Integrated assessment of perioperative changes in the blood coagulating system during uterine artery embolization
Objective. To assess the time course of perioperative changes in the blood coagulation system during uterine artery embolization (EMA) based on monitoring the indicators of the hemostatic system, including thromboelastography (TEG), and to identify a thrombogenic propensity (or absence.Syutkina I.P., Khabarov D.V., Rakitin F.A., Kochetkova M.V., Ineshina A.D.
Subjects and methods. The investigation enrolled 37 female patients with EMA, in whom the risk factors predisposing to thromboembolic events were excluded during preoperative examination. Dynamic monitoring of coagulography and TEG measures was performed in the perioperative period.
Results. A day after EMA, the patients tended to have hypercoagulation, as evidenced by TEG (decreases in R time and K time and increases in α-angle and maximum amplitude), as well as activation of fibrinolysis (an increase in LY 30 levels). At the same time, a study of coagulogram indices revealed increases in the level of hypercoagulability markers (fibrinogen, D-dimer, soluble fibrin-monomer complexes) and in prothrombin index and a decrease in activated partial thromboplastin time. The findings generally suggest that there is an increased risk for venous thromboembolic events (VTEE) in the postoperative period after EMA.
Conclusion. The revealed features of hemostasiological changes in patients after EMA suggest that there is a need for the continuous monitoring of the hemostatic system and the development of effective preventive measures for VTEE.
Keywords
Currently, uterine artery embolization (UAE) is a modern highly effective minimally invasive organ-preserving method which is widely used in the treatment of uterine fibroids [1–5]. There are a large number of publications where it is shown that the risk of developing serious complications of UAE is lower than one with other surgical treatment options for uterine fibroids [6–8].
But, like any surgical injury in response to UAE, a systemic response of the body develops, consisting of an endocrine stress response and a systemic inflammatory (immune) response [9–11]. Changes in the hemostatic system are in direct functional connection with neuroendocrine and systemic inflammatory changes [12].
The incidence of pulmonary embolism in patients undergoing this procedure, according to the literature is 0.2% - 0.4% [13–15]. According to a meta-analysis conducted by Sundeep S. Toor and co-workers [16], the incidence of deep vein thrombosis in patients after UAE was 0.2%, and pulmonary embolism - 0.4% (1 case for 250 and 500 operations, respectively).
Dobrokhotova et al. [17] pointed out the feasibility of including additional criteria in the well-known classification of the degree of risk of postoperative venous thromboembolic complications (VTEC) by C.Samama and M.Samama (as modified in 1999) [18]. The criteria increase the risk of VTEC in the early postoperative period in patients with uterine myoma. These criteria include aggravated family thrombotic history, previous missed miscarriage and complicated course of pregnancy, menometrorrhagia leading to anemia, the presence of growing fibroids, methylenetetrahydrofolate reductase (MTHFR) mutation, Leiden mutation (point mutation V factor), prothrombin G mutation 20210A, hyperhomocysteinemia, positive lupus anticoagulant (BA +).
The mechanism of UAE-associated pulmonary embolism is not clear. H. Hamoda et al. [14] suggest the theoretical possibility of blood stasis in the enlarged and crowded pelvic veins in hypervascular myomas. J. Qiu et al. call a prolonged pressure on the site of the puncture of the femoral artery a possible risk factor which may lead to blood stasis in the lower limbs [19]. According to domestic studies [17, 22, 23], no marked hypercoagulative changes were observed after UAE, except for an increase in fibrinogen and a decrease in thrombin time. Rogozhkina and co-authors reveal the progression of the activation of the coagulation potential in patients with uterine myoma after UAE, manifested by an increase in the products of paracoagulation with simultaneous activation of fibrinolysis processes.
Thus, the dynamic monitoring of the hemostatic system in the perioperative period and the issue of prevention of thromboembolic complications in uterine artery embolization in patients with uterine myoma is extremely relevant.
Routine coagulation tests evaluate only some of the individual chains of the coagulation cascade or the level of individual coagulation factors, but not the entire cascade as a whole. It seems promising to use the thromboelastography (TEG) method, which makes it possible to perform an integral assessment of the state of the hemostasis system and within one test to evaluate all links of the blood coagulation system - plasma, platelet and fibrinolysis system [24].
