Dynamic control of hemostasis during organ-sparing surgery in gynecologic practice
Siutkina I.P., Demura А.Yu., Rakitin F.A., Kochetkova M.V., Khabarov D.V.
Objective: This study aimed to examine changes in the hemostatic system and determine the presence or absence of a tendency for thrombus formation with the prophylactic use of tranexamic acid during conservative laparoscopic myomectomy.
Materials and methods: This study included 33 patients with multiple uterine fibroids, anemic syndrome, and a low risk of venous thromboembolism (VTE) who underwent surgery, including laparoscopic myomectomy. Two days before surgery, all patients received iron carboxymaltose infusion, and intraoperatively, they received tranexamic acid at a dose of 20 mg/kg. We conducted dynamic monitoring of coagulation and thromboelastography parameters, as well as ultrasound screening of veins in the lower extremities on the 6th day after surgery.
Results: The use of tranexamic acid resulted in hypercoagulable changes within the first 24 h after surgery, but these changes remained within the compensatory-adaptive limits. On the 6th day after surgery, patients still showed a tendency towards hypercoagulation, with elevated levels of fibrinogen, D-dimer, and SFMCs exceeding the reference values. This tendency was associated with the extent of surgery and initial anemia, indicating an ongoing risk of VTE, including hidden thrombosis.
Conclusion: The identified tendency towards hypercoagulation in patients with multiple uterine fibroids after laparoscopic conservative myomectomy necessitates constant monitoring of the hemostatic system and the development of effective measures to prevent VTE, including the use of low-molecular-weight heparins.
Authors' contributions: Siutkina I.P., Khabarov D.V. – conception and design of the study; Siutkina I.P., Rakitin F.A.,
Demura A.Yu. – material collection and processing; Siutkina I.P., Kochetkova M.V. – statistical analysis; Siutkina I.P. – drafting of the manuscript; Khabarov D.V. – editing of the manuscript.
Conflicts of interest: The authors have no conflicts of interest regarding the publication of this article.
Funding: Research Institute of Clinical and Experimental Lymрhology – Branch of the Institute of Cytology and Genetics, Siberian Branch of Russian Academy of Sciences.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the Research Institute of Clinical and Experimental Lymрhology – Branch of the Institute of Cytology and Genetics, Siberian Branch of Russian Academy of Sciences.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Siutkina I.P., Demura А.Yu., Rakitin F.A., Kochetkova M.V., Khabarov D.V.
Dynamic control of hemostasis during organ-sparing surgery in gynecologic practice.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2024; (4): 87-92 (in Russian)
https://dx.doi.org/10.18565/aig.2023.208
Keywords
Uterine fibroids pose both medical and social challenges in medicine and society. Imaging techniques indicate that this condition affects 80% of women and up to 30% of women of childbearing age [1, 2]. Currently, organ-sparing surgery, specifically laparoscopic conservative myomectomy, is the preferred approach [3, 4]. Although reducing the extent of surgical intervention can minimize perioperative complications, they cannot be completely eliminated [3–6].
One of the difficulties encountered during the perioperative period is the management of anemia caused by recurrent menometrorrhagia. The risk of significant intraoperative blood loss further emphasizes the need for a comprehensive approach to address this issue, including the use of hemostatic therapies, such as fibrinolysis inhibitors [7–10]. Additionally, preventing venous thromboembolism (VTE) is crucial, as the rate of VTE during gynecological interventions ranges from 0.98% to 5.3% [11–14]. This rate increases to 5.1% to 12% with the use of active diagnostic methods such as ultrasound and/or fibrinogen absorption test [12]. Moreover, surgeries involving multiple uterine fibroids typically last over 45 min [15], significantly increasing the risk of VTE according to J. Caprini scale. In some cases, VTE may not manifest clinically immediately after surgery but can appear after the patient has been discharged from the hospital [12, 14]. The true incidence of VTE following organ-sparing laparoscopic uterine surgery remains unclear, and the mechanisms underlying this complication are not yet fully understood. Thromboelastography, including its application during gynecological operations, provides valuable insights into hemostasis [16]. However, few studies have investigated the changes in hemostasis parameters during minimally invasive gynecological interventions.
Therefore, it is essential to optimize therapeutic, diagnostic, and preventive measures to address dynamic control of the hemostatic system, minimize intraoperative blood loss, and prevent VTE in patients with uterine fibroids.
This study aimed to explore changes in the hemostatic system and determine whether there is a predisposition to thrombus formation when tranexamic acid is prophylactically used during laparoscopic conservative myomectomy.
