compared with the rates in the earlier period (1995–2006): 89.8%, 98%, and 19%, respectively [14]. Besides, while partial exchange transfusions were performed in 44% of newborns 30 minutes or more after birth between 1995 and 2006 [14], today, almost all partial exchange transfusions are conducted within 20–30 minutes after birth, which prevents the adverse effects of hypoxia on the infant's brain.
In earlier years, 2 to 4 exchange transfusions were given to most newborns, while nowadays, only one transfusion is given. Late exchange transfusions performed between 1995 and 2006 in 41.6% of neonates with severe HDFN were not performed in recent years due to the successful intrauterine treatment of HDFN.
We consider it essential to note that 11 newborns with fetal hydrops whose mothers started intrauterine treatment in time (at 19–23 weeks) and received 4 to 6 IUTs received fewer partial exchange transfusions (72.7% and 87.5%), exchange transfusions (72.7% and 87.5%), and standard blood transfusions (18.2% and 37.5%), compared with eight newborns with fetal hydrops whose mothers were treated later (at 24–27 weeks) and received only 3 IUTs.
Postnatally, two children (2.1%) with fetal hydrops who underwent 3 IUTs (instead of 4) died on days 5 and 7 of life. Surviving neonates with HDFN (n=93) were transferred to the second phase of nursing on days 4–23 of life after their condition had stabilized.
Discussion
Opinions on the gestational age at which the first IUT can be performed are mixed, as the studies were primarily performed on relatively small samples. Many researchers have noted the possibility of shifting the first IUT to an earlier gestational age (16–19 weeks) [15, 16].
Our findings suggest the importance of early intrauterine treatment of severe HDFN (from 19 weeks of gestation) and the feasibility of at least four IUT in hydrops fetalis. It was found that timely intrauterine treatment of hydrops fetalis (at 19–23 weeks) and carrying out 4 to 6 IUTs, as compared with a later start of treatment (at 24–27 weeks) and carrying out only 3 IUTs, resulted in fewer neonatal partial exchange transfusions (72.7% and 87.5%), exchange transfusions (72.7% and 87.5%) and standard blood transfusions (18.2% and 37.5%).
Many studies have investigated IUT efficacy [7–9, 17–20]. We evaluated their effectiveness on a large number of conducted IUT (n=399) by hematocrit level immediately after blood transfusions. The maximum effect was observed during the first IUT, resulting in a 2.5-fold increase in fetal hematocrit. Further intrauterine transfusions were also effective (1.5–1.9-fold increase in hematocrit) but without statistically significant differences between the second and subsequent IUTs.
As the literature and our study have shown, multiple IUTs increase the survival rate of fetuses with severe forms of the disease. In the early 1990s, the survival rate of fetuses with HDFN, according to the world statistics, was 70–75% [21, 22], but to date, due to improvements in technology and the use of high-resolution fetal ultrasonographic monitoring, this rate has improved and in some studies reaches 77.5-96% [16, 19, 23–25]. We confirmed the worldwide trend of improved fetal survival from 79.2% (in 1995–2006) [14] to 96% (in 2014–2018).
In our study, 4 (4%) fetuses died antenatally, consistent with literature data of 0.8–38.8% [16, 18, 26]. The majority of those who died (75%) had a low hematocrit level before the first blood transfusion, as noted by other clinicians [27, 28].
One factor that worsens perinatal outcomes in HDFN is forced preterm termination of pregnancy and induction of early preterm birth [9, 29, 30]. Considering the problem of preterm birth prevention, we would like to emphasize that due to the intrauterine treatment, we have managed to prolong pregnancy to 32-36 weeks of gestation in 72.7% of patients with severe HDFN.
We proved the appropriateness of performing at least four intrauterine blood transfusions for hydrops fetalis. It allowed for treating hydrops fetalis in 80.9% of cases and reduced the need for partial exchange transfusions, exchange transfusions, and standard blood transfusions.