Therefore, the objective of this study was to assess the dynamics of changes in the blood coagulation system in the perioperative period with UAE based on monitoring of indicators of the hemostasis system, including TEG and the identification of tendencies (or its absence) to thrombosis.
Materials and Methods
The study included 37 patients with the diagnosis of symptomatic multiple myoma of the uterine body, who were in the gynecological department of the NIIKEL clinic. Inclusion criteria were verified diagnosis of interstitial or interstitial-subserous multiple myoma of the uterine body, performed UAE operation, anesthetic risk not higher than ASA (American Society of Anesthesiologists) grades I-II, absence of diseases associated with impaired hemostasis, including negative results of blood tests for thrombophilia genetic factors such as mutations of the MTHFR genes, factor V, prothrombin G 20210A; the degree of VTEC risk is not higher than 1A grade according to the classification of C. Samama and M. Samama in a modification of 1999 with additions by Dobrokhotova and co-authors.
All patients underwent general clinical studies, a coagulogram with the determination of prothrombin time (PT), prothrombin index (PTI), international normalized ratio (INR), activated partial thromboplastin time (APTT), fibrinogen concentrations, D-dimer level, and soluble fibrin monomeric complex (FMC). Deviations from the reference values were the criteria for exclusion from the study.
To assess the venous blood flow in the lower extremities and veins of the pelvis, as well as to exclude possible venous thrombosis, we used a comprehensive ultrasound scan (angioscanning and Doppler sonography), which was performed on a Voluson-E8 Expert BT-12 device.
Given the low risk of developing venous thromboembolic complications, prophylactic measures in the perioperative period consisted of early activation and elastic compression of the lower extremities. Drug prevention of thromboembolic complications in these patients was not used.
Angiosurgical intervention was performed under X-ray guidance. Access to the uterine arteries was carried out by puncture of the right femoral artery by the standard Seldinger technique using local anesthesia with lidocaine solution. As an embolisate, Contour PVA 700–900 microns microemboli and MeriMedical 500–700 microemboli were used. Iopamidol 370 mg/ml was used as X-ray contrast agent. After UAE, hemostasis was achieved using the ExoSeal stapling system. The pressure bandage was not applied. The mean operative time was 21 ± 0.9 min.
A comprehensive assessment of the functional activity of the hemostasis system was performed using platelet count, APTT, PTI and INR, fibrinogen level, soluble FMC and D-dimer. All patients in the dynamics recorded TEG.
When deciphering the thromboelastogram the following key parameters were taken into account:
- R (min) - the time from the moment the specimen is placed in the analyzer until the formation of the first fibrin filaments reflects the formation of thromboplastin and thrombin
- K (min) - the time from the beginning of the formation of a blood clot to the moment of the achievement of a fixed level of clot strength, when the magnitude of the amplitude (according to the characteristics of the device) reaches 20 mm; this parameter characterizes the formation of fibrin and reflects the kinetics of increasing clot strength;
- angle α (hail) – angle which is built tangentially to the thromboelastogram from the point of the beginning of the clot formation, displays the growth rate of the fibrin network and the increase in the strength of the clot;
- MA (maximum amplitude) - the greatest distance by which the branches of the thromboelastogram diverge, reflects the maximum dynamic properties of the compound of fibrin and platelets via GPIIb/IIIa receptors, that is, the maximum strength of the clot. MA is 80% due to the number and properties (ability to aggregate) of platelets, 20% - due to the amount of fibrin formed.
LY30 is an indicator of 30-minute lysis, determined by the percentage decrease of the area under the curve for 30 minutes; it characterizes the activity of the fibrinolysis system.
The coagulogram parameters were studied on an ACL Elit Pro automatic coagulometer (USA). To determine the APTT, PT/PT by Kvik test, we used clotting methods based on determining the time interval from the addition of the starting reagent, which triggers the plasma cascade, until the formation of a clot (fibrin deposition). INR was calculated automatically as the ratio of the patient’s PT to the PT of the control plasma, taking into account the international sensitivity index. Fibrinogen was determined by turbodimetric method. D-dimer was determined by the immunological method. The panel of these tests was performed with reagents company HemosIL (Instrumentation Laboratory, USA). The qualitative and quantitative determination of soluble FMC was carried out using the ortho-phenanthroline test using reagents produced by Tekhnolog-Standart, Russia.
