Clinical and diagnostic features of uterine subinvolution and postpartum endometritis
Postpartum endometritis (PE) is the leading cause of pyoinflammatory complications in the postpartum period. At the same time, almost every second case of PE is associated with reduced contractility of the myometrium, which manifests as subinvolutive dimensions and volume of the postpartum uterus. However, uterine subinvolution (USI) is not only a nosological entity in its own right but also a pre-stage of PE and has several clinical and laboratory features that require prevention, timely diagnosis, and therapeutic intervention. Objective: To investigate the clinical, anamnestic, laboratory, and instrumental diagnostic features of USI in women after spontaneous and operative delivery. Materials and methods: This study retrospectively analyzed 200 records of postpartum women treated at Voino-Yasenetsky Krasnoyarsk State Medical University clinics from 2019 to 2022. Of these, 100 had mild PE, and 100 were diagnosed with uterine subinvolution. Each group was divided into two subgroups consisting of 50 postpartum women after spontaneous birth (SB) and 50 postpartum women after abdominal delivery. Results: Significant differences were observed in the clinical course of the disease among women in the study groups. Complaints in postpartum women with USI occurred 4 days later (day 13) than those in patients with PE (day 9). After abdominal delivery, clinical manifestations were significantly more common than in the SB subgroups (p<0.05), including lower abdominal pain and bloody discharge in all postpartum women after cesarean section (CS); more than half of the patients had purulent discharge 66/100 (66%], p<0.05). In the CS subgroup, 9/50 (18%), 19/50 (38%), and 13/50 (26%) patients had lower abdominal pain, bloody vaginal discharge, and purulent vaginal discharge, respectively. More pronounced inflammatory changes in the blood tests were characteristic of postpartum women with PE (especially in the CS subgroup) due to higher levels of leukocytes, C-reactive protein, and ESR (p<0.05). Subinvolutive dimensions of the uterus were found by ultrasound in 78% of the women with PE. Conclusion: USI has several clinical and laboratory features that can be considered as an isolated postpartum complication. The presence of inflammatory changes in the blood tests confirmed the predominance of the infectious component over the reduced contractile function of the myometrium in patients with PE after CS. In women with SB, reduced contractile function of the myometrium prevails without obvious clinical manifestations or inflammatory changes in the blood tests. The results of the present study emphasize the need for timely diagnosis of subinvolutive changes in the uterus. Authors' contributions: Galkina D.E. – conception and design of the study, statistical analysis; Makarenko T.A. – manuscript editing; Fadeeva T.A., Dresvyanskaya T.V. – material collection. Conflicts of interest: The authors have no conflicts of interest to declare. Funding: There was no funding for this study. Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the Voino-Yasenetsky Krasnoyarsk State Medical University. Patient Consent for Publication: All patients provided informed consent for the publication of their data. Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator. For citation: Galkina D.E., Makarenko T.A., Fadeeva T.A., Dresvyanskaya T.V. Clinical and diagnostic features of uterine subinvolution and postpartum endometritis. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2023; (8): 67-77 (in Russian) https://dx.doi.org/10.18565/aig.2023.87Galkina D.E., Makarenko T.A., Fadeeva T.A., Dresvyanskaya T.V.
Keywords
Timely diagnosis and treatment of purulent-inflammatory postpartum disorders are among the most pressing challenges in modern obstetric-gynecological practice. Postpartum endometritis (PE) is the leading cause of these conditions, with an incidence ranging from 15% to 50% [1, 2]. Notably, in women with a history of cesarean section (CS), the prevalence of this pathology can escalate to 55–85% [3, 4].
The literature indicates a prevailing trend in recent decades towards the emergence of vague clinical presentations of PE, where classical clinical manifestations are only evident in one-third of patients [5]. Additionally, it is important to recognize that complaints and symptoms often surface after postpartum discharge, resulting in delayed diagnosis and treatment [6].
Clinical guidelines from 2016 have classified PE into three forms: mild, moderate, and severe. A vague clinical presentation of PE is also acknowledged, in which initial symptoms might not arise until the seventh postpartum day [7]. Furthermore, PE following operative delivery warrants separate consideration owing to its more severe progression, potentially showcasing symptoms such as bloating, stool retention, gas discharge, and reduced urine excretion [7].
A significant finding revealed that approximately 51% of PE cases are associated with diminished myometrial contractility, consistent with the concept of uterine subinvolution (USI). USI denotes a delayed return of the uterus to its original size and volume within six weeks of delivery [7, 8]. The development of this condition stems from insufficient myometrial contractility during the postpartum period, often attributed to factors such as obstruction of the uterine cavity outflow, accounting for 86.6% of cases. Among these cases, spasm of the internal os contributes to 20.9%; hyper ante- or retroflexion of the uterus to 11.9%; the presence of significant decidual tissue, membranes, or large blood clots to 52.2%; and retained placental fragments to 1.5% [7–9]. Notably, most of these complications arise after uterine surgery, while 13.4% are categorized as idiopathic contractility disorders without an apparent cause [7–9].
Gorin V.S. et al. (2011) introduced a classification of USI based on hysteroscopy data, identifying two types: the first involves blood clot presence in the uterine cavity (essentially a hematometra/lochiometra), and the second relates to remnants of placental tissue [10]. The presence of decidual tissue often contributes to an infectious and inflammatory process within the uterine cavity, potentially establishing a connection between USI and a pre-stage of PE.
An alternative classification distinguishes between two types of USI based on causative factors. These include true subinvolution, which arises after childbirth with a large fetus or multiple pregnancies, as well as in women with high parity. In contrast, infected subinvolution develops in the context of emerging infectious processes, often associated with prolonged ruptured membranes, polyhydramnios, chorioamnionitis, and similar conditions [10, 11]. The first type might be considered an independent form of postpartum complication, while the second type could potentially represent a precursor to PE formation.