Conclusion
Multiple IUTs for severe, very severe disease and hydrops fetalis prolong pregnancy, improve perinatal outcomes, increase fetal survival, and reduce the number of exchange transfusions in the early neonatal period. Multiple IUTs should not be considered a breakthrough in solving the problem of Rh alloimmunization. Administration of anti-D immunoglobulin should remain a mainstay in preventing maternal Rh(D) sensitization and Rh hemolytic disease of the newborn.
References
- Савельева Г.М., Курцер М.А., Сичинава Л.Г., Коноплянников А.Г., Латышкевич О.А., Сонголова Е.Н. 50 лет иммунопрофилактике резус-иммунизации: на страже перинатальной заболеваемости и младенческой смертности (исторический экскурс). Акушерство и гинекология. 2018; 12: 177-83. [Savelyeva G.M., Kurtser M.A., Sichinava L.G., Konoplyannikov A.G., Latyshkevich O.A., Songolova E.N. 50 years of immunization with Rh immunization: on guard of perinatal morbidity and infant mortality (historical excursus). Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2018; 12: 177-83. (in Russian)]. https://dx.doi.org/10.18565/aig.2018.12.177-183.
- Zwingerman R., Jain V., Hannon J., Zwingerman N., Clarke G. Alloimmune red blood cell antibodies: prevalence and pathogenicity in a Canadian perenatal population. J. Obstet. Gynaecol. Can. 2015; 37(9): 784-90. https://dx.doi.org/10.1016/S1701-2163(15)30148-1.
- Ghesquière L., Garabedian C., Coulon C., Verpillat P., Rakza T., Wibaut B. et al. Management of red blood cell alloimmunization in pregnancy. J. Gynecol. Obstet. Hum. Reprod. 2018; 47(5): 197-204. https://dx.doi.org/10.1016/j.jogoh.2018.02.001.
- Healsmith S., Savoia H., Kane S.C. How clinically important are non-D Rh antibodies? Acta Obstet. Gynecol. Scand. 2019; 98(7): 877-84. https://dx.doi.org/10.1111/aogs.13555.
- Virk M., Papakonstantino K., Cai W., Oh D., Andrews J. Blood donation during pregnancy due to anti-ku hemolytic disease of the fetus and newborn. Lab. Med. 2019; 50(4): 421-5. https://dx.doi.org/10.1093/labmed/lmz020.
- Шаробаро В.Е. Гемолитическая болезнь плода и новорожденного (лекция). Смоленский медицинский альманах. 2019; 4: 69-78. [Sharobaro V.E. Hemolytic disease of the fetus and newborn (lecture). Smolenskij medicinskij al'manah/Smolensk Medical Almanac. 2019; 4: 69-78. (in Russian)].
- Bruno A.M., Rosenbloom J.I., Woolfolk C., Conner S.N., Tuuli M.G., Macones G.A. Neonatal outcomes after percutaneous umbilical cord blood sampling†. J. Matern. Fetal Neonatal Med. 2019 Mar 25: 1-6. https://dx.doi.org/10.1080/14767058.2019.1593960.
- Bekdache G.N., Mylopoulos M., Kulasegaram K.M., Windrim R. Pedagogical strategies in teaching invasive prenatal procedures: a scoping review protocol. BMJ Open. 2019; 9(5): e024629. https://dx.doi.org/10.1136/bmjopen-2018-024629.
- Gudlaugsson B., Hjartardottir H., Svansdottir G., Gudmundsdottir G., Kjartansson S., Jonsson T. et al. Rhesus D alloimmunization in pregnancy from 1996 to 2015 in Iceland: a nation-wide population study prior to routine antenatal anti-D prophylaxis. Transfusion. 2020; 60(1): 175-83. https://dx.doi.org/10.1111/trf.15635.
- Керимова Э.А., Путилова Н.В., Чистякова Г.Н., Пестряева Л.А., Устьянцева Н.Ю. Клинико-иммунологическое обоснование внутриутробных гемотрансфузий при гемолитической болезни плода по системе резус. Акушерство и гинекология. 2016; 12: 24-7. [Kerimova E.A. Putilova N.V., Chistyakova G.N., Pestryaeva L.A., Ustyantseva N.Yu. Clinical and immunological substantiation of intrauterine blood transfusions with hemolytic disease of the fetus according to the rhesus system. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2016; 12: 24-7. (in Russian)]. https://dx.doi.org/10.18565/aig.2016.12.24-7.