Materials and methods
The study included 33 patients treated in the Gynecological Department of the Research Institute of Clinical and Experimental Lymрhology – Branch of the Institute of Cytology and Genetics Siberian Branch of the RAS. The patient had multiple symptomatic uterine fibroids with iron deficiency anemia and iron deficiency confirmed on the outpatient stage, and planned surgical treatment including laparoscopic conservative myomectomy. The inclusion criteria were hemoglobin levels from 90 to 105 g/L, no personal or family history of VTE, and VTE risk assessment according to J. The Caprini scale was no more than 2 points (low risk); the anesthetic risk was no more than II according to the American Society of Anesthesiologists (ASA) scale.
All patients underwent dynamic monitoring of a complete blood count (CBC) and a comprehensive dynamic analysis of changes in the coagulation system, with assessment of changes in both thromboelastogram (TEG) and basic coagulation parameters, including activated partial thromboplastin time (APTT), prothrombin index (PTI), fibrinogen, D-dimer, and soluble fibrin monomer complexes (SFMCs).
The coagulation study was carried out on an automatic coagulometer ACL Elit Pro using reagents from HemosIL. APTT and PTI were determined using clotting methods, fibrinogen using the turbidimetric method, and D-dimer using the immunological method. SFMCs were determined by the ortho-phenanthroline test using reagents from Tekhnologiya-Standard Company. TEG was performed with venous blood stabilized with sodium citrate using kaolin cuvettes on a TEG 5000 apparatus (HAEMONETICS). The monitored parameters included R, K, angle α°, 30-minute lysis indicator (LY30), and maximum amplitude (MA).
Scanning and Doppler examinations of the lower extremity veins were performed on the day of admission using a Voluson-E8 Expert VT-12 device. A follow-up examination to identify subclinical forms of thrombosis was performed on the 6th postoperative day six.
Monitoring of the parameters of blood flow, hemostasis, and TEG was carried out: 1 – on the day of hospitalization; 2 – after achieving surgical hemostasis during surgery; 3 – one day after myomectomy; 4 – 6 days after myomectomy.
As all patients enrolled in this study were at low risk of developing VTE, no drug prophylaxis was used. During the perioperative period, elastic compression of the lower extremities was performed in combination with early ambulation.
Two days before surgery, the patients received an intravenous infusion of iron carboxymaltose with individualized dose calculation in milligrams of elemental iron. Total iron deficiency was calculated using the Ganzoni formula: iron need (mg iron) = body weight (kg) × (target Hb – actual Hb) × 2.4 + depot iron (mg iron). The target hemoglobin level was 150 g/l, and the depot iron level was 500 mg for patients weighing more than 35 kg.
All patients underwent laparoscopic myomectomy under general anesthesia using a standard technique. To reduce intraoperative blood loss, all patients received an infusion of tranexamic acid at a dose of 20 mg/kg in the operating room after pre-medication.
Statistical analysis
Statistical analysis was performed using Statistica 10.0. The distribution of continuous variables was tested for normality using the Shapiro–Wilk test. Continuous variables showing a normal distribution were expressed as mean (M) and standard deviation (SD); otherwise, the median (Me) with interquartile range (Q1; Q3) was reported. Repeated measurements obtained at different time points were compared using Friedman’s test. Normally distributed continuous variables were compared between the two groups using Student’s t-test. Non-normally distributed data were analyzed using the Wilcoxon test. When comparing two variables, the difference was considered statistically significant at p<0.05.
The study was reviewed and approved by the Research Ethics Committee of the Research Institute of Clinical and Experimental Lymрhology – Branch of the Institute of Cytology and Genetics, Siberian Branch of the Russian Academy of Sciences.
Results
All patients in the study were younger than 40 years, with a mean age of 35.16 (3.81) years. According to the ultrasound, the number of fibroids varied: minimum, 2; maximum, 8; their location was interstitial or interstitial-subserous. The mean diameter of the fibroids was 47.51 (17.68) mm, and the maximum diameter of the dominant fibroid was 120.3 mm. The mean preoperative uterine volume was 324 (29.68) ml.
All patients had mild anemia with a mean hemoglobin level on admission of 101.9 (5.6) g/l, after administration of iron carboxymaltose on the day of surgery it was 115.7 (6.7) g/l, on the 2nd day after surgical treatment it was 105.3 (5.1) g/l.
