Regional clinical and anamnestic characteristics of the course of the postpartum period in women according to the data in Krasnoyarsk and the Krasnoyarsk region
Galkina D.E., Makarenko T.A., Bochanova E. N., Shageeva G.A., Ulyanova I.O.
Relevance: Currently there is no trend toward reduction in postpartum purulent-inflammatory diseases (PPID), and they account for 5–26% of all postpartum complications. Postpartum endometritis (PE) ranks first in the structure of PPID, and is diagnosed in more than 40% of all cases of postpartum complications.
Objective: To explore the clinical and anamnestic characteristics in women with PPID according to the data in Krasnoyarsk and in the Krasnoyarsk region.
Materials and methods: A retrospective assessment and statistical analysis of clinical and anamnestic data of 387 patients with various complications in late postpartum period and 446 puerperant women with PE in the period 2017–2022 was conducted.
Results: In the structure of postpartum complications, the rate of postoperative wound to the anterior abdominal wall is very high and reaches in general 64.2% (248/387), the rate of endometritis is 36.7% (142/387), and the rate of suppurating perineal wound is 11.1% (43/387). Manifestation of the clinical picture of preeclampsia is blurred in most cases – in 36.8% (164/446)) of women, and only one third of patients have classical clinical symptoms of the disease. On average, the debut of clinical symptoms is on day 11 of the postpartum period. At the same time, the symptoms in women after cesarean section occur one day earlier (10,5 days) in comparison to the vaginal delivery (11.2 days).
Conclusion: Thus, there is no trend toward reduction in the rate of PPID. At the same time, it is noteworthy that predominantly blurred clinical picture of PPID does not correlate with the severity of destructive processes in the site of inflammation, and therefore, delays timely diagnosis, leading to critical delayed consequences up to sepsis. The obtained results are of scientific and practical interest and are aimed at development of novel, most effective methods of diagnosis and treatment of PPID.
Authors' contributions: Galkina D.E. – article writing; Makarenko T.A., Bochanova E.N. Shageeva G.A., Ulyanova I.O – article editing.
Conflicts of interest: The authors declare that they have no conflicts of interest.
Funding: The study was conducted without any sponsorship.
Ethical Approval: The study was approved by the Local Ethics Committee of Krasnoyarsk State Medical University named after Professor V.F. Voino-Yasenetsky, Ministry of Health of the Russian Federation.
Patient Consent for Publication: The patients have signed informed consent for publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Galkina D.E., Makarenko T.A., Bochanova E. N., Shageeva G.A., Ulyanova I.O. Regional clinical and anamnestic characteristics of the course of the postpartum period in women according to the data in Krasnoyarsk and the Krasnoyarsk region.
Akusherstvo i Gynecologia/Obstetrics and Gynecology. 2023; (12): 114-124 (in Russian)
https://dx.doi.org/10.18565/aig.2023.218
Keywords
Currently there is no trend toward reduction in postpartum purulent-inflammatory diseases (PPID), and they account for 5–26% of all postpartum complications [1–3]. In recent years, prevalence of septic complications ranked third and reached 12% in the overall structure of the causes of maternal mortality [4–8]. Sepsis is a very serious delayed complication of PPID that requires timely diagnosis and immediate treatment [9–12].
Postpartum endometritis (PE) takes leading position in the structure of PPID, and is diagnosed in more than 40% of all cases of postpartum complications [13–18]. In Europe and the USA, the incidence rate of PE after vaginal delivery is 1–3%, in Russia 2–5%. The rate of PE after elective cesarean delivery in the countries of Europe and in the United States is 5–15%, in Russia 10–15% [13–15, 19–21].
Attention should be paid to the evolution of the clinical picture and the course of PPPID in the temporal aspect. Thus, in 1996, 3 288 562 birth histories were analyzed. The incidence rate of PPID was 21.7%, whereas in the period 1984–1988 the rate was only 10%. However, against the background of the total number of all PPID cases, the rate of PE increased from 33.2% to 39.5%. At the same time, in 92.4% of cases, there was a classical clinical picture of PE [11, 12, 22, 23].
The course of PPID is influenced by a number of features of the microbiological spectrum. The polyetiological nature of pathogens (especially enterobacteria and associations of enterobacteria) coexisting in the form of biofilms that have high resistance to the most important types of antimicrobial medicines was reported [24]. Moreover, according to a number of authors, opportunistic microorganisms can also be causative agents of postpartum infectious diseases against the background of blood loss, especially during abdominal delivery and in local immunosuppression [10, 24–28]. The widespread use of a wide range of antiseptics and antibacterials in obstetric practice, the rising antibiotic resistance and virulence of microorganisms lead to increasing percentage of obscure and abortive forms of postpartum diseases, that are often difficult to diagnose [29, 30].
It should also be noted that in recent years the activity in surgical delivery tends to increase. Thus, in level one obstetric care facilities, the percentage of surgical methods of delivery is 19.0–24.2%, in level 2 – 30–37%, in level 3 – 35–40%, and in specialized research centers – up to 70% [12]. Despite the use of modern suture material and antibiotic prophylaxis, cesarean section (CS) remains a serious risk factor for the incidence of PPID [20, 21]. Thus, according to a number of authors, the incidence rate of endometritis after cesarean delivery is 2.5–20%; at the same time, 10–20% in pathological delivery, and 13.3–54.3% in women at high risk of infections [16, 22, 27, 31].
According to domestic authors, the rate of postpartum endometritis (PE) in general population of puerperant women after spontaneous vaginal delivery (SVD) is 3–8%, and 10–20% after CS [16, 22], and the rate of obstetric peritonitis after CS in maternity hospitals ranges from 0.1% to 1.5%. Most often, this pathology develops after operative delivery (98%). At the same time, postpartum endometritis is one of the major causes of generalized infection and formation of uterine scar defects [16, 21, 23].
Thus, due to the perinatal focus of modern obstetrics and worsening of general health status of women of reproductive age, the frequency of abdominal delivery significantly increased, that creates additional risk for intraoperative and postoperative complications [22]. So, according to foreign and domestic authors, the general rate of all complications in abdominal delivery is 16.3–21.4% [16, 22].
The level of socio-economic development of the region and the organization of medical care have a significant impact on the frequency of postpartum infectious complications [22, 27]. At the same time, systematic organization of medical care for patients with postpartum complications is an acute issue. Thus, postpartum women undergo treatment in gynecology hospitals, and there are no conditions for adequate postpartum care and keeping mothers and newborn babies together. There are also no standardized approaches to perform surgical interventions for treatment of postpartum complications. At the same time, radical interventions predominate with underlying insufficient use of endoscopic technologies [19, 32].