TEG registration was performed using a TEG 5000 thromboelastograph with samples of venous blood stabilized with sodium citrate using a kaolin cuvette. When conducting tests, we used reagents of the company HAEMONETICS, USA.
The control of the above parameters was carried out at the following stages:
- on admission of the patient to the operating room (baseline);
- 2 hours after occlusion of the uterine arteries;
- 24 hours after the operation;
- 48 hours after the operation.
The study was approved by the local ethics committee. All people participating in the study gave informed consent.
Statistical processing of the results was carried out on a personal computer using Statistica Version 7.0. Checking the normal distribution of quantitative indicators was performed using the Shapiro-Wilk criterion. For normally distributed samples, the following characteristics were calculated: arithmetic average (M), standard deviation (SD). The data in this case are presented as mean values and standard deviation – M (SD). For samples whose distribution differed from the normal, the median (Me), the first (Q1) and third (Q3) quartiles were calculated, and the data are presented as the median and interquartile range (Me (Q1; Q3). For comparison with the initial level, the Student’s paired criterion was used when complying with the normal distribution law; in case of failure to comply with the normal distribution law, the Wilcoxon criterion was used. Two compared values were considered statistically significant at a significance level p <0.05.
Results
The average age of patients was 35.8 (3.23) years; body mass index was 26.58 (2.34). All patients corresponded to the ASA grades I-II of anesthetic risk. The accompanying pathology was characterized by a stage of compensation or remission.
The size of myoma nodes did not exceed 70 mm in diameter, averaging 39.5 (13.83) mm. Myoma was multiple and the nodes had different localization (except UAE, subserous nodes on a thin base).
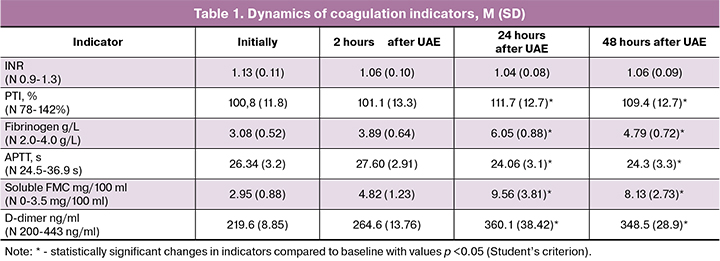
When assessing the initial indicators of coagulation hemostasis and TEG in patients before UAE, no deviations from the standard values were detected. So, the APTT indicators were 26, 34 (3.2) s, INR 1.13 (0.11), PTI 100.8% (11.8%). The markers of thrombinemia and fibrinolysis, soluble FMC and D-dimer, did not exceed the standard values and were 2.95 (0.88) mg/100 ml and 219.6 (8.85) ng/ml, respectively. TEG values indicated baseline normal coagulation.
In the postoperative period, a statistically significant shortening of the APTT was noted. The minimum values of this indicator were recorded 24 hours after the operation and amounted to 24.3 (3.1) s, which corresponded to 91.4% of the initial values (p=0.007). At the same time, a statistically significant increase in PTI was observed to 111.7% (12.7%) which corresponded to 91.4% of the initial values (p=0.009). However, it should be emphasized that the values of these indicators did not go beyond the reference values during the entire study period.
A more significant and dynamic indicator in the course of monitoring the operated patients was the concentration of fibrinogen. The maximum values of the fibrinogen concentration reached 24 hours after the UAE, exceeding the initial values almost twice, and amounted to 6.05 (0.88) g/L.
In the course of the study, we recorded a significant increase in the index of soluble FMC and D-dimer. Changes in these indicators were most pronounced, 24 hours after the UAE, so the concentration of D-dimer was 360.1 (38.42) ng/ml, 163.9% of the initial values (p<0.001), soluble FMC 9.56 (3.81) mg/ 100 ml, 324% of the initial values (p<0.001), statistically significantly exceeding the initial values, at the same time soluble FMC indicator exceeded the upper limit of reference values. The increase in the concentration of these indicators shows an intense intravascular fibrin formation and at the same time activation of the thrombolytic process. The complete data on the dynamics of coagulogram indices are given in Table 1.