In light of these considerations, various opinions exist regarding the involvement of USI in PE development. Some authors view USI as a precursor to endometritis [10, 11], whereas others classify it as a distinct nosological entity, emphasizing discrepancies in clinical presentations and pathomorphological changes compared to classical forms of PE [11, 12]. Importantly, the WHO's 9th revision includes a reference to USI, while ICD-10 assigns it code O90.8, encompassing other postpartum complications that lack specific headings. In ICD-11, it is coded as JB44.Y (other specified complications of the postpartum period) or JB44.Z (unspecified complications during the postpartum period).
The incidence of USI is considerable, accounting for 11–51.5% of all postpartum complications [13]. Echography and hysteroscopy findings indicate USI in 10–31% of postpartum women; notably, in 15–86.7% of cases, USI presents as a full-fledged clinical manifestation of endometritis [13, 14]. It is crucial to acknowledge that conditions such as lochiometra and hematometra are often equated with subinvolutive changes in the uterus, essentially reflecting histopathological alterations associated with reduced contractility.
There is a lack of comprehensive data in the domestic and international literature regarding the primary cause of Uterine Subinvolution (USI). It is generally accepted that contractile hypofunction of the myometrium is linked to several factors, including somatic elements such as concomitant infectious-inflammatory and endocrine status, age-related factors like youth or advanced age, and constitutional factors like obesity, pregnancy complications (such as preeclampsia, polyhydramnios, multiple pregnancies, large fetuses, etc.), and childbirth factors (like rapid, prolonged, premature, or operative labor, labor anomalies, increased blood loss, etc.) [15–17].
However, recent decades have witnessed an increasing number of studies highlighting the genetic, cellular, molecular, and morphological triggers for the development of subinvolutive changes in the postpartum uterus [10]. Several studies have demonstrated that the subinvolutive myometrium experiences myocyte hypotrophy and a reduced number of myocytes per unit of total myometrial area when compared to the normal myometrium [17, 18]. Other studies have proposed that USI is associated with subinvolution of the placental bed vessels, with the expression of the Bcl-2 oncoprotein gene, an apoptosis inhibitor [18, 19]. The role of telocytes in transmitting electrical signals along the smooth muscle fibers of the myometrium is also considered significant. These cells, situated between blood vessels, myocytes, and nerve endings, initiate a slow wave that triggers contraction. Damage to these cells (due to inflammation or death) disrupts impulse conduction, leading to impaired contractile function and ultimately the development of USI [20–22].
Furthermore, the postpartum myometrial receptor apparatus plays a crucial role in ensuring adequate contractility. The myometrium contains receptors for estrogen, progesterone, prostaglandin, oxytocin, histamine, α- and β-adrenergic, and m-cholinergic receptors. Reduced expression of these receptors could stem from abnormal labor activity and decreased synthesis of the multifunctional protein β-arrestin, which is responsible for oxytocin production [23, 24]. The effect of oxytocin on the uterus depends on the level of oxytocin receptors and is related to cAMP and calcium levels [25].
A distinctive group of postpartum patients affected by USI includes those who have undergone operative delivery. Under optimal conditions, with regenerative endometrial function, the uterine cavity naturally expels decidual and necrotic tissues after childbirth. Typically, robust myometrial contractility during the puerperal period results in abundant bloody discharge from the genital tract in the initial 5 days, transitioning to a brownish hue from days 6th to 10th day, while maintaining substantial volume. By the 15th day, lochia assume a moderate yellowish tone, becoming mucous by the third week. However, a delay in evacuating uterine contents (such as blood clots, remnants of placental tissue, or cervical canal rigidity after cesarean section) can lead to an infectious process. Failure to diagnose and prevent this may result in PE [26, 27]. Notably, in postpartum women with endometritis after abdominal delivery, normal uterine regression is observed in only 46–67.2% of patients [27–29].
Despite the incomplete understanding of the comprehensive influences of all factors contributing to USI's development, it is crucial from a clinical standpoint to distinguish USI from other postpartum complications, as the terms 'subinvolution of the uterus' and 'postpartum endometritis' are often used interchangeably.
The aim of this study was to investigate the clinical, anamnestic, laboratory and instrumental diagnostic features of USI in women following spontaneous and operative delivery.
Materials and methods
This study retrospectively analyzed the medical records of 200 postpartum women treated at the Voino-Yasenetsky Krasnoyarsk State Medical University clinics between 2019 and 2022. Of these, 100 had mild PE, and the remaining 100 were diagnosed with uterine subinvolution (Fig. 1). Each group was then divided into two subgroups, consisting of 50 postpartum women who had a spontaneous birth (SB) and 50 postpartum women who had an abdominal delivery.