The studies by Oboskalova T.A. and Glukhova E.Yu. aimed at investigation of regional characteristics of PPID, reported the data on the system for prevention of PPID in maternity hospitals in Ekaterinburg and the program for providing medical care to women with complications of puerperium [33]. Since 2006, when financing of maternity hospitals in Ekaterinburg has been improved, there was increased delivery of medications and consumables; and improved technique for abdominal delivery was used; the frequency of severe purulent-septic complications, which previously ended with a surgical organ transplant, reduced by almost half. However, due to the increased frequency of abdominal delivery (from 14.8% to 31.2% in the period from 1998 to 2013), there was a trend toward the increase in the incidence of complications, such as postoperative wound suppuration in the anterior abdominal wall and uterine scar failure (by 2 times) [33].
The authors showed that to prevent PPID, a certain organizational structure of maternity hospitals is important, that provides maximum separation between parturient and puerperant women, but at the same time ensures keeping mothers and babies together. It is also relevant that there should be interchangeable, cyclically functioning units with high infectious risk in maternity hospitals – resuscitation and intensive care units, operating and birth rooms [33].
Based on the new regional data on the characteristics of PPID, future development of organizational measures in the obstetric and gynecological service of the city and region are possible, that will reduce the incidence of severe forms of postpartum complications.
The purpose of the study was to explore the clinical and anamnestic characteristics in women with postpartum purulent-inflammatory diseases (PPID according to the data obtained in Krasnoyarsk and the Krasnoyarsk region.
Materials and methods
A retrospective descriptive epidemiological study of clinical and anamnestic data of 387 birth histories of patients with various late postpartum complications and 446 histories of puerperant women with PE from 2017 to 2022 was conducted in the clinical hospitals of Krasnoyarsk State Medical University named after Professor V. F. Voino-Yasenetsky.
Group 1 in the study was arranged to determine the structure of postpartum complications, evaluation of the somatic status and clinical and anamnestic data of the general cohort of parturient and puerperant women obtained from data repository in maternity hospitals in Krasnoyarsk. According to the data of group 2, evaluation of clinical picture and anamnesis, specific features of the cause of PE depending on the type of delivery was performed. At the same time, group 2 was divided into two subgroups: 205 birth histories of puerperant women after spontaneous vaginal delivery (SVD) and 241 after CS. The archival data were collected from data repositories in Krasnoyarsk inter-district clinical hospital of emergency care named after N.S. Karpovich Regional Clinical Hospital, Maternity Hospital No.1, Krasnoyarsk Inter-District Clinical Hospital No. 4 (Fig. 1).
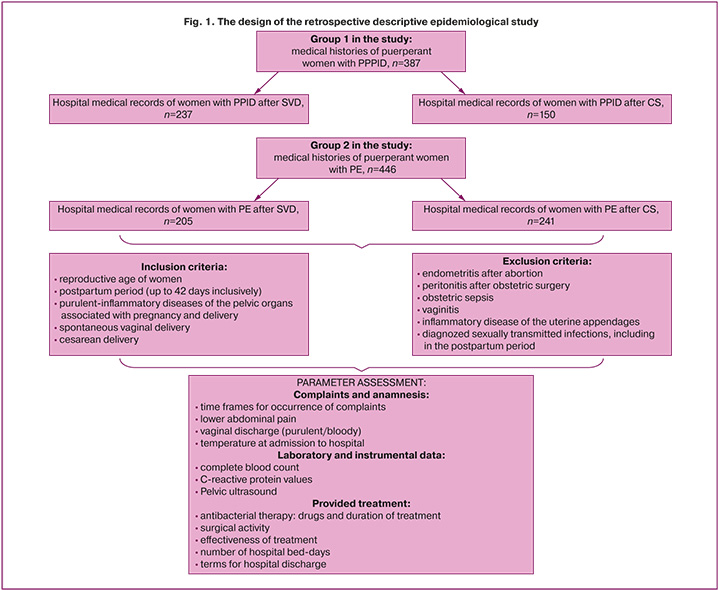
Inclusion criteria were reproductive age of women, postpartum period (up to 42 days inclusively), purulent-inflammatory diseases of the pelvic organs associated with pregnancy and delivery, SVD or CS.
Exclusion criteria were: endometritis after abortion, peritonitis after obstetric surgery, obstetric sepsis, vaginitis, inflammatory disease of the uterine appendages, diagnosed sexually transmitted infections, including in the postpartum period.
The research process included assessment of clinical and anamnestic data (time frames for occurrence of complaints, lower abdominal pain, vaginal discharge (purulent/bloody), temperature at admission to hospital), laboratory and instrumental data (complete blood count, C-reactive protein values, pelvic ultrasound), as well as the analysis of treatment and its effectiveness in comparison groups (Fig. 1).
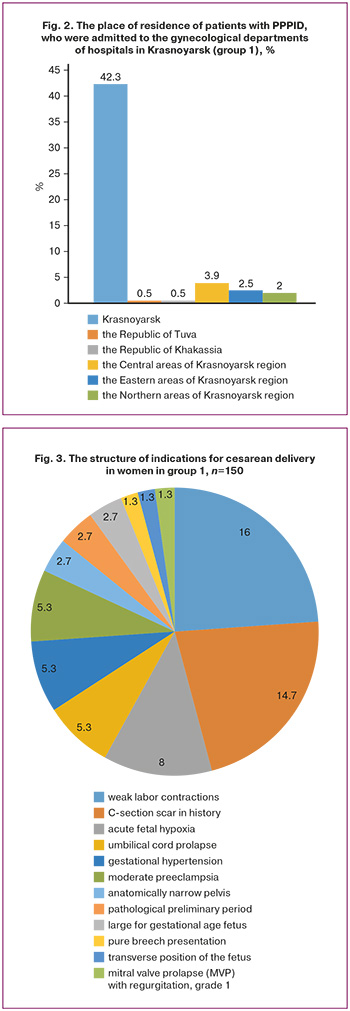
Geographic distribution of the patient’ places of residence is shown in Figure 2. It should be noted, that most of puerperant women with postpartum complications (42.3% (164/387)) were in Krasnoyarsk.
Statistical analysis
Statisitical analysis was performed using IBM SPSS Statistics. Table sampling method was used to determine the required sample size. The normality of distribution was assessed by using the Lilliefors-corrected Kolmogorov–Smirnov test with critical value at the level of significance р>0.05. In this case, it was considered that the parameters follow normal distribution. The descriptive statistics is presented as qualitative data in percentage (%).