Comorbidities in patients were in the stage of compensation or remission. The risk of VTE when assessed according to the J. The Caprini scale score was no more than 2 points.
Surgical intervention (laparoscopic conservative myomectomy) was performed as planned under general anesthesia using endotracheal anesthesia with sevoflurane, fentanyl, and rocuronium. The mean duration of the surgical interventions was 1.43 (0.26) hours. Considering the patients’ weight of 69.47 (5.36) kg, the estimated circulating blood volume (CBV) was 4.86 (0.38) l. The mean intraoperative blood loss was 0.383 (0.047) ml, 7.9 (0.97) of the total volume. All patients had drainage installed during surgery, which was removed on the 1st day postoperatively. The mean drainage loss was 30 (6.3) ml. No postoperative complications occurred.
In the preoperative period, the basic coagulation parameters, APTT, and PTI were within the reference values. Indicators of SFMCs and D-dimer levels within the normative values indicated the absence of thrombinemia and fibrinolysis. According to the TEG data, coagulation was normal.
Changes in coagulation hemostasis parameters in the postoperative period were characterized by a 15% decrease in aPTT and a 22% increase in PTI from the preoperative level. 24 hours after the operation, the minimum aPTT was 22.56 (21.55; 24.87), and the maximum increase in PTI was 118.30 (104.77; 126.34). The study revealed a dynamic increase in the concentrations of fibrinogen, SFMCs, and D-dimer. An increase in the concentration of these indicators was recorded intraoperatively against the background of the administration and peak effect of tranexamic acid with a further increase and reaching maximum values on the 6th day postoperatively: fibrinogen was 6.41 (5.02; 6.88) g/l, SFMCs was 10 .65 (7.83; 13.47) mg/100 ml, D-dimer was 545 (498.54; 601.24) ng/ml. The changes in coagulation parameters are presented in Table 1.
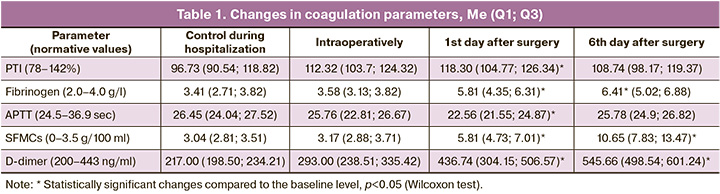
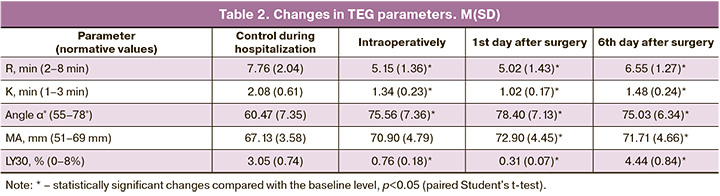
Cumulative evaluation of TEG parameters showed intraoperative increases in thromboplastin, thrombin, and fibrinogen formation rate – decrease of R and K parameters to 5.02 (1.43) and 1.02 (0.17), respectively); increase of overall clot formation rate and clot strength – increase of α angle to 78.4 (7.13), increase of MA value to 72.9 (4.45) mm, which generally indicates perioperative increase in blood viscosity. Statistically significant changes in these parameters from baseline persisted on day 6 after myomectomy.
The observed decrease in LY30 to 0.76 (0.18) % during surgery and to 0.31 (0.07) % on day 1 after surgery, with baseline values of 3.05 (0.74) % (p <0.05), indicates a blockade of fibrinolysis and the formation of a complete clot against the background of a specific targeted effect of tranexamic acid. The changes in the TEG parameters are listed in Table 2.
During the study, clinical manifestations of lower-extremity venous thrombosis were not observed in any patient during hospitalization. Ultrasound examination of the lower extremity veins revealed no thrombosis.
Discussion
The presence of anemic syndrome, the prevalence of which in patients with uterine fibroids reaches 60–80% [8, 9], combined with the risk of significant intraoperative blood loss aggravating the severity of anemia, determines the need for an integrated approach, including the use of modern antianemic agents and gentle surgical techniques with perioperative administration of antifibrinolytic drugs.
Based on the concept of an integrated approach, we first used iron carboxymaltose, which has been shown to be highly effective in correcting preoperative anemia in patients with recurrent BPL metromenorrhagia [9, 17]. The advantage of this drug is its relative safety when administered intravenously, with the possibility of rapid replenishment of iron deficiency and a rapid increase in hemoglobin and red blood cells [9].
