В настоящее время в мире заболевания почек и мочевыводящих путей занимают второе место после сердечно-сосудистой патологии в структуре экстрагенитальной патологии у беременных. Следует отметить, что наличие инфекции мочевыводящих путей при беременности сопровождается повышенным уровнем материнской и перинатальной заболеваемости и смертности. Кроме того, актуальность проблемы обусловлена ростом удельного веса этих заболеваний, изменением характера микрофлоры и ее резистентности к антибактериальным препаратам, разработкой и применением новых современных методов диагностики и лечения инфекции органов мочеполовой системы. Наиболее часто встречающейся патологией среди беременных является хронический рецидивирующий неосложненный цистит [1, 2].
Цистит – это воспаление стенки мочевого пузыря разнообразной этиологии. Стенка мочевого пузыря состоит из трех оболочек: слизистой, собственной мышечной и серозной. При цистите преимущественно поражается слизистая оболочка, обладающая сенсорной и барьерной функциями, которые нарушаются при инфекционно-воспалительном процессе [2, 3].
В большинстве случаев цистит встречается у женщин, при этом у них отсутствуют структурные изменения почек, нарушения оттока мочи и серьезные сопутствующие заболевания. Кроме того, каждый год примерно 10% женщин обращаются с впервые возникшим эпизодом, более 50% – имеют хотя бы один эпизод цистита в течение жизни [3, 4].
Наиболее частыми возбудителями неосложненного цистита являются уропатогенный штамм Escherichia coli, встречающийся в 75–80% случаев, следующие по выявляемости – Staphylococcus saprophyticus и Klebsiella spp. – в 5–10% случаев. Реже выделяются Enterobacteriaceae [5].
Патогенез цистита обусловлен колонизацией промежности, мочеиспускательного канала и влагалища уропатогенами кишечной флоры с дальнейшим попаданием в мочевой пузырь. Однако, в отличие от кишечных штаммов Escherichia coli, уропатогенные обладают рядом факторов вирулентности, позволяющих им проникать в слизистую оболочку мочевого пузыря, адгезироваться к уроэпителиальному слою клеток и противостоять защитным механизмам [6–8]. Обострение цистита может быть связано с повышенной восприимчивостью рецепторов эпителия мочевыводящих путей к кишечной палочке [8].
Среди женщин часто наблюдается рецидивирующая форма неосложненного цистита, а предрасполагающими к развитию данного состояния факторами являются их анатомические особенности: короткая и широкая уретра, близкое расположение ануса и мочеиспускательного канала, что способствует попаданию инфекционного агента в мочевой пузырь. При беременности структурные и гормональные изменения способствуют учащению эпизодов цистита, предрасполагающими факторами являются прогестерон-индуцированное изменение уродинамики, расширение мочеточников и релаксация детрузора, увеличение объема мочевого пузыря и изменение состава мочи (глюкозурия и увеличение pH), приводящие к нарушению пассажа мочи, что предрасполагает к развитию пузырно-мочеточникового рефлюкса [1, 9].
Диагностика цистита не представляет трудности и основывается на клинико-анамнестических и лабораторных данных. Типичные симптомы при беременности и вне ее не отличаются и включают болезненное учащенное мочеиспускание, боль над лоном, мочеиспускание малыми порциями, повелительные позывы к мочеиспусканию, наличие крови в моче. Может отмечаться субфебрильная температура тела, однако лихорадка и озноб для цистита не характеры. При лабораторном исследовании основаниями для постановки диагноза являются лейкоцитурия (более 10 лейкоцитов в поле зрения) в общем анализе мочи и рост не более 2 видов бактерий при микробиологическом исследовании мочи ≥105 КОЕ/мл в средней порции [3, 10, 11].
Для верификации диагноза мочевой инфекции у беременных при сборе анамнеза следует выяснить, имеются ли другая патология почек, структурные изменения почек и мочевыводящих путей, присутствуют ли в организме очаги хронической инфекции, иммунодефицит, возникший вследствие какого-либо заболевания или индуцированный лекарственными препаратами [12].
Отсутствие лечения цистита у беременных может быть причиной развития острого пиелонефрита, что повышает риск материнских и неонатальных осложнений, таких как невынашивание беременности, преэклампсия, задержка развития плода, преждевременные роды [1, 3, 13].
В лечении рецидивирующего неосложненного цистита выбор препарата должен учитывать безопасность его использования во время беременности (включая триместр беременности). Препаратами 1-й линии, проявившими максимальную активность в отношении элиминации возбудителей, являются фосфомицин (порошок или гранулы для приготовления раствора для приема внутрь однократно на ночь 3 г) и нитрофурантоин (100 мг 3 раза в сутки в течение 7 дней) [14, 15]. Цефалоспорины III поколения (цефиксим) также относятся к препаратам выбора в эмпирической терапии цистита. При установленной чувствительности микроорганизмов к амоксициллину+клавулановой кислоте и ампициллину данные препараты также могут использоваться в лечении [16, 17].