The quantitative data with normal distribution were described as M (SD) – the arithmetic mean (M) and standard deviation (SD. When distribution differed from normal, the data were presented as median (Me) and interquartile range Me (Q1; Q3).
Results
The analysis of clinical and anamnestic data of patients in group 1 showed, that the mean age of puerperant women with PPID was 27.9 (27.4; 28.5) years. The women gave birth on average at 39.5 weeks (38.3; 40.5) of pregnancy. At the same time 38.8% of women (150/387) gave birth via cesarean section, and 61.2% of women (237/387) had spontaneous vaginal delivery. It should be noted that only in 33.9% of cases (131/387) the patients with PPID were transferred from maternity hospitals to gynecology hospitals, in all other cases (66.1%), the puerperant women were transferred from home to hospital by the ambulance crew or they were admitted to hospital as self-referred patients (256/387).
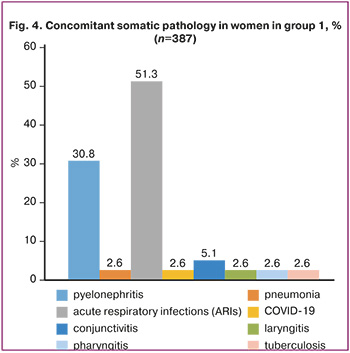
In the structure of indications for cesarean delivery in group 1 in the study, weak labor contractions ranked first and were in 16% of women (24/150), C-section scar in history was in 14.7% (22/150), and acute fetal hypoxia was in 8% (12/150) of women. At the same time, premature rupture of membranes was in 33.3% of women (50/150)
Based on the results of histological findings of placentas in 15% of cases (58/387) of clinically diagnosed chorioamnionitis, the diagnosis was confirmed in 3.5% of puerperant women after СS, and in 4.6% of women after abdominal delivery.
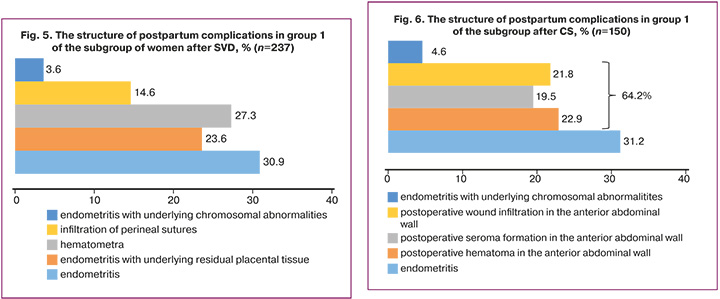
The analysis of the frequency of concomitant somatic diseases in women of the study groups showed that pyelonephritis was most common disease in 30.8% of women (119/387), and acute respiratory infections (ARIs) were in 51.3% of women (199/387), while COVID-19 was only in 2.6% of women (10/387) (Fig.4).
Late postpartum complications in the group of puerperant women with PPID were in 25.9% of patients (100/387). In the structure of late postpartum complications, the rate of endometritis was the highest – 36.7% (142/387). The rate of perineal wound suppuration, as well as hematometra was 33.6% (130/387), which may be considered as a risk factor for endometritis; at the same time, with underlying residual placental tissue, it was diagnosed in 23.6% (31/130) of cases.
Most common complications in group 1 in women after SVD were hematometra in 27.3% (66/237) of women and residual placental tissue in 24% (57/237), while postoperative wound complications in the anterior abdominal wall were diagnosed most often in patients who underwent CS, and their rate reached 64.2% (96/150). Among them, infiltration was in 21.8% (21/96), seroma was in 19.8% (19/96), and hematoma was in 22.9% (22/96) of women (Fig. 5, 6).
All patients with the above complications were transferred from maternity hospitals to gynecological departments of multidisciplinary hospitals.
The mean age of patients with PE (group 2), who were admitted to gynecological departments of hospitals in Krasnoyarsk was 28.1 (27.2; 29.2) years. Among them, first delivery was in 17.7% (79/446) of women, second delivery was in 7.2% (32/446), and third delivery was in 2.5% (11/446) of women, and only one patient became mother for sixth time. The complaints with which the patients were admitted to hospital were manifested on average on day 10.8 (9.9; 11.9) of the postpartum period. It should be noted that paucisymptomatic PE prevailed, and only in one third of cases, uterine pain was diagnosed by palpation in 34.1% of women (70/205) after SVD, and in 34% of women (82/241) after CS, bloody vaginal discharge in 35.1% of women (72/205) after SVD and in 27.8% of women (67/241) after CS. At the same time, purulent vaginal discharge, that was more specific for the classical manifestation of endometritis, was only in 18.5% of patients (38/205) with PE after SVD (Table 1), and more often after abdominal delivery – in 21.2% of women (51/241).
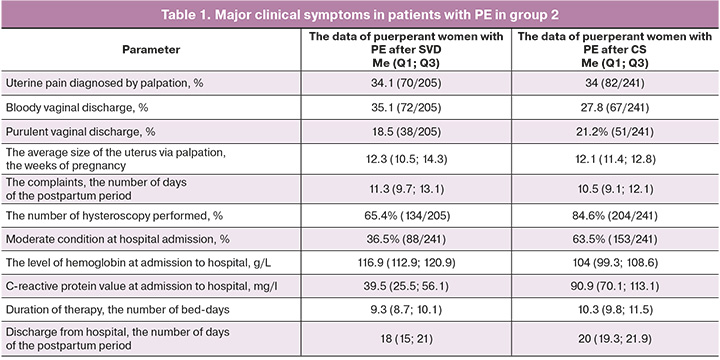
In group 2, only 38,1% of women (170/446) were admitted to hospital in satisfactory condition, moderate condition was in 57.4% of women (256/446), severe condition was diagnosed in 1.1% (5/446), sepsis was in 3,4% of women (15/446). At the same time, in the group of patients with PE after CS, moderate condition was significantly more often – in 63.5% (153/241) of women versus the women after SVD – 36% (88/241).
According to evaluation of indicators of coagulation hemostasis, fibrinogen level was slightly high – 4.7 g/l, and prothrombin time was slightly high 15.7 sec. It should be noted, that the differences between the groups of women after CS and SVD in the levels of hemoglobin (116.9 (112.9; 120.9) g/l in group with SVD and 104 (99.3; 108.6) g/l in the group after CS) and the values of C-reactive protein (39.5 (25.5; 56.1) mg/l in the group with SVD, and 90.9 (70.1; 113.1) mg/l in the group after CS) at hospital admission were statistically significant. At the same time, the group of patients after CS, in whom late postpartum complication was endometritis, had lower levels of hemoglobin, that can be explained by the volume of blood loss, and higher values of C-reactive protein (p<0.0001).