Second, to reduce intraoperative blood loss, we used the fibrinolysis inhibitor, tranexamic acid. Regarding the effectiveness of the use of fibrinolysis blockers during laparoscopic myomectomy, the opinions of researchers vary [18–23]. Considering the morphological changes in the myometrium caused by multiple fibroid growth, changes in peri-fibroid and intra-fibroid vessels with disruption of their architecture and motility leading to increased bleeding, including intraoperative bleeding, we considered the administration of tranexamic acid, which blocks fibrinolysis and promotes the formation of a full-blown clot, which is pathogenetically justified and appropriate. The choice of a relatively high dose of 20 mg/kg is due to the fact that fibroid removal and surgical hemostasis in laparoscopic myomectomy is a more technically complex and time-consuming procedure than in open surgery. We have previously shown that the use of tranexamic acid can reduce intraoperative blood loss and avoid significant worsening of anemic syndrome [22].
On the other hand, natural concomitant use of drugs that affect the components of hemostasis increases the risk of developing VTE. It is known that the effective antifibrinolytic concentration of tranexamic acid in tissues lasts for 17 h, in plasma, up to 7–8 hours. During the study, we found that during the period of action of tranexamic acid, data from a cumulative assessment of coagulation and TEG parameters indicated a tendency towards hypercoagulation (decrease in APTT, increase in PTI) and increase in the rate of formation and strength of the clot (decrease in R, K, increase in angle α, MA, decrease in L30), but remained within the reference values. Simultaneously, the concentrations of the main markers of thrombinemia and fibrinolysis (SFMCs and D-dimer) did not change significantly. The data obtained indicate that hypercoagulable changes due to the action of tranexamic acid do not go beyond the scope of compensatory-adaptive reactions.
The persistent tendency of hypercoagulation on the 6th day of the postoperative period, including an increase in the concentrations of markers of activation of the coagulation system (fibrinogen, D-dimer, and SFMCs) exceeding the reference values and an increase in viscosity according to TEG, is apparently associated with the extent of surgery for multiple fibroids against the background of an anemic syndrome at baseline, accompanied by an increase in immature rigid forms of red blood cells, endothelial dysfunction, impaired rheological properties, and activation of platelet aggregation. Data from the study indicate that patients with an anemic syndrome and an initially low J. Caprini Scale score indicates a risk for VTE, including the risk of developing a hidden thrombosis in the long-term postoperative period.
Conclusions
- Dynamic monitoring of coagulation tests and TEG parameters indicated that the use of tranexamic acid to limit intraoperative blood loss does not increase the risk of thromboembolic complications during laparoscopic myomectomy.
- The tendency for hypercoagulation that persists on the 6th day after laparoscopic conservative myomectomy in patients with multiple uterine fibroids and concomitant iron deficiency anemia suggests the need for comprehensive control of the coagulation system, as well as the need for a system of effective measures to prevent VTE, including the use of low-molecular-weight heparins in combination with nonpharmacologic prophylaxis.
References
- Giuliani E., As-Sanie S., Marsh E.E. Epidemiology and management of uterine fibroids. Int. J. Gynaecol. Obstet. 2020; 149(1): 3-9. https://dx.doi.org/10.1002/ijgo.13102.
- Савельева Г.М., Сухих Г.Т., Серов В.Н., Радзинский В.Е., Манухин И.Б., ред. Гинекология. Национальное руководство. М.: ГЭОТАР-Медиа; 2022. 1008с. [Savel’eva G.M., Sukhikh G.T., Serov V.N., Radzinsky V.E., Manukhin I.B., eds. Gynecology. National Guide. Moscow: GEOTAR-Media; 2022. 1008p. (in Russian)].
- Tanos V., Berry K.E., Frist M., Campo R., De Wilde R.L. Prevention and management of complications in laparoscopic myomectomy. BioMed. Res. Int. 2018; 2018: 8250952. https://dx.doi.org/10.1155/2018/8250952.
- Ordás P., Spagnolo E., Fernández L.G., Diestro Tejeda M.D., Lafuente P., Salas P. et al. Comparison of surgical and obstetric outcomes in women with uterine leiomyomas after laparoscopic vs. abdominal myomectomy: a single-center cohort study. Front. Surg. 2022; 9: 997078. https://dx.doi.org/10.3389/fsurg.2022.997078.