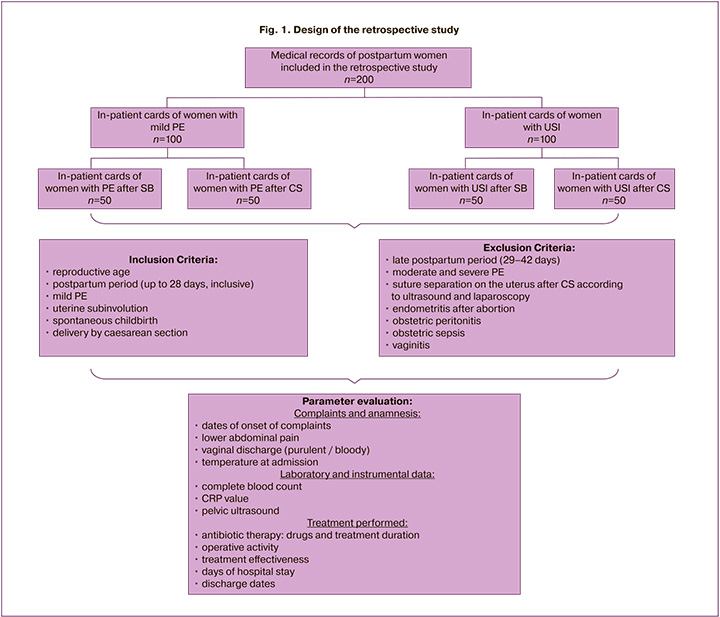
The inclusion criteria were reproductive age, postpartum period (up to 28 days, inclusive), the presence of a diagnosis of mild PE or USI, spontaneous delivery (SB), or delivery by CS.
Exclusion criteria were late postpartum period (days 29–42), moderate and severe PE, rupture of uterine sutures after abdominal delivery according to ultrasound and subsequent laparoscopy, endometritis after abortion, obstetric peritonitis, obstetric sepsis, vaginitis, inflammatory adnexal disorders, and sexually transmitted diseases, including in the postpartum period.
During the study, clinical and anamnestic data (the timing of complaint appearance, lower abdominal pain, purulent or bloody vaginal discharge, fever at admission), laboratory and instrumental data (complete blood count, C-reactive protein (CRP], pelvic ultrasound), as well as an analysis of the treatment and its effectiveness in the comparison groups (Fig. 1).
Statistical analysis
Statistical analyses were performed using the IBM SPSS Statistics software. The sample size was determined using a Sample Size Table. The distribution of continuous variables was tested for normality using the Kolmogorov–Smirnov test with the Lilliefors correction with a critical significance level of p>0.05; in this case, the variables did not meet the normality assumption. Categorical variables were described as counts with percentages (%). Quantitative variables showing normal distribution were expressed as mean (M) and standard deviation (SD) and presented as M (SD); otherwise, the median (Me) with interquartile range (Q1; Q3) was reported. The Kruskal–Wallis test was used to compare non-normally distributed data between three or more groups, followed by post hoc pairwise comparisons using the Mann–Whitney test with the Bonferroni correction to clarify which groups had statistically significant differences.
Results
The mean age of postpartum women with USI and in the group with PE was similar, 28.5 (23.8;35) and 27 (23;33.3) years, respectively, and the gestational ages at the time of delivery were also identical, 39.4 (38.6; 40) and 39 (39; 41) weeks of gestation). It should be noted that in the group of patients with USI, SB occurred in 65/100 (65%) postpartum women and CS was performed in 35/100 (35%) cases, of which 16/35 (45.7%) were emergency Cs. In the group of postpartum women with mild PE, SB was somewhat more common, 54/100 (54%), and CS was performed in 46/100 (46.2%) postpartum women (p>0.05).
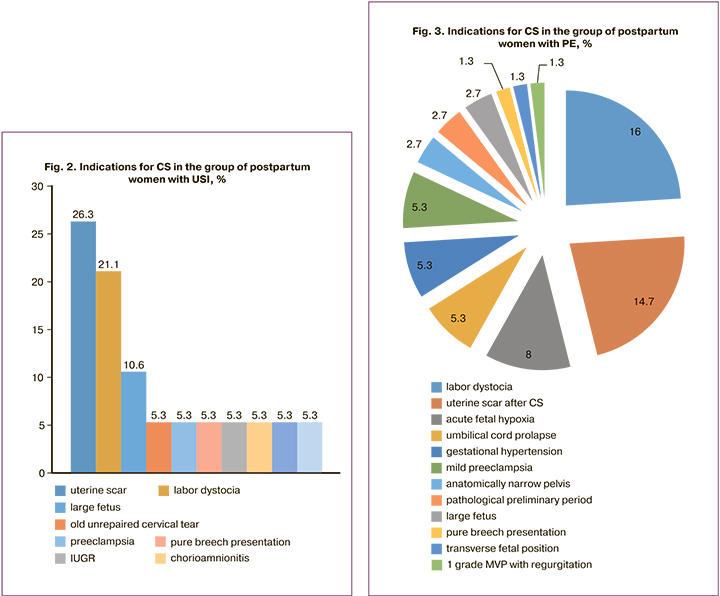
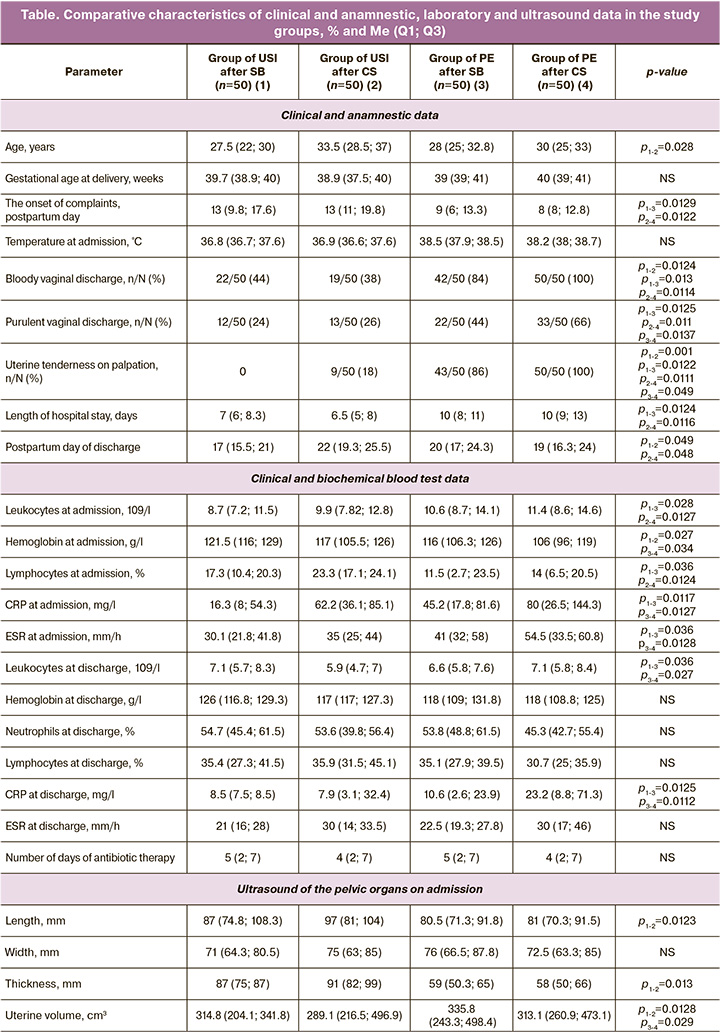
Among the indications for abdominal delivery in patients with USI, the most common was a uterine scar after previous births, 26/100 (26%) and labor dystocia, 21/100 (21%). In the control group, the most common indication for abdominal delivery was labor dystocia, 16/100 (16%), whereas premature rupture of the membranes occurred in 33% (33/100) of women (Fig. 2, 3).