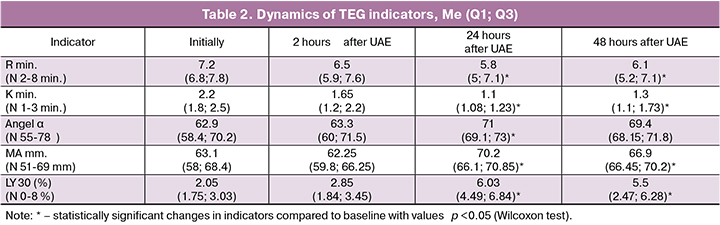
In the dynamic assessment of TEG indicators, the estimated indicators did not go beyond the reference values, nevertheless, a clear trend was observed showing an increase in blood viscosity over time and high functional activity of platelets. Thus, 24 hours after UAE we revealed a statistically significant shortening of R time to 5.8 (5; 7.1)* min, 80.5% of the initial values (p=0.009), K to 1.1 ± (1.08; 1.23) minutes, 50% of the initial values (p=0.002), increasing the angle α to 71 (69.1; 73)* degrees, 112.9 % of the initial values (p=0.03), an increase in the MA value to 70.2 (66.1; 70.85) mm, 111.3% of the initial values (p=0.02). All the above data are suggestive of the activation of the enzymatic part of the coagulation cascade, acceleration of first fibrin strands formations, an increase in growth rate of fibrin network, an enhancement of fibrin polymerization and an increase in clot strength in comparison with the initial values. The estimation of the area under the thromboelastometry curve over the next 30 minutes after reaching the maximum amplitude indicates an increase in fibrinolotic activity and the development of compensatory secondary fibrinolysis. The clot lysis at 30 minutes after 24 hours after the UAE was 6.03% (4.49%; 6.84%), 294.1% of the initial values (p=0.002). The full data of the dynamics of TEG parameters are given in Table 2.
Discussion
The results indicate that the postoperative period after UAE is accompanied by activation of the hemostatic system, which is characterized by a tendency to hypercoagulation and thrombus formation. In patients after UAE, the level of hypercoagulable markers (fibrinogen, D-dimer, soluble FMC) increased in the blood, PTI increased and APTT decreased. All patients after one day after UAE, had is a tendency to hypercoagulation according to TEG data (a decrease in R, K and an increase in α, an increase in MA) with simultaneous activation of fibrinolysis (an increase in LY 30), which generally indicates an increase in risk of VTEC in the postoperative period after UAE.
The data presented by us in general do not contradict studies previously conducted in patients subjected to UAE [21, 22, 23, 24].
In the overwhelming majority of VTEC cases described in the literature, including fatal and non-fatal pulmonary embolism after UAE, there were no predisposing factors in the history, including mutations leading to thrombophilia, during the perioperative examination. [14, 15, 19, 25, 26].
On the first day after UAE, there is a significant decrease in the maximum blood flow velocity in the uterine arteries [27, 28]. Reducing the flow of arterial blood to the uterus primarily leads to organ ischemia with the subsequent development of necrobiotic and necrotic changes in the leiomyoma tissues and to a lesser extent in the myometrium [29]. Slowing blood flow affects the uterine venous system, to some extent altered due to the presence of fibroids. These changes are the pathological basis for the development of surgical stress response, manifested as immunobiochemical changes and the activation of the hemostasis system with the development of hypercoagulable shifts. According to Virchow’s theory, hypercoagulation, stasis and damage to the vessel wall are the main causes of thrombosis. Tissue factor present in the damage zone triggers the formation of active forms of coagulation factors, which in turn can diffuse into the bloodstream. The appearance of thrombin in nanomolar quantities in some area of the blood flow is enough for sudden activation of the factors XI, IX, VIII. And they continue the cascade of reactions leading again to thrombin [30]. The process of thrombosis can be started both in the veins of the lower extremities and in the pelvic veins. In the latter case, compression stockings will not have a preventive effect. In view of the above, it is necessary to consider the feasibility of using direct anticoagulants for thromboprophylaxis with UAE.
Thus, additional studies are needed to develop effective measures for the prevention of VTEC in patients with uterine artery embolization.