- Макогон А.В., Андрюшина И.В. Гемолитическая болезнь плода: мониторинг, лечение плода и родоразрешение. Вопросы гинекологии, акушерства и перинатологии. 2018; 17(3): 45-52. [Makogon A.V., Andryushina I.V. Hemolytic disease of the fetus: monitoring, treatment of the fetus and delivery. Voprosy ginekologii, akusherstva i perinatologii/Questions of gynecology, obstetrics and perinatology. 2018; 17(3): 45-52. (in Russian)]. https://dx.doi.org/10.20953/1726-1678-2018-3-45-52.
- Туриченко О.В., Корчагина Е.Е., Лапин А.А. Анализ диагностики и лечения тяжелых форм гемолитической болезни плода. Кубанский научный медицинский вестник. 2018; 25(4): 102-5. [Turichenko O.V., Korchagin E.E., Lapin A.A. Analysis of diagnosis and treatment of severe hemolytic disease of the fetus. Kubanskij nauchnyj medicinskij vestnik / Kuban Scientific Medical Bulletin. 2018; 25(4): 102-5. (in Russian)]. https://dx.doi.org/10.25207/1608-6228-2018-25-4-102-105.
- Mari G., Deter R.L., Carpenter R.L., Rahman F., Zimmerman R., Moise K.J. Jr. et al. Noninvasive diagnosis by Doppler ultrasonography of fetal anemia due to maternal red-cell alloimmunization. Collaborative Group for Doppler Assessment of the Blood Velocity in Anemic Fetuses. N. Engl. J. Med. 2000; 342(1): 9-14. https://dx.doi.org/10.1056/NEJM200001063420102.
- Савельева Г.М., Коноплянников А.Г., Курцер М.А., Панина О.Б. Гемолитическая болезнь плода и новорожденного. Руководство. M.: ГЭОТАР-Медиа; 2013. 144с. [Savelyeva G.M., Konoplyannikov A.G., Kurtser M.A., Panina O.B. Hemolytic disease of the fetus and newborn: a guide. M: GEOTAR-Media; 2013. 144 p. (in Russian)].
- Al-Riyami A.Z., Al-Salmani M., Al-Hashami S.N., Al-Mahrooqi S., Al-Marhoobi A., Al-Hinai S. et al. Intrauterine fetal blood transfusion: descriptive study of the first four years' experience in Oman. Sultan Qaboos Univ. Med. J. 2018; 18(1): e34-42. https://dx.doi.org/10.18295/squmj.2018.18.01.006.
- Potdar O., Narkhede H.R., Satoskar P.R. Perinatal outcome after intrauterine transfusion in Rh isoimmunized mothers. J. Obstet. Gynaecol. India. 2019; 69(2): 123-8. https://dx.doi.org/10.1007/s13224-018-1108-6.
- Donepudi R.V., Moise K.J. Jr. Intrauterine transfusion complicated by umbilical artery thrombosis. Case Rep. Obstet. Gynecol. 2019; 2019: 5952326. https://dx.doi.org/10.1155/2019/5952326.
- Sánchez-Durán M.Á., Higueras M.T., Halajdian-Madrid C., Avilés García M., Bernabeu-García A., Maiz N. et al. Management and outcome of pregnancies in women with red cell isoimmunization: a 15-year observational study from a tertiary care university hospital. BMC Pregnancy Childbirth. 2019; 19(1): 356. https://dx.doi.org/10.1186/s12884-019-2525-y.
- Şavkli A.Ö., Çetin B.A., Acar Z., Özköse Z., Behram M., Çaypinar S.S. et al. Perinatal outcomes of intrauterine transfusion for foetal anaemia due to red blood cell alloimmunisation. J. Obstet. Gynaecol. 2020; 40(5): 649-53. https://dx.doi.org/10.1080/01443615.2019.1647521.