Parity was comparable between the groups, while in women with USI, the first birth occurred in 44/100 (44%), the second in 39/100 (39%), the third in 13/100 (13%), and the fourth in 4/100 (4%). In patients with PE, the first birth occurred in 42/100 (42%), the second in 34/100 (34%), and the third in 24/100 (24%) women (p>0.05).
Analysis of the structure of complications during childbirth revealed that in patients with USI, labor dystocia was diagnosed in 7/100 (7%), fetal hypoxia in 8/100 (8%), premature rupture of membranes in 1/100 (1%), and chorioamnionitis in 2/100 (2%) cases. When studying surgical activity during childbirth in the same group, stripping of the membranes was performed in 5/100 (5%) patients, and vacuum extraction of the fetus was performed in 4/100 (4%) patients. In patients with PE, labor dystocia was diagnosed in 8/100 (8%), preeclampsia in 8/100 (8%), and fetal hypoxia in 25/100 (25%) (p>0.05).
When studying the medical history in the study groups, it was noted that in postpartum women with a history of USI, weakness of labor was more common (21.2% vs. 16%) as an indication for abdominal delivery (p=0.031), which undoubtedly reflects a reduced contractile ability of the uterus and directly affects the occurrence of postpartum subinvolutive changes.
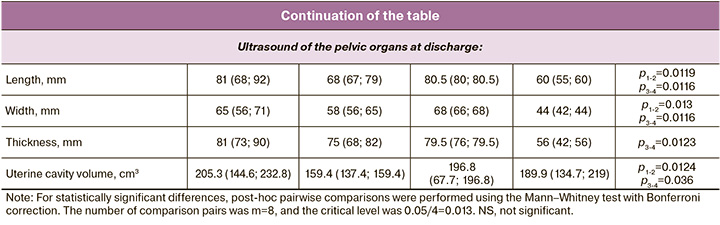
In the group of women with USI, complaints on average occurred on the 13th postpartum day (after SB – on 13 (9.8; 17.6), after CS – on 13 (11; 19.8)). In the control group, postpartum women showed clinical manifestations earlier, on day 9 (6; 13.3), and after abdominal delivery on day 8 (8; 12.8) (p<0.05).
Among the clinical manifestations in women with USI, uterine tenderness on palpation, 18/100 (18%) cases, and bloody vaginal discharge, 41/100 (41%) cases, while the frequency of complaints of purulent vaginal discharge did not exceed 24% (24/100). At the same time, the presence of the above complaints in a quarter of patients with postpartum subinvolutive changes allows us to classify this form of USI as infected, which is undoubtedly a high-risk factor for the formation of PE.
In the comparison group, the average temperature at admission was 38.5 (38–38.5°C), which was somewhat higher than that in postpartum women with USI (p=0.62). Lower abdominal pain was noted in 94/100 (94%) postpartum women with PE, spotting 89/100 (89%); every second had purulent vaginal discharge, 55/100 (55%), which was significantly more common than in women in the USI group (p<0.05).
When evaluating the results of a detailed blood test upon hospital admission, postpartum women with USI had relatively low leukocytosis, 9.4 (7.6; 11.9) × 109/l, CRP, 55.9 (29.8; 80,3) mg/l, ESR, 35 (25; 43) mm/h, as well as the presence of mild anemia in most postpartum women of this group (noted in 54/100 (54%) cases); while the average value of hemoglobin was 118.5 (106.8; 126) g/l. At admission, women with PE had significantly higher levels of leukocytes (11 [8.6; 14.4) × 109/l, CRP (43 [9; 105.7]) mg/l, and ESR, 47.5 (32.8; 60] mm/h), and lower hemoglobin values, especially in the group after abdominal delivery (106 [96; 119] g/l) (p<0.05).
All postpartum women underwent ultrasound assessment of the size, volume, and contents of the uterine cavity before and after treatment. Therefore, on admission, according to the results of ultrasound, the uterine dimensions in the USI group were 96 (80.5;106) mm in length, 74 (64;85) mm in width, and 90 (80;99) mm in thickness. There was expansion of the uterine cavity due to heterogeneous avascular content up to 15.5 (9.7; 27.8) mm, uterine volume – 301.3 (213.4; 415) cm3. It should be noted that the presence of hematometra/lochiometra was diagnosed in almost every third of patients with USI (26/100 (26%)).