Pelvic ultrasound was performed when the patients were admitted to hospital. Ultrasound assessment showed subinvolutive dimensions of the uterus, the parameters which were: the mean length 77.2 (61.8; 93.4) mm, thickness 57.2 (48.4; 66.0) mm, width 76.7 (64; 88.4) mm.
According to a number of authors, the clinical symptoms of PE after operative delivery occur on day 6±2 of the postpartum period, which after spontaneous vaginal delivery on day 4±2 [17, 23, 34]. The results of our study showed that PE was diagnosed on day 10.8 (9.9; 11.9). At the same time the clinical picture after CS occurred a bit earlier – on day 10.5 (9.1; 12.1) than after SVD – on day 11.3 (9.7; 13.1). Therefore, the debut of the clinical picture of PE usually occurs when the puerperant women is already discharged from maternity hospital, that may lead to untimely referral for medical care, delayed diagnosis and development of severe types of PPID.
Currently, the specific feature of postpartum infectious diseases is their polyetiological nature. According to the published data, the leading role in the etiological structure belongs to opportunistic microorganisms – non-fermenting gram-negative rods (predominantly Escherichia coli, Klebsiella pneumonia), enterococci, (predominantly Enterococcus faecalis), staphylococci (predominantly Staphylococcus aureus, Staphylococcus haemoliticus), massive colonization of which in the form of aerobic and anaerobic associations is present in the uterus in most cases [18, 21, 28, 29].
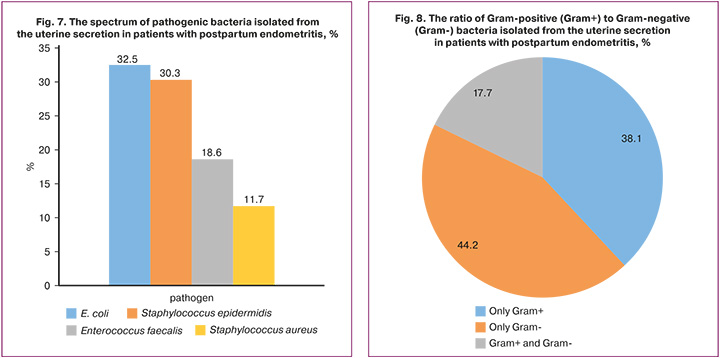
The results of our analysis of the microbial spectrum at the site of inflammation showed that most common, causative agents of PE are Escherichia coli (32.5% (145/446), Staphylococcus epidermidis (30.3% (135/446)), Enterococcus faecalis (18.6% (83/446)), Staphylococcus aureus (11.7% (52/446)). Monoculture of gram-positive bacteria was observed in 38.1% of cases (170/446), and gram-negative bacteria was in 44.2% of cases (197/446), and a mixture of gram-positive and gram-negative bacteria was diagnosed in 17.7% of cases (79/446) (Fig. 7, 8).
The investigation of antibiotic resistance of most common bacteria to antimicrobial agents showed that resistance of Escherichia coli to ampicillin was in every third case (35.8% (52/145)), to doxycycline in 21.4% of cases (31/145). Moreover, in 21.4% (31/145) of Escherichia coli strains, the production of extended spectrum β-lactamases (ESBLs), the enzymes that confer resistance to first–fifth-generation of cephalosporins, were detected.
Gentamicin and amikacin resistant Escherichia coli were in 31.7% (46/145) и 33% (48/145) of cases, respectively.
Erythromycin resistant Staphylococcus epidermidis was in 45.9% (62/135) of cases, and in almost equal proportions ciprofloxacin and levofloxacin resistant S. epidermidis was in 16.3% (22/135) and 14.8% (20/135) of cases, respectively. Resistance of Methicillin-Resistant Staphylococcus epidermidis (MRSE) to cefoxitin in 15.6% (21/135) of cases indicates resistance to all β-lactam antibiotics except ceftaroline and ceftobiprole.
It should be noted that in 1992, the results of antibiogram of bacterial isolates of uterine discharge in puerperant women women with PE were the following: gram-negative bacteria were most sensitive to gentamicin (72.8%), carbenicillin (70.9%), monomycin (64.1%), kanamycin (62.7%), levomycitin (57.8%). Staphylococci were most sensitive to gentamicin (83.5%), carbenicillin (79.7%), tetracycline (75.8%), oxacillin (75.1%), methicillin (67.6%). Moreover, sensitivity of the groups of streptococcal bacteria proved to be most sensitive to monomycin (36%) and to ampicillin (96.3%) [30, 35–38].
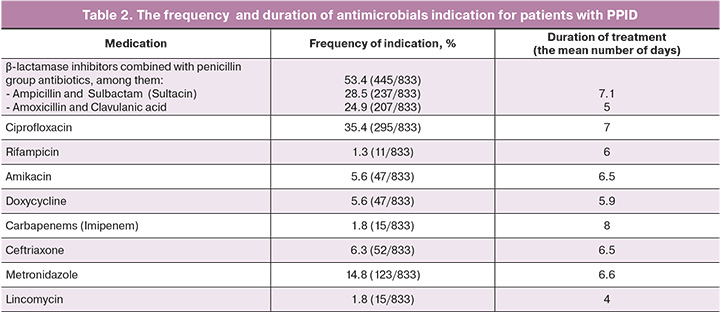
However, the results of our analysis of the microbial spectrum from the uterine cavity proved the resistance of Escherichia coli to ampicillin in every third patient; and extended-spectrum β-lactamases (ESBL+) producing Escherichia coli, were resistant to extended-spectrum penicillins and first–fourth generation cephalosporins. At the same time, β-lactamase inhibitors combined with penicillin group antibiotics were indicated in almost every second clinical case (in 53.4% of cases).
The retrospective analysis showed that the average duration of antimicrobial therapy for patients with PPID, including PE was 7.9 (7.4; 8.4) days. Moreover, β-lactamase inhibitors combined with penicillin group antibiotics were indicated in 53.4% (238/446) of cases, monotherapy was indicated in 11.2% of cases, and therapy with ciprofloxacin was in other cases.
Since the species were collected from the uterine cavity before starting antimicrobial therapy, the subsequent antimicrobial therapy adjustment strategy was based on the results of bacterial culture and antibiogram. At the same time, treatment regimens changed in 30% (134/446) of cases – ceftriaxone in 6.3% (28/446), doxycycline in 5.6% (25/446)) and metronidazole in 14.8% (66/446) of cases (Table 2). However, in 11.7% (52/446) of cases, the results of bacterial culture showed no bacterial growth.