Conclusions
- In patients who underwent UAE, in the absence of medical prophylaxis of thrombotic complications on the 2nd day of the postoperative period, a statistically significant increase in fibrinogen occurred; the level of hypercoagulation markers increased (D-dimer, soluble FMC), PTI increased and APTT decreased. In all patients 24 hours after UAE, there was a tendency to hypercoagulation according to TEG data (a decrease in R, K time and an increase in α, an increase in MA), which is generally suggestive of an increase in the risk of VTEC in the postoperative period after UAE.
- The revealed features of changes in hemostasiological parameters in patients after UAE, indicate the need for continuous monitoring of the hemostasis system and the development of effective measures for the prevention of VTEC.
References
- de Bruijn A.M., Ankum W.M., Reekers J.A., Birnie E., van der Kooij S.M., Volkers N.A., Hehenkamp W.J. Uterine artery embolization vs hysterectomy in the treatment of symptomatic uterine fibroids: 10-year outcomes from the randomized EMMY trial. Am. J. Obstet. Gynecol. 2016; 215(6): 745. e1–745. e12. https://dx.doi.org/10.1016/j.ajog.2016.06.051.
- Доброхотова Ю.Э., Капранов С.А., ред. Эмболизация маточных артерий в практике акушера-гинеколога. М.: Литтерра; 2011. [Dobrokhotova Yu.E., Kapranov S.A., eds. Embolizatsiya matochnykh arterii v praktike akushera-ginekologa. Moscow: Litterra; 2011. (in Russian)]
- Гришин И.И., Хачатрян А.С., Ибрагимова Д.М., Доброхотова Ю.Э. Лечение субмукозных миоматозных узлов методом эмболизации маточных артерий. Акушерство и гинекология. 2014; 10: 48–51. [Grishin I.I., Khachatryan A.S., Ibragimova D.M., Dobrokhotova Yu.E. Submucosal myomatous nodules treated by uterine artery embolization. Akusherstvo i ginekologiya/Obstetrics and Gynegology. 2014; (10): 48–51. (in Russian)]
- Ситкин И.И. Эмболизация маточных артерий – эффективный и безопасный метод лечения миомы матки. Вестник репродуктивного здоровья. 2011; 2: 11–7. [Sitkin I.I. Embolization of uterine arteries is an effective and safe method of treating myoma of uterus. Vestnik reproduktivnogo zdorov’ya/Bulletin of Reproductive Health. 2011; (2): 11–7. (in Russian)]
- Савельева Г.М., Сухих Г.Т., Серов В.Н., Радзинский В.Е., ред. Гинекология. Национальное руководство. М.: ГЭОТАР-Медиа; 2019. [Savel’eva G.M., Sukhikh G.T., Serov V.N., Radzinskii V.E., eds. Ginekologiya. Natsional’noe rukovodstvo. Moscow: GEOTAR-Media; 2019. (in Russian)]
- Савельева Г.М., Бреусенко В.Г., Краснова И.А., Капранов С.А., Шиповский В.Н., Бобров Б.Ю., Арютин Д.Г., Аксенова В.Б., Ваганов Е.Ф. Эмболизация маточных артерий в лечении миомы матки, современное состояние вопроса. Журнал акушерства и женских болезней. 2010; 59(2): 81–7. [Savel’eva G.M., Breusenko V.G., Krasnova I.A., Kapranov S.A., Shipovskii V.N., Bobrov B.Yu., Aryutin D.G., Aksenova V.B., Vaganov E.F. Embolizatsiya matochnykh arterii v lechenii miomy matki, sovremennoe sostoyanie voprosa. Zhurnal akusherstva i zhenskikh boleznei/Journal of Obstetrics and Women’s Diseases. 2010; 59(2): 81–7. (in Russian)]
- Walker W.J., Pelage J.P. Uterine artery embolisation for symptomatic fibroids: clinical results in 400 women with imaging follow up. BJOG. 2002; 109(11): 1262–72. https://dx.doi.org/10.1046/j.1471-0528.2002.01449.x.
- Fonseca M.C.M., Castro R., Machado M., Conte T., Girao M.J.B.C. Uterine artery embolization and surgical methods for the treatment of symptomatic uterine leiomyomas: a systemic review and meta-analysis followed by indirect treatment comparison. Clin. Ther. 2017; 39(7): 1438–55. https://dx.doi.org/10.1016/j.clinthera.2017.05.346.