- Chen R., Su Z., Yang L., Xin L., Yuan X., Wang Y. The effects and costs of laparoscopic versus abdominal myomectomy in patients with uterine fibroids: a systematic review and meta-analysis. BMC Surg. 2020; 20(1): 55. https://dx.doi.org/10.1186/s12893-020-00703-0.
- Buckley V.A., Nesbitt-Hawes E.M., Atkinson P., Won H.R., Deans R., Burton A. et al. Laparoscopic myomectomy: clinical outcomes and comparative evidence. J. Minim. Invasive Gynecol. 2015; 22(1): 11-25. https://dx.doi.org/10.1016/j.jmig.2014.08.007.
- Catanese A., Siesto G., Cucinella G., Chiantera V., Culmone S., Schiattarella A. et al. Factors influencing surgical outcomes of laparoscopic myomectomy. A propensity-score matched analysis. Prz. Menopauzalny. 2022; 21(3): 149-56. https://dx.doi.org/10.5114/pm.2022.118970.
- Ye M., Zhou J., Chen J., Yan L., Zhu X. Analysis of hidden blood loss and its influential factors in myomectomy. J. Int. Med. Res. 2020; 48(5): 300060520920417. https://dx.doi.org/10.1177/0300060520920417.
- Тихомиров А.Л., Сарсания С.И. Лейомиома матки и ЖДА. Вариант предоперационной подготовки. Медицинский совет. 2019; 13: 178-82. [Tikhomirov A.L., Sarsaniya S.I. Uterine leiomyoma and IDA. Variant of preoperative preparation. Medical Council. 2019; (13): 178-82. (in Russian)]. https://dx.doi.org/10.21518/2079-701X-2019-13-178-182.
- Barbosa P.A., Villaescusa M., Andres M.P., Fernandes L.F.C., Abrão M.S. How to minimize bleeding in laparoscopic myomectomy. Curr. Opin. Obstet. Gynecol. 2021; 33(4): 255-61. https://dx.doi.org/10.1097/GCO.0000000000000725.
- Jorgensen E.M., Li A., Modest A.M., Leung K., Moore Simas T.A., Hur H.C. Incidence of venous thromboembolism after different modes of gynecologic surgery. Obstet. Gynecol. 2018; 132(5): 1275-84. https://dx.doi.org/10.1097/AOG.0000000000002918.
- Jorgensen E.M., Hur H.C. Venous thromboembolism in minimally invasive gynecologic surgery: a systematic review. J. Minim. Invasive Gynecol. 2019; 26(2): 186-96. https://dx.doi.org/10.1016/j.jmig.2018.08.025.
- Сяо Я. Инцидентность венозных тромбозов в европейской популяции: роль хирургических вмешательств. Медицина неотложных состояний. 2017; 4: 24-9. [Siao Y. The incidence of venous thrombosis in the European population: the role of surgical interventions. Emergency Medicine. 2017; (4): 24-9. (in Russian)]. https://dx.doi.org/10.22141/2224-0586.4.83.2017.107421.
- Nicholson M., Chan N., Bhagirath V., Ginsberg J. Prevention of venous thromboembolism in 2020 and beyond. J. Clin. Med. 2020; 9(8): 2467. https://dx.doi.org/10.3390/jcm9082467.
- Kathopoulis N., Prodromidou A., Zacharakis D., Chatzipapas I., Diakosavvas M., Kypriotis К. et al. The effect of intravenous tranexamic acid on myomectomy: a systematic review and meta-analysis of randomized controlled trials. J. Pers. Med. 2022; 12(9): 1492. https://dx.doi.org/10.3390/jpm12091492.
- Сюткина И.П., Хабаров Д.В., Ракитин Ф.А., Кочеткова М.В., Инешина А.Д. Комплексная оценка изменений свертывающей системы крови в периоперационном периоде при эмболизации маточных артерий. Акушерство и гинекология. 2019; 12: 133-9. [Syutkina I.P., Khabarov D.V., Rakitin F.A., Kochetkova M.V., Ineshina A.D. Integrated assessment of perioperative changes in the blood coagulating system during uterine artery embolization. Obstetrics and Gynecology. 2019; (12): 133-9. (in Russian)]. https://dx.doi.org/10.18565/aig.2019.12.133-139.
- Waskowski J., Schefold J.C, Stueber F. Prophylaktische Anwendung von Tranexamsäure in der nichtkardialen Chirurgie: update 2017. [Prophylactic use of tranexamic acid in noncardiac surgery: update 2017]. Med. Klin. Intensivmed. Notfmed. 2019; 114(7): 642-9. (in German). https://dx.doi.org/10.1007/s00063-018-0402-5.