Concomitant therapy with uterotonics (oxytocin) was indicated for 8.6 days in 58.9% (263/446) of cases, nadroparin calcium (fraxiparin) for 7.9 days in 46.4% (207/446), and iron preparations (sorbifer) for 9.6 days in 43.1% (192/446) of cases. Due to this, there was statistically significant increase in the level of hemoglobin from 111.2 to 118.3 g/l.
The analysis of surgical activity with regard to postpartum women with PPID in group 1 showed that 45.5% (176/387) of women underwent hysteroscopy in late postpartum period, and almost one third of patients (31.7% (123/387) 387)) underwent uterine aspiration for hematometra and residual placental tissue. On average, hysteroscopy was performed on day 14.5 (12.3; 16.5) of the postpartum period. Moreover, in 1.7% (3/176) of cases, hysteroscopy was combined with laparoscopy for suspected suture dehiscence after abdominal delivery according to ultrasound data. It should be noted that in the group of women with PE after abdominal delivery, hysteroscopy was performed significantly more often – in 84.6% (204/241) of cases versus the group of patients with PE after SVD –in 65.4% (134/205) of cases.
The average number of hospital bed-days for patients with PPID was 9.9 (9.4; 10.4). At the same time, treatment duration in hospital for the patients with PE after CS was significantly longer – 10.3 (9,8;11,5) bed-days versus the women after SVD – 9.3 (8,7;10,1) bed-days. On average, the puerperant women were discharged from hospital after conservative therapy on day 18 (15; 21) of the postpartum period, and on day 20 (19.3; 21.9) after operative treatment. According to ultrasound assessment, uterine size has decreased by 54.6%, the total number of white blood cells measured by WBC count was 6.2 (5.5; 6.8)×109/l, and the C-reactive protein value was 17.7 (5.9; 33.2) mg/l.
All patients with PPID were discharged from hospital in satisfactory condition for subsequent outpatient follow-up by obstetrician-gynecologist.
Therefore, cesarean section takes leading position in the general structure of all risk factors for development of PE, despite the use of modern suture material and legitimate practices of antibiotic prophylaxis and treatment regimens. The results of our study showed that most cases of PPID were diagnosed in women who underwent cesarean delivery. At the same time, PE takes the leading position in the structure of PPID and reaches 36.7% among all cases of postpartum complications, while complications of postoperative wound in the anterior abdominal wall take the leading position in the group of puerperant women after abdominal delivery (64.2%).
It should be noted that the symptoms of PE appear on average on day 11 of the postpartum period, when the puerperant women are already discharged from maternity hospitals. The clinical symptoms of PE after CS appear on day 10, that is one day earlier than in patients with SVD. Due to obscure clinical forms, diagnostic delay of PPID occurs rather often leading to moderate severity of the disease in 57.4% of cases, and development of sepsis in 3.4% of cases.
It is important to note operative activity in the group of patients with PE after abdominal delivery: virtually in every second case (45.4%), the women underwent hysteroscopy. At the same time, the frequency of surgical treatment reaches 84.6%, and is significantly higher versus the group of women with PE after SVD (65.4%).
In the spectrum of pathogens isolated from the uterine cavity, gram-negative bacteria take leading position (in 44.2% of cases), and among most often identified bacteria, Escherichia coli (32,5%) is diagnosed, (among them extended-spectrum beta-lactamases (ESBLs)+Escherichia coli are found in 21.4% of cases, Enterococcus faecalis in 18.6%, Staphylococcus epidermidis in 30%.
Also it is noteworthy that Escherichia coli isolates express resistance to ampicillin (35.8%). At the same time, it should be noted that ESBL+ Escherichia coli cultured in 21.4% of cases has extended spectrum of resistance, that may result in ineffective therapy with penicillins and first–fourth generation cephalosporins. However, according to the data in our study, most common antibiotic regimens for treatment included indication of the group of drugs co-administered with β-lactamase inhibitors combined with penicillins (in 53.4% of cases).
Conclusion
Thus, the cause of PE in women after abdominal deliveer is more severe in comparison to puerperant women after SVD: the clinical picture occurs one day earlier, there are no inflammatory markers in patients at admission to hospital, and comorbidity of anemia is more often (especially in cases of emergency C-section), antibacterial therapy lasts longer versus women with PE after SVD, and operative activity with regard to these patients is 84.6% versus 65.4% in puerperant women after SVD.
Given a gradual increase in the number of resistant microorganisms, the issue of antibiotic resistance is becoming more acute every day. At the same time, optimization of using antimicrobial agents is a key issue in the global strategy to stop spread of superbugs.
In this regard, further researches are required to study the regional characteristics of the microbial landscape in the focus of inflammation and determine antimicrobial sensitivity and resistance of isolated microorganisms to modern antibacterial drugs, as well as asses the immunological resistance of macroorganisms on the local and systemic levels. These researches will help in future to develop rational regimens of antimicrobial therapy and immunocorrection in patients with PPID, reduce the frequency of severe destructive forms, preserve fertility, as well as achieve a significant socio-economic effect by reducing duration of therapy and hospital stay for this category of patients.
References
- Сайдалиева Д.А., Додхоева М.Ф., Абдуллаева Р.А. Эпидемиология и факторы риска развития материнского сепсиса. Вестник Авиценны. 2023; 25(2): 248-59. [Saydalieva D.A., Dodkhoeva M.F., Abdullaeva R.A. Epidemiology and risk factors for maternal sepsis. Avicenna Bulletin. 2023; 25(2): 248-59(in Russian)]. https://dx.doi.org/10.25005/2074-0581-2023-25-2-248-259.
- Серов В.Н., Сухих Г.Т., ред. Клинические рекомендации. Акушерство и гинекология. 4-изд., перераб. и доп. М.: ГЭОТАР-Медиа; 2014: 545-607. [Serov V.N., Sukhikh G.T., ed. Clinical Guidelines. Obstetrics and Gynecology. 4th ed., rev. and add. M.: GEOTAR-Media; 2014: 545-607. (in Russian)].
- Российское общество акушеров-гинекологов. Ассоциация акушерских анестезиологов-реаниматологов. Септические осложнения в акушерстве. Клинические рекомендации (протокол лечения). М.; 2017. 63 с. [Russian society of obstetricians and gynecologists. Association of obstetric anesthesiologists and resuscitators. Septic complications in obstetrics. Clinical guidelines (treatment protocol). Moscow; 2017. 63 p. (in Russian)].