In women with PE, ultrasound findings at admission were similar. Patients in the comparison group had the following values: length – 89 (83.5; 108) mm; width – 66 (59.5; 75) mm; thickness – 88 (72; 105.5) mm; expansion of the uterine cavity up to 12.5 (6.5; 15) mm; and total volume of the uterus – 325.8 (180.2; 476.3) cm3 (p>0.05). It should be noted that in patients with PE, subinvolutive changes in the uterus were diagnosed in of the 78/100 (78%) cases. Notably, the uterine volume was significantly smaller in the subgroups of women with USI and PE after abdominal delivery (p<0.05).
In the USI group, 75/100 (75%) of the postpartum women received antibiotic therapy, with a median number of days of antibiotic therapy of 4.5 (2.3; 7), and the most frequently prescribed drugs were metronidazole, 58/100 (58%), ceftriaxone, 32/100 (32%), and amoxicillin+clavulanic acid, 10/100 (10%). All postpartum women received uterotonic therapy, which had a positive effect.
It was noted that the mean number of days of antibiotic therapy in women with USI and PE were comparable (Table), while in the group with PE, in half of the cases (54/100 (54%)), antibiotic therapy included inhibitor-protected aminopenicillins, 36% (36/100) ciprofloxacin, and 10/100 (10%) cephalosporins in combination with metronidazole.
When evaluating the treatment in postpartum women with USI, it was noted that surgical interventions took place in only a quarter of patients in this group and included hysteroscopy in 26/100 (26%) and in 3/100 (3%) women, placental tissue remnants in the uterine cavity were visualized during hysteroscopy. In 2% (2/100) of the cases, hysteroscopy was combined with diagnostic laparoscopy, and the indication for the intervention was the suspicion of a uterine scar defect according to ultrasound data in the group of women after abdominal delivery.
In the group of women with PE, hysteroscopy was performed in 45% (45/100) of the patients, and in every third of them, 32/100 (32%), vacuum aspiration of the contents of the uterine cavity for hematometra and placental tissue remnants were performed. On an average, hysteroscopy was performed on postpartum day 16 (13; 18); in 2/100 (2%) cases, it was combined with laparoscopy. It should be noted that in the group of women with PE after abdominal delivery, hysteroscopy was performed significantly more often in 43/50 (86%) patients than in the group of patients with PE after SB, 33/50 (66%], p=0.041).
In the USI group and in patients with PE, the size and volume of the uterus significantly decreased on ultrasound against the background of ongoing conservative therapy; however, by the time of discharge, the total volume had decreased by an average of 40–50% (p<0.05). It should be noted that the positive changes during treatment were comparable in both groups (p>0.05), which confirms the high receptivity of the myometrium to uterotonic drugs, both in women with USI and in patients with PE.
Comparison of the two groups of postpartum women with USI showed statistically significant differences in clinical and anamnestic data and laboratory parameters depending on the delivery mode (Table). Therefore, in patients in the group after abdominal delivery, bloody vaginal discharge was slightly less common, 22/50 (44%); however, uterine tenderness on palpation was noted in 9/50 (18%) postpartum women after CS. The women with USI after SB had no such complaints. Analysis of laboratory parameters showed slightly higher levels of leukocytes, CRP, and ESR in the group of women with USI after surgical delivery; at the same time, their hemoglobin levels were lower than those in the comparison group. Ultrasound findings showed statistically significantly smaller uterine volume in the group of women after CS (289.1 vs. 314.8 cm3), p=0.043.
The results obtained allow us to conclude that mechanical massage of the uterus after fetal extraction during CS has a positive effect on the amount of lochia, uterine size, and volume in the postpartum period. However, the very fact of operative delivery is a leading infectious risk factor, which is confirmed by indicators of both complete blood count and biochemical blood tests. The presented data make it possible to classify USI after CS surgery as a pre-stage of PE and to classify this subinvolution as infected.
Discussion
Undoubtedly, women with subinvolutive changes in the uterus have a number of similar clinical and anamnestic features characteristic of PE. However, patients diagnosed with PE have more pronounced clinical and laboratory symptoms: complaints appear almost 4 days earlier, and the frequency of bloody and purulent discharge, as well as uterine tenderness on palpation, is significantly more common (86–100%) than in patients with USI (p<0.05). According to the results of complete blood count and biochemical blood tests in the group of postpartum women with PE (especially after CS surgery), higher levels of leukocytes, CRP, and ESR, and lower levels of hemoglobin were noted, which is due to greater blood loss, especially in cases of emergency delivery.
However, pelvic ultrasound findings showed that the uterine volume in postpartum women who delivered by surgery was significantly smaller than that in women after SB (p<0.05). Based on the data obtained, it can be concluded that manual massage of the uterus after fetal extraction during CS can stimulate contractility of the myometrium, which leads to a decrease in its size and volume.
Undoubtedly, knowledge of the features of the course of USI (early manifestation, data from bimanual examination and ultrasound, subclinical manifestations) will allow timely preventive measures to be taken to prevent subsequent purulent-inflammatory complications.
To date, there are several preventive measures aimed at preventing the progression of the process and the development of pyo-inflammatory complications in women with subinvolutive uterine changes. Thus, physical methods of influencing the contractility of the myometrium (electrical stimulation, electrophoresis, diadynamic currents, etc.) are widely used [22, 23]; there are also methods of complex prevention of USI using physiotherapy with a low-frequency alternating magnetic field and sinusoidally modulated current in combination with intramuscular administration of oxytocin at 2.5 IU every 30 minutes for 4 injections [27, 28]. However, the advisability of using uterotonic agents to improve myometrial contractility in the case of subinvolutive changes in the uterus (especially in the case of true USI) has a number of controversial opinions that are based on the reduced expression of oxytocin receptors in the uterus [23].