При рецидивирующем течении цистита происходят изменения иммунной системы, в развитии данного состояния важное значение отводится дисбалансу в системе цитокинов. Иммунный ответ характеризуется секрецией и продукцией провоспалительных интерлейкинов (ИЛ-1, ИЛ-6, ИЛ-8), цитокинов, интерферонов (ИФН-α и ИФН-β), фактора некроза опухоли. В месте повреждения происходит инфильтрация макрофагами CD8+ и CD4+, Т-лимфоцитами, секретирующими цитокины. Повышение продукции провоспалительных цитокинов приводит к хронизации инфекции и иммунопатологическим процессам в тканях [18–20]. Исходя из вышеизложенных данных, обоснованным является включение в комплексную терапию хронического рецидивирующего неосложненного цистита иммуномодулирующего препарата. Одним из наиболее эффективных является препарат «Суперлимф», который применяется в комплексной терапии для удлинения периода ремиссии и является уникальным иммуномодулирующим препаратом, в состав которого входит комплекс природных противомикробных пептидов и цитокинов. Противомикробная и противовирусная активность этого средства обусловлена стимуляцией функциональной активности клеток врожденного иммунитета – моноцитов и нейтрофилов. Под действием «Суперлимфа» активируются фагоцитоз и выработка противовоспалительных цитокинов, усиливаются активность естественных киллеров, их миграция в очаг воспаления. Препарат снижает развитие воспалительных реакций, стимулирует регенерацию и эпителизацию. Эффективность данного препарата неоднократно была продемонстрирована в клинических исследованиях [19, 21].
Цель исследования – оценить эффективность комплексной терапии хронического рецидивирующего неосложненного цистита при беременности.
Материалы и методы
Проведено клиническое исследование, в которое были включены 50 беременных, перенесших рецидив хронического неосложненного цистита во II или III триместре беременности (с 16-й по 32-ю недели беременности).
Критериями отбора являлись: возраст 18–40 лет, одноплодная беременность, отсутствие пороков развития мочевыводящей системы, аутоиммунных заболеваний, повышенной чувствительности к компонентам лекарственных препаратов, хроническое течение неосложненного цистита (не менее 2 рецидивов в течение последних 6 месяцев или 3 эпизода в течение 1 года) с эпизодом во II или III триместре беременности, клиническая картина обострения цистита (болезненное и учащенное мочеиспускание, жжение и рези при мочеиспускании, императивные позывы, недержание мочи, чувство неполного опорожнения мочевого пузыря, боли внизу живота и над лоном), отсутствие температуры тела выше 38°C; лейкоцитурия (более 10 лейкоцитов в поле зрения) по данным общего анализа мочи, при бактериологическом исследовании мочи выявление роста возбудителя >105 КОЕ/мл.
Пациентки с подтвержденным циститом методом произвольной выборки были разделены на 2 равные группы:
- группа 1 – основная, включающая 25 женщин, которые получали лечение антибактериальным препаратом в сочетании с иммуномодулирующим средством «Суперлимф».
- группа 2 – сравнения, включающая 25 женщин, получающих монотерапию антибактериальным препаратом.
В качестве антибактериального препарата всем пациенткам назначался фосфомицин 3 г, антибиотик широкого спектра действия, производное фосфоновой кислоты. Схема приема: однократно на ночь после мочеиспускания.
Иммуномодулирующая терапия проводилась препаратом «Суперлимф» 10 ЕД ректально по 1 суппозиторию 2 раза в сутки в течение 10 дней. Курс лечения начинали одновременно с антибактериальной терапией.
Всем пациенткам было проведено стандартное исследование согласно клиническим рекомендациям, а также анализ клинико-анамнестических показателей. Эффективность лечения оценивалась на 11-й день от начала проведения терапии (контрольный визит), анализировались общий анализ мочи, микробиологическое исследование мочи, выраженность симптомов цистита оценивалась по шкале ACSS (The Acute Cystitis Symptom Score, Шкала симптомов острого цистита) при первом и контрольном обращении. Для оценки иммуномодулирующей эффективности терапии изучали фенотипическую характеристику основных популяций лимфоцитов (CD3+, CD4+, CD8+).
Все пациентки динамически наблюдались в течение минимум 3 месяцев после проведенного лечения с целью оценки межрецидивного периода.
Также анализировались исходы родов у пациенток с хроническим рецидивирующим неосложненным циститом и особенности перинатальных исходов в обеих группах в зависимости от схем их лечения.