- Гранатович Н.Н., Волков В.Г. Сепсис в родах и послеродовом периоде как причина региональной материнской смертности. Архив акушерства и гинекологии им. В.Ф. Снегирева. 2017; 4(1): 36-9. [Granatovich N.N., Volkov V.G. Sepsis in childbirth and the postnatal period as a cause of the regional maternal mortality rate. V.F. Snegirev Archives of Obstetrics and Gynecology, Russian journal. 2017; 4(1): 36-9. (in Russian)].https://dx.doi.org/10.18821/2313-8726-2017-4-1-36-39.
- Бойко Ю.П., Шаповалова М.А., Щербин А.В., Угурчиева Х.Ю., Кашкарова И.А. Анализ материнской смертности в Российской Федерации. Основные тенденции. Прикаспийский вестник медицины и фармации. 2020;1(3-4): 8-16. [Boyko Yu.P., Shapovalova M.A., Shherbin A.V.,Ugurchieva Kh.Yu., Kashkarova I.A. Analysis of maternal mortality in the Russian Federation. The main trends. Caspian journal of medicine and pharmacy. 2020; 1(3-4): 8-16. (in Russian)]. https://dx.doi.org/10.17021/2020.1.3-4.8.16.
- Knowles S.J., O'Sullivan N.P., Meenan A.M., Hanniffy R., Robson M. Maternal sepsis incidence, aetiology and outcome for mother and fetus: a prospective study. BJOG. 2015; 122(5): 663-71. https://dx.doi.org/10.1111/1471-0528.12892.
- Надеев А.П., Жукова В.А., Новоселов В.П., Низовцев К.А., Логинова А.Б. Гнойно-септические заболевания в нозологической структуре материнской смерти. Вестник судебной медицины. 2021; 10(2): 26-33. [Nadeev A.P., Zhukova V.A., Novoselov V.P., Nizovtsev K.A., Loginova A.B. Purulent septic diseases in the nosological structure of maternal death. Bulletin o f Forensic Medicine. 2021;10(2): 26-33. (in Russian)].
- Say L., Chou D., Gemmill A., Tunçalp Ö., Moller A.B., Daniels J. et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob. Health. 2014; 2(6): e323-33. https://dx.doi.org/10.1016/S2214-109X(14)70227-X.
- Кукуруза И.Л., Титаренко Н.В., Дацюк А.И., Столярчук О.В. Анализ случаев материнской смертности от сепсиса в Винницкой области. Пути улучшения диагностики и лечения с позиции доказательной медицины. Медицина неотложных состояний. 2017; 3(82): 34-9. [Kukuruza I.L.,Titarenko N.V., Datsyuk O.I., Stolyarchuk O.V. Analysis of maternal mortality from sepsis in the Vinnytsia region. Ways to improve the diagnosis and treatment from the standpoint of evidence-based medicine. Emergency medicine Journal. 2017; 3(82): 34-9. (in Russian)]. https://dx.doi.org/10.22141/2224-0586.3.82.2017.102321.
- Коробков Н.А., Бакулина Н.В., Лодягина Н.С. Анемия воспаления - предиктор развития послеоперационной инфекции в акушерстве. Вестник Северо-Западного государственного медицинского университета им. И.И. Мечникова. 2022; 14(4): 53-61. [Korobkov N.A., Bakulina N.V., Lodyagina N.S. Anemia of inflammation is a predictor of the progression of postoperative infection in obstetrics. Herald of North-Western State Medical University named after I.I. Mechnikov. 2022; 14(4): 53-61. DOI:https://dx.doi.org/10.17816/mechnikov114844.
- Галкина Д.Е., Макаренко Т.А., Окладников Д.В. Иммунологические аспекты нормальной и патологически протекающей беременности. Вестник Российской академии медицинских наук. 2022; 77(1): 13-24. [Galkina D.E., Makarenko T.A., Okladnikov D.V. Immunological aspects of normal and pathological pregnancy. Annals of the Russian Academy of Medical Sciences. 2022; 77(1): 13-24 (in Russian)]. https://dx.doi.org/10.15690/vramn1507.
- Милованов А.П., Миханошина Н.А., Лебеденко Е.Ю. Клинико-морфологическая дифференциация сепсиса во время родов и в послеродовом периоде. Доктор.Ру. 2018; 6(150): 11-6. [Milovanov A.P., Mikhanoshina N.A., Lebedenko E.Yu. Clinical and morphological differential diagnosis of sepsis in labor and postpartum. Doctor.Ru. 2018; 6(150): 11-6.(in Russian)]. https://dx.doi.org/10.31550/1727-2378-2018-150-6-11-16.
- Чечнева М.А., Титченко Ю.П., Реброва Т.В., Бирюкова Н.В., Матвеев М.О. Новые подходы к ранней диагностике послеродового эндометрита. Российский вестник акушера-гинеколога. 2020; 20(1): 68-72. [Chechneva M.A., Titchenko Iu.P., Rebrova T.V., Biriukova N.V., Matveev M.O. New approaches to the early diagnosis of postpartum endometritis. Russian Bulletin of Obstetrician-Gynecologist. 2020; 20(1): 68 72. (in Russian)]. https://dx.doi.org/10.17116/rosakush20202001168.
- Сахаутдинова И.В., Тихонова Т.Ф., Яркина Е.И., Шульженко М.С. Клинические аспекты хирургических методов лечения послеродового эндометрита. Вестник Башкирского государственного медицинского университета. 2018; 1: 172-7. [Sakhautdinova I.V., Tikhonova T.F., Yarkina E.I., Shulzhenko M.S. Clinical aspects of surgical methods of local treatment of postpartum endometriosis. Bulletin of Bashkir State Medical University. 2018; 1: 172-7. (in Russian)].
- Баринов С.В., Блауман Е.С., Лазарева О.В., Долгих В.Т., Попова Л.Д., Новиков Д.Г., Пьянова Л.Г. Новый метод лечения больных с послеродовым эндометритом. Российский вестник акушера-гинеколога. 2019; 19(4): 65-71. [Barinov S.V., Blauman E.S., Lazareva O.V., Dolgikh V.T., Popova L.D., Novikov D.G., P'ianova L.G. A new method of treatment of patients with postpartum endometritis. Russian Bulletin of Obstetrician-Gynecologist. 2019; 19(4): 65 71. (in Russian)]. https://dx.doi.org/10.17116/rosakush20191904165.
- Министерство здравоохранения Российской Федерации. Послеродовой эндометрит. Клинические рекомендации. 2016. [Ministry of Health of the Russian Federation. Postpartum endometritis. Clinical guidelines. 2016.(in Russian)].