- Yüksel İ.T., Acar D., Turhan U., Çetİn B.A., Köroğlu N., Şenol G. et al. Assessment of fetal right ventricular myocardial performance index changes following intrauterine transfusion. J. Matern. Fetal Neonatal Med. 2021; 34(18): 3046-9. https://dx.doi.org/10.1080/14767058.2019.1677595.
- Rodeck C.H., Nicolaides K.H., Warsof S.L., Fysh W.J., Gamsu H.R., Kemp J.R. The management of severe rhesus isoimmunization by fetoscopic intravascular transfusions. Am. J. Obstet. Gynecol. 1984; 150(6): 769-74.
- Ney J.A., Socol M.L., Dooley S.L., MacGregor S.N., Silver R.K., Millard D.D. Perinatal outcome following intravascular transfusion in severely isoimmunized fetuses. Int. J. Gynaecol. Obstet. 1991; 35(1): 41-6.
- Garabedian C., Philippe M., Vaast P., Wibaut B., Salleron J., Delsalle A. et al. Is intrauterine exchange transfusion a safe procedure for management of fetal anaemia? Eur. J. Obstet. Gynecol. Reprod. Biol. 2014; 179: 83-7. https://dx.doi.org/10.1016/j.ejogrb.2014.05.008.
- Ducellier-Azzola G., Pontvianne M., Weingertner A.S., Kohler M., Viville B., Weil M., Sananès N. Devenir obstétrical après transfusion in utero pour allo-immunisation fœto-maternelle [Outcome of in utero transfusion in case of fœtomaternal red blood cell incompatibility]. Gynecol. Obstet. Fertil. Senol. 2018; 46(1): 14-9. French. https://dx.doi.org/10.1016/j.gofs.2017.11.007.
- Melekoglu R., Celik E., Kural H. Sonographic demonstration of intracranial hemorrhage in a fetus with hydrops fetalis due to Rh alloimmunization after intrauterine intravascular transfusion: A case report and review of the literature. Case Rep. Obstet. Gynecol. 2018; 2018: 8412139. https://dx.doi.org/10.1155/2018/8412139.
- Zwiers C., Oepkes D., Lopriore E., Klumper F.J., de Haas M., van Kamp I.L. The near disappearance of fetal hydrops in relation to current state-of-the-art management of red cell alloimmunization. Prenat. Diagn. 2018; 38(12): 943-50. https://dx.doi.org/10.1002/pd.5355.
- Lindenburg I.T.M., van Kamp I.L., Oepkes D. Intrauterine blood transfusion: current indications and associate risks. Fetal Diagn. Ther. 2014; 36(4): 263-71. https://dx.doi.org/10.1159/000362812.
- Deka D., Dadhwal V., Sharma A.K., Shende U., Agarwal S., Agarwal R. Perinatal survival and procedure-related complications after intrauterine transfusion for red cell alloimmunization. Arch. Gynecol. Obstet. 2016; 293(5): 967-73. https://dx.doi.org/10.1007/s00404-015-3915-7.
- Бойко Н.В., Модель Г.Ю., Алехина В.И. Особенности адаптации новорожденных, перенесших внутриутробные гемотрансфузии. Кубанский научный медицинский вестник. 2018; 25(3): 34-9. [Boyko N.V., Model G.Yu., Alekhina V.I. Features of adaptation of newborns who have had intrauterine blood transfusions. Kubanskij nauchnyj medicinskij vestnik/Kuban Scientific Medical Bulletin. 2018; 25(3): 34-9. (in Russian)]. https://dx.doi.org/10.25207/1608-6228-2018-25-3-34-39.
- Сайфуллина Д.Н., Петрова Ж.В., Геворкян Д.В., Воропаев И.В., Савилова Т.В., Сенникова Ж.В., Степанов С.Ю., Дёмина Л.М. Ранний неонатальный период у новорожденных с гемолитической болезнью, перенесших внутриутробное внутрисосудистое переливание крови. Оренбургский медицинский вестник. 2018; 6(4): 37-9. [Saifullina D.N., Petrova Zh.V., Gevorkyan D.V., Voropaev I.V., Savilova T.V. et al. Early neonatal period in newborns with hemolytic disease who have undergone intrauterine intravascular blood transfusion. Orenburgskij medicinskij vestnik/Orenburg medical bulletin. 2018; 4(24): 37-9. (in Russian)].