Статистический анализ
Полученные результаты проходили статистическую обработку на персональном компьютере при помощи программы Microsoft Office 2021. Для статистического анализа использовали программу StatSoft (STATISTICA), в расчете средних величин использовались непараметрические методы.
Результаты и обсуждение
На фоне лечения у всех беременных отмечалось улучшение самочувствия, и все пациентки имели высокую приверженность к проводимому лечению. Побочные реакции на фоне приема лекарственных средств не были отмечены ни в одном из случаев [1, 17]. Пациентки обеих групп заполняли опросник ACSS, согласно которому оценивались эффективность проведенной терапии, выраженность клинических проявлений, качество жизни пациенток. Данное анкетирование включает 18 вопросов, поделенных на 4 группы: 1-я включает типичные симптомы, характерные для острого цистита; 2-я – симптомы для постановки дифференциального диагноза; в 3-й группе оценивается качество жизни; вопросы 4-й группы характеризуют основное заболевание. О наличии острого цистита свидетельствует значение, равное 6 баллам и более, при оценке типичных симптомов.
Данные валидизированной русской версии ACSS при первом и контрольном визитах пациенток представлены в таблице 1.

Исходя из данных, представленных в таблице 1, можно отметить, что в основной группе пациенток, получавших антибактериальную терапию + препарат «Суперлимф», отмечались статистически значимое снижение клинических проявлений (боли и жжение при мочеиспускании, учащенные и срочные позывы к мочеиспусканию, наличие крови в моче) и повышение качества жизни по сравнению с пациентами 2-й группы, получавшими только фосфомицин (p<0,05). В 1-й группе исследования было отмечено уменьшение суммы баллов характерных признаков цистита в 4 раза, во 2-й группе – в 2,5 раза, отличительных (дифференциальных) – в 2,1 и 1,5 раза, качество жизни улучшилось в 4,5 и 2,0 раза по группам соответственно, дополнительные не имели статистического значения.
При анализе результатов лабораторного исследования у всех пациенток отмечалась лейкоцитурия по данным общеклинического анализа мочи. Результаты общего анализа мочи представлены в таблице 2.
Как видно из таблицы 2, у всех беременных с эпизодом рецидивирующего цистита отмечалась пиурия до лечения, однако в 1-й группе по сравнению с 2-й на фоне терапии при контрольном посещении количество лейкоцитов было в 2,7 раза ниже (p<0,05).
Анализ микробиологического исследования показал рост одного или двух возбудителей в титре ≥105 КОЕ/мл. Рост Escherichia coli отмечался наиболее часто и составил 78,4% случая, Klebsiella встречалась в 9,5%, рост Staphylococcus – в 6,7% и Proteus – в 5,4% случаев [2, 15]. При повторном микробиологическом исследовании мочи на 11-й день от начала лечения отмечалось, что в группе 1 полная элиминация возбудителя была достигнута у 24 пациенток (96%), у 1 (4%) – снижение микробной колонизации до 103 КОЕ/мл; в группе 2 полная элиминация возбудителя отмечалась у 20 женщин (80%), у 5 (20%) – снижение микробной колонизации до 103–104 КОЕ/мл. Данные результаты согласуются с литературными данными и отражают наибольшую эффективность антибактериальной терапии в сочетании с иммуномодулирующим препаратом «Суперлимф» (p<0,05) [19, 21].
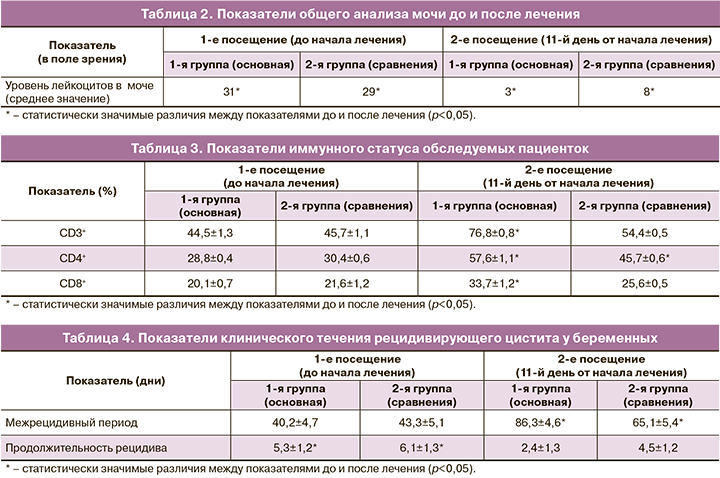
При обследовании пациенток обеих групп выявлены признаки специфического иммунодефицита, сформированного персистированием микробного агента в организме. При анализе влияния проводимой терапии в основной группе отмечалось увеличение количества субпопуляций лимфоцитов. Данные продемонстрированы в таблице 3.