- Анохова Л.И., Белокриницкая Т.Е., Патеюк А.В. Эффективность, безопасность и возможности иммунотропной терапии при послеоперационных эндометритах. Медицинский совет. 2018; 13: 50-6. [Anokhova L.I., Belokrinitskaya T.E., Pateyuk A.V. Efficiency, safety and possibilities of immunotropic therapy after surgical treatment of endometriosis. Medical Council. 2018; (13): 50-6. (in Russian)]. https://dx.doi.org/10.21518/2079-701X-2018-13-50-56.
- Докудаева Ш.А. Современные представления об этиологии, патогенезе, клинике и диагностике послеродового эндометрита. Вестник национального медико-хирургического центра им. Н.И. Пирогова. 2016; 11(4): 109-15. [Dokudaeva Sh.A. Current concepts of etiology, pathogenesis, clinical presentation and diagnosis of postpartum endometritis. Bulletin of the N.I. Pirogov National Medical and Surgical Centre. 2016; 11(4): 109-15(in Russian)].
- Самчук П.М., Ищенко А.И., Розалиева Ю.Ю. Органосохраняющие технологии при гнойных воспалительных осложнениях кесарева сечения. Вопросы гинекологии, акушерства и перинатологии. 2020; 19(2): 96-103. [Samchuk P.M., Ishhenko A.I., Rozalieva Yu.Yu. Organ-sparing surgery for suppurative inflammatory complications of caesarean section. Gynecology, obstetrics and perinatology. 2020; 19(2): 96-103. (in Russian)].https://dx.doi.org/10.20953/1726-1678-2020-2-96-103.
- Доброхотова Ю.Э., Кузнецов П.А, Копылова Ю.В., Джохадзе Л.С. Кесарево сечение: прошлое и будущее. Гинекология. 2015; 17(3): 64-6. [Dobrokhotova Yu.E., Kuznetsov P.A., Kopylova Yu.V., Dzhokhadze L.S. Caesarean section: past and future. Gynecology. 2015; 17(3): 64-6. (in Russian)].
- Краснопольский В.И., Логутова Л.С., Буянова С.Н. Несостоятельный рубец на матке после кесарева сечения: причины формирования и лечебная тактика. Акушерство и гинекология. 2013; 12: 28-33. [Krasnopolsky V.I., Logutova L.S., Buyanova S.N. Inconsistent uterine scar after cesarean section: causes of formation and treatment policy. Obstetrics and Gynecology. 2013; (12): 28-33 (in Russian)].
- Тирская Ю.И., Баринов С.В., Долгих Т.И., Новиков А.А., Иванова О.В., Овчинникова Е.М. Профилактика развития послеродового эндометрита у родильниц группы инфекционного риска. Акушерство и гинекология. 2013; 3: 75-9. [Tirskaya Yu.I., Barinov S.V., Dolgikh T.I.,Novikov A.A., Ivanova O.V., Ovchinnikova E.M. Prevention of postpartum endometritis in puerperas at risk for infections. 2013; (3): 75-9(in Russian)].
- Вересова А.А., Тютюнник В.Л., Кан Н.Е., Балушкина А.А. Современные представления о развитии послеродовых инфекционно-воспалительных осложнений. Вопросы гинекологии, акушерства и перинатологии. 2013; 12(4): 30-7. [Veresova A.A., Tyutyunnik V.L., Kan N.E., Balushkina A.A. Current review of the development postpartum infectious and inflammatory complications. Obstetrics, Gynecology and Perinatology. 2013; 12(4): 30-7.(in Russian)].
- Кузнецова Д.Е., Макаренко Т.А., Аверчук Е.С. Особенности микробного пейзажа в очаге воспаления при послеродовых гнойно-воспалительных заболеваниях (обзор литературы). Проблемы репродукции. 2021; 27(2): 101-7. [Kuznetsova D.E., Makarenko T.A., Averchuk E.S. Features of the microbial landscape in the focus of inflammation in postpartum purulent-inflammatory diseases (literature review). Russian Journal of Human Reproduction. 2021; 27(2): 101-7. (in Russian)]. https://dx.doi.org/10.17116/repro202127021101.
- Рустамова Ш.Б., Худоярова Д.Р., Элтазарова Г.Ш. Особенности течения беременности и исход родов на фоне цервицита шейки матки. Достижения науки и образования. 2019; 13(54): 70-2. [Rustamova S.B., Khudoyarova D.R., Eltazarova G.S. Features of the course of pregnancy and the outcome of childbirth against cervical cervicitis. Advances in science and education. 2019; 13(54): 70-2. (in Russian)].
- Токарева Е.П., Путалова И.Н., Кравченко Е.Н. Возможности лимфатической коррекции в комплексном лечении острого послеродового эндометрита. Мать и дитя в Кузбассе. 2014; 2(57): 141-3.[Tokareva E.P., Putalova I.N., Kravchenko E.N. Possibilities of lymphaticТcorrection in complex treatment of the sharp postnatal endometritis. Mother and Child in Kuzbass. 2014; 2(57):141-3. (in Russian)].
- Агарков Н.М., Аксёнов В.В., Иванов А.В., Иванов В.А., Кича Д.И., Субботина Т.И. Диагностическая значимость и кластеризация параметров системного гуморального иммунитета при остром эндометрите. Клиническая лабораторная диагностика. 2017; 62(12): 750-3. [Agarkov N.M., Aksyonov V.V., Ivanov A.V., Ivanov V.A., Kicha D.I., Subbotina T.I. The diagnostic significance and clustering of parameters of systemic humoral immunity under acute endometritis. Klin. Lab. Diagn. 2017; 62(12): 750-3. (in Russian)]. https://dx.doi.org/10.18821/0869-2084-2017-62-12-750-753.
- Агарков Н.М., Афанасова Е.П., Будник И.В. Диагностика и прогнозирование острого эндометрита по информативным параметрам клеточного иммунитета. Журнал акушерства и женских болезней. 2014; 63(6): 15-20. [Agarkov N.M., Afanasova E.P., Budnik I.V. Diagnosis and prediction of acute endometritis according to informative features of cell immunity. Journal of Obstetrics and Women's Diseases. 2014; 63(6): 15-20(in Russian)].
- Смирнова С.С., Егоров И.А., Голубкова А.А. Гнойно-септические инфекции у родильниц. Часть 2. Клинико-патогенетическая характеристика нозологических форм, этиология и антибиотикорезистентность (обзор литературы). Журнал микробиологии, эпидемиологии и иммунобиологии. 2022; 99(2): 244-59. [Smirnova S.S., Egorov I.A., Golubkova A.A. Purulent-septic infections in puerperas. Part 2. Clinical and pathogenetic characteristics of nosological forms, etiology and antibiotic resistance (literature review)). Journal of microbiology, epidemiology and immunobiology. 2022; 99(2): 244-59. (in Russian)]. https://dx.doi.org/10.36233/0372-9311-227.