- Opoku-Anane J., Vargas M.V., Marfori C.Q., Moawad G., Maasen M.S., Robinson J.K. Intraoperative tranexamic acid to decrease blood loss during myomectomy: a randomized, double-blind, placebo-controlled trial. Am. J. Obstet. Gynecol. 2020; 223(3): 413.e1-413.e7. https://dx.doi.org/10.1016/j.ajog.2020.02.019.
- Hunt B.J. The current place of tranexamic acid in the management of bleeding. Anaesthesia. 2015; 70(Suppl. 1): 50-3, e18. https://dx.doi.org/10.1111/anae.12910.
- Shaaban M.M., Ahmed M.R., Farhan R.E., Dardeer H.H. Efficacy of tranexamic acid on myomectomy-associated blood loss in patients with multiple myomas: a randomized controlled clinical trial. Reprod. Sci. 2016; 23(7): 908-12. https://dx.doi.org/10.1177/1933719115623646.
- Wang D., Wang L., Wang Y, Lin X. The efficiency and safety of tranexamic acid for reducing blood loss in open myomectomy: a meta-analysis of randomized controlled trials. Medicine (Baltimore). 2017; 96(23): e7072. https://dx.doi.org/10.1097/MD.0000000000007072.
- Кочеткова М.В., Сюткина И.П., Королева Е.Г., Смагин А.А., Хабаров Д.В., Демура А.Ю. Использование карбоксимальтозата железа и транексамовой кислоты при органосохраняющих операциях в гинекологической практике. Современные проблемы науки и образования. 2017; 5: 118. [Kochetkova M.V., Syutkina I.P., Koroleva E.G., Smagin A.A., Khabarov D.V., Demura A.Yu. The use of iron carboxymaltose and tranexamic acid in organ-preserving operations in gynecological practice. Modern Problems of Science and Education. 2017; (5): 118. (in Russian)].
- Жорова В.Е. Терапия железодефицитной анемии у пациенток гинекологического профиля. Медицинский совет. 2019; 7: 148-52. [Zhorova V.E. Therapy of iron-deficiency anemia in gynecological patients. Medical Council. 2019; (7): 148-52. (in Russian)]. https://dx.doi.org/10.21518/2079-701X-2019-7-148-152.
Received 28.08.2023
Accepted 20.03.2024
About the Authors
Irina P. Siutkina, PhD, Researcher, Surgical Lymphology and Lymphodetoxication Laboratory, Department of Anesthesiology and Intensive Care, Research Institute of Clinical and Experimental Lymрhology – Branch of the Institute of Cytology and Genetics, Siberian Branch of Russian Academy of Sciences, Novosibirsk, Russia, komarok777@mail.ru, https://orcid.org/0000-0002-3941-4521Alexander Yu. Demura, Researcher, Surgical Lymphology and Lymphodetoxication Laboratory, Department of Anesthesiology and Intensive Care, Research Institute of Clinical and Experimental Lymрhology – Branch of the Institute of Cytology and Genetics, Siberian Branch of Russian Academy of Sciences, Novosibirsk, Russia, dx_@bk.ru,
https://orcid.org/0000-0001-8470-5400
Fedor A. Rakitin, Head of the Department of Gynecology, Research Institute of Clinical and Experimental Lymрhology – Branch of the Institute of Cytology and Genetics, Siberian Branch of Russian Academy of Sciences, Novosibirsk, Russia, rakitinfedorr@mail.ru
Marya V. Kochetkova, PhD, Researcher, Surgical Lymphology and Lymphodetoxication Laboratory, Department of Anesthesiology and Intensive Care, Research Institute of Clinical and Experimental Lymрhology – Branch of the Institute of Cytology and Genetics, Siberian Branch of Russian Academy of Sciences, Novosibirsk, Russia,
masha0112@mail.ru, https://orcid.org/0000-0002-8752-4151
Dmitriy V. Khabarov, Dr. Med. Sci., Leading Researcher of Surgical Lymphology and Lymphodetoxication Laboratory, Head of the Department of Anesthesiology and Intensive Care, Research Institute of Clinical and Experimental Lymрhology – Branch of the Institute of Cytology and Genetics, Siberian Branch of Russian Academy of Sciences, Novosibirsk, Russia; Professor, Department of Anesthesiology, Novosibirsk State University, hdv@ngs.ru, https://orcid.org/0000-0001-7622-8384