Проанализировав результаты, можно отметить, что в основной группе у пациенток, получавших иммуномодулирующую терапию, повышение лимфоцитов CD3+, CD4+, CD8+ было более значимым, чем в группе сравнения. Полученные данные подтверждают эффективность и действенность препарата «Суперлимф» в повышении иммунного ответа.
При наблюдении всех пациенток в течение 3 месяцев после проведенного лечения (в том числе, после родоразрешения) оценивались межрецидивный период и длительность рецидива [3, 14]. Данные полученных результатов отображены в таблице 4.
Как видно из данных, представленных в таблице 4, проведенная терапия способствовала статистически значимому уменьшению частоты и снижению продолжительности рецидивов у пациенток обеих групп. Однако стоит отметить, что в группе 1 (фосфомицин + «Суперлимф») длительность межрецидивного периода была значимо выше по сравнению с группой 2 (фосфомицин) и составила 86,3±4,6 дня.
У всех пациенток оценивались акушерские и перинатальные исходы. Произошло 50 родоразрешений. В основной группе, где применялся препарат «Суперлимф», частота своевременных родов составляла 92% (n=23), соответственно преждевременных – 8% (n=2), в группе сравнения своевременные роды произошли в 80% (n=20), преждевременные – в 20% (n=5).
Частота оперативного родоразрешения путем кесарева сечения составила соответственно по группам – 24% (n=6) и 44% (n=11), р<0,05, что значимо выше в группе 2. Вакуум-экстракция плода не применялась ни в одном случае. Показания к оперативному родоразрешению в превалирующем большинстве (40%) были сочетанными (со стороны матери и плода), только со стороны матери отмечались в 19%, со стороны плода – в 31% случаев.
Осложненное течение родов имело место в обеих группах исследования, наиболее частым являлось преждевременное излитие околоплодных вод, которое составляло 24% (n=6) и 40% (n=10) по группам соответственно. Средний объем кровопотери в родах составил 280,3±46,5 мл. Общая длительность своевременных родов составила 8 ч 35 минут + 1 ч 34 минуты, преждевременных родов – 7 ч 40 минут + 1 ч 25 минут.
У 50 детей от матерей из обеих групп исследования проанализированы перинатальные исходы. Гестационный возраст новорожденных варьировался от 34 до 41 недели беременности. Оценка состояния по шкале Апгар колебалась в группе 1 от 7 до 9 баллов, в группе 2 – от 6 до 9 баллов, асфиксия наблюдалась в 16% (n=4) и 24% (n=6) по группам соответственно. Учитывая наибольшее число преждевременных родов в группе 2, высокий процент недоношенных детей встречался также в данной группе и составил 20%. Масса доношенных новорожденных в среднем составила 3557±84 г, недоношенных – 2178±76 г. Кроме того, достоверно чаще (р<0,05) в группе 2 – 24% (n=6) по сравнению с группой 1 – 12% (n=3) дети рождались с признаками внутриутробной инфекции.
В результате исследования получены данные, демонстрирующие статистически значимо более высокую эффективность применения антибактериальной терапии в комплексе с иммуномодулирующим препаратом «Суперлимф» в лечении хронического рецидивирующего неосложненного цистита во II и III триместрах беременности. При включении в схему терапии препарата «Суперлимф» наиболее значимо снижались лейкоцитоз в общем анализе мочи, рост возбудителей при микробиологическом исследовании, положительная динамика показателей врожденного иммунитета была более выраженной, существенно удлинялся период между обострениями цистита и уменьшалась длительность рецидивов, а также достоверно улучшалось качество жизни в соответствии с опросником ACSS. Кроме того, в данной когорте пациенток регистрировались наиболее благоприятные акушерские и перинатальные исходы.
Заключение
Таким образом, в лечении хронического рецидивирующего неосложненного цистита у беременных наиболее высокую эффективность продемонстрировала комплексная терапия антибактериальным препаратом с иммуномодулирующим средством «Суперлимф». Благодаря его действию на клетки, участвующие в иммунном ответе, происходят активация нейтрофилов, моноцитов, макрофагов, элиминация внутриклеточных микроорганизмов, вследствие чего снижается выраженность воспалительной реакции.
В ходе исследования не было обнаружено побочного и токсического эффектов препарата, отмечалась высокая приверженность пациенток к лечению. Исходя из полученных данных, иммуномодулирующее средство «Суперлимф» можно рекомендовать к использованию в терапии рецидивирующего неосложненного цистита у беременных в комбинации с антибактериальным препаратом, что значительно повышает эффективность проводимого лечения.