Received 03.12.2021
Accepted 15.12.2021
About the Authors
Galina M. Savelyeva, Dr. Med. Sci., Academician of the RAS, Professor at the Department of Obstetrics and Gynecology of Pediatric Faculty, Pirogov Russian National Research Medical University, Ministry of Health of Russia, +7(495)718-34-72, gms@cfp.ru, https://orcid.org/0000-0001-8735-1281,117997, Russia, Moscow, Ostrovityanova str., 1.
Alexander G. Konoplyannikov, Dr. Med. Sci., Professor at the Department of Obstetrics and Gynecology of Pediatric Faculty, Pirogov Russian National Research Medical University, Ministry of Health of Russia, +7(499)723-04-20, npo.med@gmail.com, https://orcid.org/0000-0001-9923-8833, 117997, Russia, Moscow, Ostrovityanova str., 1.
Elena Ya. Karaganova, MD, PhD, Associate Professor at the Department of Obstetrics and Gynecology of Pediatric Faculty, Pirogov Russian National Research Medical University, Ministry of Health of Russia, +7(916)684-95-05, karaganova22@gmail.com, https://orcid.org/0000-0002-3199-4867, 117997, Russia, Moscow, Ostrovityanova str., 1.
Aleksandra A. Smirnova, MD, Head of the Consulting and Diagnostic Department, Centre for Family Planning and Reproduction, Moscow Healthcare Department,
+7(916)189-40-72, salexandra2006_0@mail.ru, https://orcid.org/0000-0002-8852-1980, 117209, Russia, Moscow, Sevastopolsky Ave., 24A.
Maria M. Astrakhantseva, MD, Ph.D., Teaching Assistant at the Department of Obstetrics and Gynecology of Pediatric Faculty, Pirogov Russian National Research Medical University, Ministry of Health of Russia, +7(495)718-34-72, agpf. gms@gmail.com, https://orcid.org/0000-0003-1482-6279, 117997, Russia, Moscow, Ostrovityanova str., 1.
Natalia G. Martynova, Clinical Resident at the Department of Obstetrics and Gynecology, Faculty of Fundamental Medicine, Lomonosov Moscow State University, martynovanatalia96@yandex.ru, 119991, Russia, Moscow, GSP-1, Leninskie gory, 1, p. 51.
Оleg А. Latyshkevich, MD, Ph.D., Teaching Assistant at the Department of Obstetrics and Gynecology of Pediatric Faculty, Pirogov Russian National Research Medical University, Ministry of Health of Russia, 117997, Russia, Moscow, Ostrovityanova str., 1; Chief physician, Centre for Family Planning and Reproduction, Moscow Healthcare Department, 117209, Russia, Moscow, Sevastopolsky Ave., 24A, +7(495)718-20-70, latishkevich@mail.ru, https://orcid.org/0000-0002-3467-4236
Authors' contributions: Savelyeva G.M., Konoplyannikov A.G. – concept and design of the study; Smirnov A.A., Astrakhantseva M.M., Martynova N.G., Latyshkevich О.А. – data collection and analysis; Smirnov A.A. – statistical analysis; manuscript drafting; Savelyeva G.M., Konoplyannikov A.G., Karaganova E.Ya. - manuscript editing.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical approval: in the process of review and approval.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Savelyeva G.M., Konoplyannikov A.G., Karaganova E.Ya., Smirnov A.A., Astrakhantseva M.M., Martynova N.G., Latyshkevich О.А. Our experience with multiple intrauterine blood transfusions in severe Rh hemolytic disease of the fetus and newborn.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2022; 2: 65-71 (in Russian)
https://dx.doi.org/10.18565/aig.2022.2.65-71