- Хабаров Д.В., Сюткина И.П., Королева Е.Г., Смагин А.А., Кочеткова М.В., Демура А.Ю. Динамика маркёров стресс-реакции при эмболизации маточных артерий. Бюллетень сибирской медицины. 2017; 16(3): 156–65. [Khabarov D.V., Siutkina I.P., Koroleva E.G., Smagin A.A., Kochetkova M.V., Demura A.Yu. The dynamics of the stress response markers in uterine artery embolization. Byulleten’ cibirskoi meditsiny//Bulletin of Siberian Medicine. 2017; 16(3): 156–65. (in Russian)]
- Сюткина И.П., Хабаров Д.В., Королёва Е.Г., Смагин А.А.Оптимизация периоперационного обезболивания при эмболизации маточных артерий с помощью маркёров стресс-реакции. Анестезиология и реаниматология. 2018; 63(1): 32-7. [Syutkina I.P., Khabarov D.V., Koroleva E.G., Smagin A.A. Optimization of perioperative analgesia techniques during uterine artery embolization by means of the analysis of stress-response markers. Anesteziologiya i reanimatologiya/ Russian Journal of Anаеsthesiology and Reanimatology. 2018; 63(1): 32–7. (in Russian)]
- Сюткина И.П., Хабаров Д.В., Ракитин Ф.А., Щедрова В.В. Комплексная оценка периоперационного периода при эмболизации маточных артерий на основе анализа маркёров стресс-реакции и допплерометрического контроля редукции маточного кровотока. Акушерство и гинекология. 2018; 10: 64–70. https://dx.doi.org/10.18565/aig.2018.10.64–70. [Syutkina I.P., Khabarov D.V., Rakitin F.A., Shchedrova V.V. Comprehensive assessment of the postoperative period after uterine artery embolization based on stress response markers and Doppler imaging of uterine blood flow reduction. Akusherstvo i ginekologiya/Obstetrics and Gynegology. 2018; (10): 64–70. https://dx.doi.org/10.18565/aig.2018.10.64–70. (in Russian)]
- Любошевский П.А., Артамонова Н.И., Овечкин А.М. Нарушения гемостаза как компонент хирургического стресс-ответа и возможности его коррекции. Анестезиология и реаниматология. 2012; 3: 44–8. [Lyuboshevsky P.A., Artamonova N.I., Ovechkin A.M. Haemostasis disturbances as the component of the surgical stress-response and possibilities of their correction. Anesteziologiya i reanimatologiya/ Russian Journal of Anаеsthesiology and Reanimatology. 2012; (3): 44–8. (in Russian)]
- Memtsa M., Homer H. Complications associated with uterine artery embolization for fibroids. Obstet. Gynecol. Int. 2012; 2012: 290542. https://dx.doi.org/10.1155/2012/290542.
- Hamoda H., Tait P., Edmonds D.K. Fatal pulmonary embolus after uterine artery fibroid embolisation. Cardiovasc. Intervent. Radiol. 2009; 32(5): 1080–2. https://dx.doi.org/10.1007/s00270-009-9589-4.
- So-Young Park, Hyuk Jung. Fatal pulmonary thromboembolism after uterine artery embolisation for uterine myoma: a case report. Korean J. Obstet. Gynecol. 2011; 54: 62–5. https://dx.doi.org/10.5468/KJOG.2011.54.1.62.
- Toor S.S., Jaberi A., Macdonald D.B., McInnes M.D., Schweitzer M.E., Rasuli P. Complication rates and effectiveness of uterine artery embolization in the treatment of symptomatic leiomyomas: a systematic review and meta-analysis. AJR Am. J. Roentgenol. 2012; 199(5): 1153–63. https://dx.doi.org/10.2214/AJR.11.8362.