In addition, several authors have proposed surgical methods for emptying the uterine cavity in cases of hematometra or lochiometra, such as curettage and vacuum aspiration of the contents of the uterine cavity under hysteroscopic control [28, 30, 31]. Vacuum aspiration is recommended in the presence of a heterogeneous suspension according to ultrasound data, and curettage is recommended in the presence of denser structures. To date, it is recommended to perform intrauterine interventions under hysteroscopy control [7].
Thus, from the standpoint of diagnosis, USI is a complex isolated postpartum complication; however, despite several clinical and diagnostic parameters similar to PE, slow regression of the uterus does not always lead to the formation of an infectious process.
Conclusion
Uterine subinvolution exhibits several clinical and laboratory characteristics that classify it as an isolated postpartum complication. In patients with PE after cesarean section, the presence of inflammatory changes in the blood tests confirmed the predominance of the infectious component over the reduced contractile function of the myometrium. In contrast, in women after spontaneous birth, reduced contractile function of the myometrium prevails without obvious clinical manifestations or inflammatory changes in blood tests. The results of the present study underscore the importance of timely diagnosis of subinvolutive changes in the uterus.
References
- Баев О.Р., Орджоникидзе Н.В., Тютюнник В.Л., Ушкалова Е.А., Шмаков Р.Г. Антибиотикопрофилактика при проведении абдоминального родоразрешения (кесарево сечение). Клинический протокол. М.; 2011. 5с. [Baev O.R., Ordzhonikidze N.V., Tyutyunnik V.L., Ushkalova E.A., Shmakov R.G. Antibiotic prophylaxis during abdominal delivery (caesarean section). Clinical protocol. Moscow; 2011. 5p. (in Russian)].
- Коротких И.Н., Бригадирова В.Ю., Корг М.А., Чернов А.В. Анализ факторов риска развития гнойно-септических осложнений у пациенток после самостоятельных и оперативных родов. Системный анализ и управление в биомедицинских системах. 2011; 10(2): 349-54. [Korotkich I.N., Brigadirova V.Yu., Korg M.A., Chernov A.V. The risk factors analysis of purulent-septic complications development at pations after independent and operative sorts. Systems Analysis and Control in Biomedical Systems. 2011; 10(2): 349-54. (in Russian)].
- Верес И.А., Пересада О.А., Небышинец Л.М., Сокол В.П., Барсуков А.Н., Руткевич С.А., Мазитова С.Э. Послеродовая субинволюция матки: клинические, эхографические и биохимические аспекты. Медицинский журнал. 2023; 1: 84-93. [Veres I., Peresada O., Nebyshinets L.M., Sokol V.P., Barsukov A.N. et al. Postpartum subinvolution of the uterus: clinical, sonographic and biochemical aspects. Medical Journal. 2023; (1): 84-93.(in Russian)]. https://dx.doi.org/10.51922/1818-426x.2023.1.84.
- Агарев А.Е., Коваленко М.С., Здольник Т.Д. Факторы риска развития донозологических форм послеродовых гнойно-воспалительных заболеваний. Вестник Авиценны. 2019; 21(4): 550-5. [Agarev A.E., Kovalenko M.S., Zdolnik T.D. Risk factors of the development of prenosological forms of postpartum pyoinflammatory diseases. Avicenna Bulletin. 2019; 21(4): 550-5. (in Russian)]. https://dx.doi.org/10.25005/2074-0581-2019-21-4-550-554.
- Докудаева Ш.А. Современные представления об этиологии, патогенезе, клинике и диагностике послеродового эндометрита. Вестник национального медико-хирургического центра им. Н.И. Пирогова. 2016; 11(4): 109-15. [Dokudaeva Sh.A. Current concepts of etiology, pathogenesis, clinical presentation and diagnosis of postpartum endometritis. Bulletin of the N.I. Pirogov National Medical and Surgical Centre. 2016; 11(4): 109-15(in Russian)].
- Беженарь В.Ф., Шапкайц В.А., Добровольская И.А. Возможности ранней диагностики современного акушерского сепсиса. Акушерство, гинекология и репродукция. 2021; 15(2): 121-31. [Bezhenar V.F., Shapkaitz V.A., Dobrovolskaya I.A., Rukoyatkina E.A., Nesterov I.M. Opportunities for early diagnostics of contemporary obstetric sepsis. Obstetrics, Gynecology and Reproduction. 2021; 15(2): 121-31. (in Russian)].https://dx.doi.org/10.17749/2313-7347/ob.gyn.rep.2021.183.
- Адамян Л.В., Кан Н.Е., Ломова Н.А., Тютюнник В.Л., Серов В.Н., Шмаков Р.Г. Послеродовой эндометрит. Клинические рекомендации. М.; 2016. 31с. [Adamyan L.V., Kan N.E., Lomova N.A., Tyutyunnik V.L., Serov V.N.,Shmakov R.G. Postpartum endometritis. Clinical guidelines. Moscow; 2016. 31p. (in Russian)].
- Баринов С.В., Блауман Е.С., Тирская Ю.И., Шкабарня Л.Л. Факторы риска развития и особенности течения послеродового эндометрита. Мать и дитя в Кузбассе. 2017; 2: 22-8. [Barinov S.V., Blauman E.S., Tirskaya Yu.I., Shkabarnya L.L. Risk factors and peculiarities of postpartum endometritis. Mother and Child in Kuzbass. 2017; (2): 22-8. (in Russian)].