- Козлов Р.С. Проблема антибиотикорезистентности в акушерстве и гинекологии. РМЖ. 2014; 1: 79. [Kozlov R.S. The problem of antibiotic resistance in obstetrics and gynaecology. Russian Medical Journal. 2014; (1): 79.(in Russian)].
- Каримова Г.Н., Муравьева В.В., Припутневич Т.В., Шмаков Р.Г. Роль микрофлоры цервикального канала родильниц в раннем пуэрперии в развитии послеродового эндометрита. Акушерство и гинекология. 2016; 11: 71-8. [Karimova G.N., Muravyeva V.V., Priputnevich T.V., Shmakov R.G. Role of the microflora from the cervical canal of purperant women in the early puerperium in the development of postpartum endometritis. Obstetrics and Gynecology. 2016; (11): 71-8. (in Russian)]. https://dx.doi.org/10.18565/aig.2016.11.71-8.
- Жабченко И.А. Современные подходы к профилактике акушерского травматизма и его последствий. Репродуктивная медицина. 2020; 2(43): 50-5. [Zhabchenko I.A. Modern going near prophylaxis of obstetric traumatism and its consequences. Reproductive Medicine. 2020: 2(43): 50-5 (in Russian)].https://dx.doi.org/10.37800/RM2020-1-15.
- Обоскалова Т.А., Глухов Е.Ю., Харитонов А.Н. Динамика и структура инфекционно-воспалительных заболеваний позднего послеродового периода. Уральский медицинский журнал. 2016; 5: 5-9. [Oboskalova T.A., Glukhov E.Yu., Kharitonov A.N. Dinamics and structure of inflammatory infections in late postanatal period. Ural Medical Journal. 2016; 5: 5-9.(in Russian)].
- Анохова Л.И., Белокриницкая Т.Е., Патеюк А.В., Кохан С.Т. Послеродовый эндометрит и его профилактика (обзор литературы). Научное обозрение. Медицинские науки. 2016; 4: 6-13. [Anokhova L.I., Belokrinitskaya T.E., Pateyuk A.V., Kokhan S.T. Postnatal endometritis and its prevention (review of literature). Science Review. Medical Science. 2016; 4: 6-13. (in Russian)].
- Saddam N. Treatment of postpartum endometrities. Rev. Fac. Agron. (LUZ). 2018; 35: 527-37.
- Беженарь В.Ф., Шапкайц В.А., Добровольская И.А., Рукояткина Е.А., Нестеров И.М. Возможности ранней диагностики современного акушерского сепсиса. Акушерство, гинекология и репродукция. 2021; 15(2): 121-31. [Bezhenar V.F., Shapkaitz V.A., Dobrovolskaya I.A., Rukoyatkina E.A., Nesterov I.M. Opportunities for early diagnostics of contemporary obstetric sepsis. Obstetrics, Gynecology and Reproduction. 2021; 15(2): 121-31.(in Russian)]. https://dx.doi.org/10.17749/2313-7347/ob.gyn.rep.2021.183.
- Торобаева М.Т., Рыскельдиева В.Т. Современный взгляд на профилактику послеродовых гнойно-септических осложнений. Научные исследования. 2017; 1(12): 73-81. [Torobaeva M.T., Ryskeldieva V.T. Modern view on the prevention of postpartum purulent-septic complications. Scientific research. 2017; 1(12): 73-81. (in Russian)].
- Егорова А.Т., Глебова Т.К., Маисеенко Д.А., Шапошникова Е.В. Гнойно-воспалительные осложнения в акушерской практике по материалам краевой клинической больницы г. Красноярска. Сибирское медицинское обозрение. 2015; 4: 47-51. [Egorova A.T., Glebova T.K., Maiseenko D.A., Shaposhnikova E.V. Pyoinflammatory complications in obstetric practice according to the materials of the Regional Clinical Hospital of Krasnoyarsk. Siberian Medical Review. 2015; (4): 47-51. (in Russian)].
Received 07.09.2023
Accepted 17.10.2023
About the Authors
Darya E. Galkina, PhD, Associate Professor at the Department of Operative Gynecology of the Institute of Postgraduate Education, Krasnoyarsk State Medical University named after Prof. V.F. Voino-Yasenetsky, Ministry of Health of the Russian Federation, 660022, Russia, Krasnoyarsk Territory, Krasnoyarsk, Partizan Zheleznyak str., 1, +7(923)376-94-33, dashsemch@mail.ru, https://orcid.org/0000-0001-7516-5203Tatyana A. Makarenko, Dr. Med. Sci., Professor, Head of the Department of Operative Gynecology of the Institute of Postgraduate Education, Krasnoyarsk State Medical University named after Prof. V.F. Voino-Yasenetsky, Ministry of Health of the Russian Federation, 660022, Russia, Krasnoyarsk Territory, Krasnoyarsk,
Partizan Zheleznyak str., 1, +7(904)895-47-99, makarenko7777@yandex.ru, https://orcid.org/0000-0002-2899-8103
Elena N. Bochanova, Dr. Med. Sci., Associate Professor, Head of the Department of Microbiology named after Associate Professor B.M. Zelmanovich, Krasnoyarsk State Medical University named after Prof. V.F. Voino-Yasenetsky, Ministry of Health of the Russian Federation, 660022, Russia, Krasnoyarsk Territory, Krasnoyarsk, Partizan Zheleznyak str., 1, +7(913)522-16-58, bochanova@list.ru, https://orcid.org/0000-0003-4371-2342
Galina A. Shageeva, PhD, Consultant of the Department of Organization of Pediatric and Obstetric-gynecological Care, Ministry of Health of the Krasnoyarsk Territory, Russia, Krasnoyarsk Territory, Krasnoyarsk, Red Army str., 3, +7(908)023-06-87, shageeva@kraszdrav.ru
Inga O. Ulyanova, PhD, Associate Professor at the Department of Operative Gynecology of the Institute of Postgraduate Education, Krasnoyarsk State Medical University named after Prof. V.F. Voino-Yasenetsky, Ministry of Health of the Russian Federation, 660022, Russia, Krasnoyarsk Territory, Krasnoyarsk, Partizan Zheleznyak str., 1, +7(913)534-97-33, Inga_Ulyanova@mail.ru, https://orcid.org/0000-0001-5354-6021
Corresponding author: Darya E. Galkina, dashsemch@mail.ru