- Доброхотова Ю.Э., Бенедиктова М.Г., Леонтьев С.Г., Аллахвердиев С.А. Прогнозирование и профилактика тромботических осложнений у больных с миомой матки после эмболизации маточных артерий и гистерэктомии. Вестник Российского государственного медицинского университета. 2009; 1: 35–9. [Dobrokhotova Yu.E., Venediktova M.G., LeontyevS.G., AIIakhverdiyev S.A. Prognostication and prophylaxis of thrombotic complication after uterine artery embollisation and hysterectomy in patients with hysteromyoma. Vestnik Rossiiskogo gosudarstvennogo meditsinskogo universiteta (RSMU)/Bulletin of RSMU. 2009; (1): 35–9. (in Russian)]
- Samama Ch.M., Samama M.M. Prevention of venous thromboembolism. In: 7th Congress of European Society of Anaesthesiology. Amsterdam, The Netherlands, May 29 – June 1 1999. Amsterdam; 1999: 39–43.
- Qiu J., Fu Y., Huang X., Shu L., Xu J., Lu W. Acute pulmonary embolism in a patient with cesarean scar pregnancy after receiving uterine artery embolization: a case report. Ther. Clin. Risk Manag. 2018; 14: 117–20. https://dx.doi.org/10.2147/TCRM.S147754.
- Антропова Е.Ю., Коробов В.В., Куртасанова Е.С. Усовершенствование лечебно-профилактических мероприятий с целью предупреждения развития тромботических осложнений у больных миомой матки после эмболизации маточных артерий. Медицинский альманах. 2010; 3: 86–9. [Antropova E.Yu., Korobov V.V., Kurtasanova E.S. The study of risk factors of development of thrombotic complications of patients with hysteromyoma after embolization of uterine arteries. Meditsinskii al’manakh/Medical Almanac. 2010; (3): 86–9. (in Russian)]
- Рогожина И.Е., Хворостухина Н.Ф. Влияние эмболизации маточных артерий на систему гемостаза у больных миомой матки. Известия высших учебных заведений. Поволжский регион. Медицинские науки. 2011; 3: 96–105. [Rogozhina I.E., Khvorostukhina N.F. Vliyanie embolizatsii matochnykh arterii na sistemu gemostaza u bol’nykh miomoi matki. Izvestiya vysshikh uchebnykh zavedenii. Povolzhskii region. Meditsinskie nauki. 2011; (3): 96–105. (in Russian)]
- Johansson P.I., Stissing T., Bochsen L., Ostrowski S.R. Thromboelastography and thromboelastometry in assessing coagulopathy in trauma. Scand. J. Trauma Resusc. Emerg. Med. 2009; 17: 45. https://dx.doi.org/10.1186/1757-7241-17-45.
- Nikolic B., Kessler C.M., Jacobs H.M., Abbara S., Ammann A.M., Neeman Z. et al. Changes in blood coagulation markers associated with uterine artery embolization for leiomyomata. J. Vasc. Intervent. Radiol. 2003; 14(9, Pt. 1): 1147–53. https://dx.doi.org/10.1097/01.rvi.0000086540.44800.fe.
- Freed M.M., Spies J.B. Uterine artery embolization for fibroids: a review of current outcomes. Semin. Reprod. Med. 2010; 28(3): 235–41. https://dx.doi.org/10.1055/s–0030-1251480.
- Czeyda-Pommersheim F., Magee S.T., Cooper C., Hahn W.Y., Spies J.B. Venous thromboembolism after uterine fibroid embolization. Cardiovasc. Intervent. Radiol. 2006; 29(6): 1136–40. https://dx.doi.org/10.1007/s00270-005-0245-3.
- Ватутин Н.Т., Тарадин Г.Г., Костогрыз В.Б., Костогрыз А.И., Колесников В.С., Столика О.И., Борт Д.В. Успешное лечение тромбоэмболии легочной артерии, возникшей после эмболизации маточной артерии, у пациентки с фибромой матки. Акушерство и гинекология. 2017; 1: 103–7. https://dx.doi.org/10.18565/aig.2017.1.103–7. [Vatutin N.T., Taradin G.G., Kostogryz V.B., Kostogryz A.I., Kolesnikov V.S., Stolika O.I., Bort D.V. Successful treatment for pulmonary embolism arising after uterine artery embolization in a patient with uterine fibroid. Akusherstvo i ginekologiya/Obstetrics and Gynegology. 2017; (1): 103–7. https://dx.doi.org/10.18565/aig.2017.1.103–7. (in Russian)]
- Титова Г.П., Дамиров М.М., Коков Л.С., Олейникова О.Н.,Белозеров Г.Е. Структурные изменения в тканях матки после эмболизации маточных артерий у больных лейомиомой, осложненной маточным кровотечением. Гинекология. 2018; 20(1): 78–82. [Titova G.P., Damirov M.M., Kokov L.S., Oleynikova O.N., Belozerov G.E.Structural changes in uterine tissues after uterine artery embolization in patients with leiomyoma complicated by uterine bleeding. Ginekologiya/ Gynecology. 2018; 20(1): 78–82. (in Russian)].