- Шатунова Е.П., Линева О.И., Тарасова А.В., Неганова О.Б. Послеродовые воспалительные заболевания матки: клинические и диагностические грани проблемы. Российский вестник акушера-гинеколога. 2021; 21(1): 79-83. [Shatunova E.P., Lineva O.I, Tarasova A.V., Neganova O.B. Clinical and diagnostic aspects of postpartum inflammatory diseases. Russian Bulletin of Obstetrician-Gynecologist. 2021; 21(1): 79-83. (in Russian)].https://dx.doi.org/10.17116/rosakush20212101179.
- Горин В.С., Матвеева И.В., Шаклеин А.В., Попова Ж.Ю., Кугушев А.В. Оптимизация диагностики и лечения субинволюции матки как одной из форм послеродового эндометрита. Российский вестник акушера-гинеколога. 2011; 11(3): 27-34. [Gorin V.S., Matveeva I.V., Shaklein A.V., Popova Zh.Yu., Kugushev A.V. Optimisation of diagnosis and treatment of uterine subinvolution as a form of postpartum endometritis. Russian Bulletin of Obstetrician-Gynecologist. 2011; 11(3): 27-34.(in Russian)].
- Верес И.А., Пересада О.А., Юрага Т.М. Биохимические критерии послеродовой субинволюции матки. Лабораторная диагностика. Восточная Европа. 2019; 8(4): 515-25. [Veres I.A., Peresada O.A., Yuraga T.M. Biochemical criteria of postpartum uterine subinvolution. Laboratory Diagnostics. Eastern Europe. 2019; 8(4): 515-25. (in Russian).]
- Верес И.А. Анализ клинических проявлений послеродовой субинволюции матки как предстадии гипотонического послеродового эндометрита. Российский вестник акушера-гинеколога. 2020; 20(5): 84-90. [Veres I.A. Analysis of the clinical manifestations of postpartum subinvolution of the uterus as a pre-stage of hypotonic postpartum endometritis. Russian Bulletin of Obstetrician-Gynecologist. 2020; 20(5): 84-90. (in Russian)].https://dx.doi.org/10.17116/rosakush20202005184.
- Ramkumar S., Kharshiing T. Vessel subinvolution of the placental implantation site: A case report and review of supportive literature. Cureus. 2021; 13(2): e13472. https://dx.doi.org/10.7759/cureus.13472.
- Triantafyllidou O., Kastora S., Messini I., Kalampokis D. Subinvolution of the placental site as the cause of hysterectomy in young woman. BMJ Case Rep. 2021; 14(2): e238945. https://dx.doi.org/10.1136/bcr-2020-238945.
- Гребенкин Б.Е., Черемискин В.Г. Группы риска послеродовых гнойно-септических заболеваний в условиях стационара высокой степени риска. В кн.: Материалы IX Российского форума «Мать и дитя». М.; 2007: 55-6. [Grebenkin B.E., Cheremiskin V.G. Risk groups of postpartum purulent-septic diseases in a high-risk hospital. In: Materials of the IX Russian Forum "Mother and Child". Мoscow; 2007: 55-6. (in Russian)].
- Шульженко В.Т., Зарицкая Э.Н., Мирлас Е.М., Борзунов М.Н., Мостовая Т.А., Болгова Е.Д., Петров С.Н. Субинволюция матки и послеродовый эндометрит. Амурский медицинский журнал. 2014; 2(6): 97-100. [Shuljenko V.T., Zaritskaya E.N., Mirlas E.M., Borzunov M.N., Bolgova E.D., Petrov S.N. Subinvolution of uterus and puerperal endometritis. Amur Medical Journal. 2014; 2(6): 97-100. (in Russian)].
- Рыскельдиева В.Т. Субинволюция матки как фактор риска развития послеродового эндометрита. Таврический вестник. 2012; 15(1): 57. [Ryskeldieva V.T. Subinvolution of the uterus as a risk factor for the development of postpartum endometritis. Taurian Bulletin. 2012; 15(1): 57.(in Russian)].
- Верес И.А., Пересада О.А., Иванишкина-Кудина О.Л., Куликов А.А., Зновец Т.В., Шиптенко И.Л., Соколовская М.Н. Эффективность немедикаментозной профилактики послеродовой субинволюции матки. Репродуктивное здоровье. Восточная Европа. 2020; 20(1): 22-30. [Veres I.A., Peresada O.A., Ivanishkina-Kudina O.L., Kulikov A.A., Znovets T.V., Shiptenko I.L., Sokolovskaya M.N. Efficiency of non-medicinal prevention of the postpartum subinvolution of uterus. Reproductive Health. Eastern Europe. 2020; 10(1): 22-30. (in Russian)]. https://dx.doi.org/10.34883/pi.2020.10.1.017.
- Keirse M.J. Discovering the Holy Grail in postpartum uterine involution. Birth. 2011; 38(1): 80-3. https://dx.doi.org/10.1111/j.1523-536X.2010.00450.x.
- Farrugia G. Interstitial cells of Cajal in health and disease. Neurogastroenterol Motil. 2008; 20(Suppl. 1): 54-63. https://dx.doi.org/10.1111/j.1365-2982.2008.01109.x
- Новиков Б.Н., Коробков Н.А., Рябцева И.Т. Хирургическая санация полости послеродовой матки в лечении эндометрита. Журнал акушерства и женских болезней. 2011; 60(6): 45-50. [Novikov N.B., Korobkov N.A., Ryabtseva I.T. Surgical sanation postpartum uterine cavity treatment of endometritis. Journal of Obstetrics and Women's Diseases. 2011; 60(6): 45-50. (in Russian)].