- Ибрагимова Д.М., Литвинова Н.А., Алиева А.А., Гришин И.И., Доброхотова Ю.Э. Неинвазивные методы оценки состояния мио- и эндометрия пациенток с миомой матки до и после эмболизации маточных артерий в репродуктивном периоде. Журнал акушерства и женских болезней. 2009; 58(5): 113–4. [Ibragimova D.M., Litvinova N.A., Alieva A.A., Grishin I.I., Dobrokhotova Yu.E. Neinvazivnye metody otsenki sostoyaniya mio- i endometriya patsientok s miomoi matki do i posle embolizatsii matochnykh arterii v reproduktivnom periode. Zhurnal akusherstva i zhenskikh boleznei/Journal of Obstetrics and Women’s Diseases. 2009; 58(5): 113–4. (in Russian)].
- Озерская И.А. Эхография в гинекологии. М.: Видар-М; 2013. [Ozerskaya I.A. Ekhografiya v ginekologii. Moscow: Vidar-M; 2013. (in Russian)].
- Атауллаханов Ф.И., Румянцев А.Г. Новые представления о свертывании крови. Российский журнал детской гематологии и онкологии. 2018; 5(3): 13–22. [Ataullakhanov F.I., Rumyantsev A.G.New insights into the blood clotting. Rossiiskii zhurnal detskoi gematologii i onkologii/ Russian Journal of Pediatric Hematology аnd Oncology. 2018; 5(3): 13–22. (in Russian)].
Received 18.04.2019
Accepted 19.04.2019
About the Authors
Irina P. Siutkina, MD, Researcher, Surgical Lymphology and Lymphodetoxication Laboratory, Department of Anesthesiology and Intensive Care, Research Institute of Clinical and Experimental Lymрhology – Branch of the Institute of Cytology and Genetics, Siberian Branch of Russian Academy of Sciences, 2, Timakova str., Novosibirsk, Russia 630117. E-mail: komarok777@mail.ruDmitriy V. Khabarov, MD, PhD, Senior Researcher of Surgical Lymphology and Lymphodetoxication Laboratory, Head of Department of Anesthesiology and Intensive Care, Research Institute of Clinical and Experimental Lymрhology – Branch of the Institute of Cytology and Genetics, Siberian Branch of Russian Academy of Sciences, 2,
Timakova str., Novosibirsk, Russia 630117. E-mail: hdv@ngs.ru
Fedor A. Rakitin, MD, Head of Department of Gynecology, Research Institute of Clinical and Experimental Lymрhology – Branch of the Institute of Cytology and Genetics, Siberian Branch of Russian Academy of Sciences, 2, Timakova str., Novosibirsk, Russia 630117. E-mail: rakitinfedorr@mail.ru
Marya V. Kochetkova, MD, Researcher, Surgical Lymphology and Lymphodetoxication Laboratory, Department of Anesthesiology and Intensive Care, Research Institute
of Clinical and Experimental Lymphology Siberian Branch of Russian Academy of Sciences, 2, Timakova str., Novosibirsk , Russia 630117. E-mail: masha0112@mail.ru
Alisa D. Inyoshina, student, Novosibirsk State University, V.Zelman Institute for Medicine and Psychology, 3 course, 1, Pirogova str., Novosibirsk , Russia 630090.
For citation: Syutkina I.P., Khabarov D.V., Rakitin F.A., Kochetkova M.V., Ineshina A.D. Integrated assessment of perioperative changes in the blood coagulating system during uterine artery embolization.
Akusherstvo i Ginekologiya/ Obstetrics and gynecology. 2019; 12: 133-9. (In Russian).
https://dx.doi.org/10.18565/aig.2019.12.133-139