- Popescu L.M., Faussone-Pellegrini M.S. Telocytes – a case of serendipity: the winding way from Interstitial Cells of Cajal (ICC), via Interstitial Cajal-Like Cells (ICLC) to telocytes. J. Cell. Mol. Med. 2010; 14(4): 729-40.https://dx.doi.org/10.1111/j.1582-4934.2010.01059.x.
- Grotegut C.A., Feng L., Mao L., Heine R.P., Murtha A.P., Rockman H.A. β-Arrestin mediates oxytocin receptor signaling, which regulates uterine contractility and cellular migration. Am. J. Physiol. Endocrinol. Metab. 2011; 300(3): E468-77. https://dx.doi.org/10.1152/ajpendo.00390.2010.
- Wakasa T., Wakasa K., Nakayama M., Kuwae Y., Matsuoka K., Takeuchi M. et al. Change in morphology and oxytocin receptor expression in the uterine blood vessels during the involution process. Gynecol. Obstet. Invest. 2009; 67(2):137-44. https://dx.doi.org/10.1159/000172805.
- Bae H.S., Ahn K.H., Oh M.J., Kim H.J. Postpartum uterine involution: sonographic changes in the endometrium between 2 and 6 weeks postpartum related to delivery mode and gestational age at delivery. Ultrasound Obstet. Gynecol. 2012; 39(6): 727-8. https://dx.doi.org/10.1002/uog.11069.
- Belachew J., Axelsson O., Mulic-Lutvica A., Eurenius K. Longitudinal study of the uterine body and cavity with three-dimensional ultrasonography in the puerperium. Acta Obstet. Gynecol. Scand. 2012; 91(10): 1184-90.https://dx.doi.org/10.1111/j.1600-0412.2012.01418.x.
- Коноводова Е.Н., Закревская И.В., Кесова М.И., Занозин А.С. Современные представления о послеродовой субинволюции матки. Вопросы гинекологии, акушерства и перинатологии. 2015; 14(1): 48-56. [Konovodova E.N., Zakrevskaya I.V., Kesova M.I., Zanozin A.S. Current views on postpartum subinvolution of the uterus. Gynecology, Obstetrics and Perinatology. 2015; 14(1): 48-56 (in Russian)].
- Верес И.А., Пересада О.А., Сокол В.П., Зновец Т.В., Юрага Т.М. Применение электромагнитной стимуляции у родильниц с послеродовой субинволюцией матки. Медицинский журнал. 2022; 2: 59-66. [Veres I.A., Peresada O.A., Sokol V.P., Znovets T.V., Yuraga T.M. The use of electromagnetic stimulation in labouring women with postpartum uterine subinvolution. Medical Journal. 2022; (2): 59-66. (in Russian)]. https://dx.doi.org/10.51922/1818-426x.2022.2.59.
- Du R., Davies R., Supramaniam P.R. Fertility preserving management for postpartum haemorrhage secondary to subinvolution of the placental implantation site. BMJ Case Rep. 2021; 14: e245009. https://dx.doi.org/10.1136/bcr-2021-245009.
- Zubor P., Szunyogh N., Dokus K., Scasny P., Kajo K., Galo S. et al. Application of uterotonics on the basis of regular ultrasonic evaluation of the uterus prevents unnecessary surgical intervention in the postpartum period. Arch. Gynecol. Obstet. 2010; 282(3): 261-7. https://dx.doi.org/10.1007/s00404-009-1227-5.
- Егорова А.Т., Глебова Т.К., Маисеенко Д.А., Шапошникова Е.В. Гнойно-воспалительные осложнения в акушерской практике по материалам краевой клинической больницы г. Красноярска. Сибирское медицинское обозрение. 2015; 4: 47-51. [Egorova A.T., Glebova T.K., Maiseenko D.A., Shaposhnikova E.V. Pyoinflammatory complications in obstetric practice according to the materials of the Regional Clinical Hospital of Krasnoyarsk. Siberian Medical Review. 2015; (4): 47-51.(in Russian)].
Received 03.04.2023
Accepted 01.08.2023
About the Authors
Daria E. Galkina, PhD, Associate Professor at the Department of Operative Gynecology of the Institute of Postgraduate Education, Voyno-Yasenetsky Krasnoyarsk State Medical University, Ministry of Health of the Russian Federation, +7(923)376-94-33, dashsemch@mail.ru, https://orcid.org/0000-0001-7516-5203, 660022, Russia, Krasnoyarsk Territory, Krasnoyarsk, Partizan Zheleznyak str., 1.Tatyana A. Makarenko, Dr. Med. Sci., Professor, Head of the Department of Operative Gynecology of the Institute of Postgraduate Education, Voyno-Yasenetsky Krasnoyarsk State Medical University, Ministry of Health of the Russian Federation, +7(904)895-47-99, makarenko7777@yandex.ru, https://orcid.org/0000-0002-2899-8103,
660022, Russia, Krasnoyarsk Territory, Krasnoyarsk, Partizan Zheleznyak str., 1.
Tatyana A. Fadeeva, obstetrician-gynecologist, Krasnoyarsk Inter-district Clinical Hospital No. 4, +7(913)587-52-06, tanyachyst9@bk.ru
Tatyana V. Dresvyanskaya, Head of the Second Gynecological Department, Krasnoyarsk Inter-district Clinical Hospital No. 4, +7(902)991-82-98, dtw19691@mail.ru
Corresponding author: Daria E. Galkina, dashsemch@mail.ru



