Clinical and diagnostic aspects of non-traumatic fractures in metabolic bone disease of prematurity
Objective. To determine the incidence and analyze the clinical and diagnostic characteristics of the metabolic bone disease of prematurity (MBDP) presenting with non-traumatic fractures.Kukhartseva M.V., Narogan M.V., Kozlova A.V., Ryumina I.I., Orlovskaya I.V., Parnas A.Yu., Semenova O.E., Zubkov V.V., Degtyarev D.N.
Materials and methods. The study was conducted in 2013–2017 and comprised all premature infants with MBDP-associated bone fractures, who had no surgical diseases.
Results. Fractures were diagnosed in 7 children; femoral and rib fractures were more common than humerus fractures. All children received total parenteral nutrition lasting from 9 to 43 (median 19) days; in 5 of them, enteral nutrition did not meet their nutritional needs. In the 1st month of life, all infants had severe hypophosphatemia; in 5 patients the level of alkaline phosphatase increased.
Conclusion. MBDP-associated bone fractures were observed in 2.1% of children with birth weight less than 1500g (6.25% with ELBW; 0.4% with VLBW). All children had multiple risk factors for MBDP. In 3 infants, bone fractures were diagnosed belatedly, when they were in the stage of consolidation.
Keywords
Recent advances in neonatal intensive care have markedly improved the survival rates of very low birth weight (VLBW) and extremely low birth weight (ELBW) infants that has led to the emergence of new conditions and disorders occurring in this category of patients. One of these diseases that carry significant comorbidity in VLBW and ELBW neonates is a metabolic bone disease or osteopenia of prematurity [1–4].
Metabolic bone disease of prematurity (MBDP) is characterized by increased bone fragility due to a decreased bone mineral content as a result of inadequate calcium and phosphorus stores and vitamin D deficiency associated with prematurity [3, 5].
Osteopenia occurs in 23–30% and 31–55% of VLBW and ELBW infants, respectively, while bone fracture rates in preterm ELBW babies can reach 7–10%. However, the incidence of osteopenia seems to have decreased in recent years [6– 8].
To date, the diagnosis of MBDP continues to be based mainly on radiologic findings. However, radiation exposure and the lack of unified radiological criteria for osteopenia of prematurity restrict a wide use of this method [1, 4–6, 9]. The limited use of X-ray radiography in premature babies combined with a nonspecific clinical picture and the absence of effective biomarkers of MBDP makes it difficult to verify the diagnosis.
This study aimed to determine the incidence and analyze the clinical and diagnostic characteristics of MBDP presenting with non-traumatic fractures.
Material and methods
The study was conducted at the V.I. Kulakov NMRC for OG&P of Minzdrav of Russia from January 2013 to December 2017 and comprised all premature infants with MBDP-associated bone fractures, who had no surgical diseases. Exclusion criteria of the study were syndromic and hereditary disorders and surgical diseases.
The study analyzed the following patient characteristics: gestational age (GA), birth weight and length in relation to gestational age (according to Fenton preterm grows chart, 2013), gender; nutritional data (duration of total parenteral nutrition, age of achieving full enteral feeding, if physiological needs of the preterm neonate are met), the presence of necrotizing enterocolitis of non-surgical stages (NEC), moderate-to-severe and severe bronchopulmonary dysplasia and cholestasis.
Clinical evaluation included an estimation of the following clinical signs of MBDP: cranial deformity (“tower” skull, “Olympic” forehead, flattening of the skull from the sides, craniotabes), chest deformity (Harrison’s sulcus), relatively short limbs, and growth restriction.
Fractures were diagnosed using radiologic imaging (X-ray, multislice spiral computed tomography (MSCT)). Radiologic characteristics of osteopenia were defined by the grading offered by Koo W.W. et al. (1982–1984): grade 1- loss of the density of the white line in the metaphyses and thinning of the cortex; grade 2 - cup like lucid metaphyses and sub-periosteal growths; grade 3 - grade 2 changes plus fractures [4, 5, 10].
Retrospectively analyzed were serum levels of total calcium, phosphate, alkaline phosphatase (blood biochemistry tests), and vitamin D concentrations (enzyme immunoassay method).
Drug therapy was analyzed to identify medications that may adversely affect phosphate-calcium metabolism (caffeine, furosemide, dexamethasone, and phenobarbital).
Results
Over five years of follow-up (2013–2017), non-traumatic fractures were diagnosed in 7 premature babies with MBDP including 6 ELBW and 1 VLBW infant (Fig. 1).

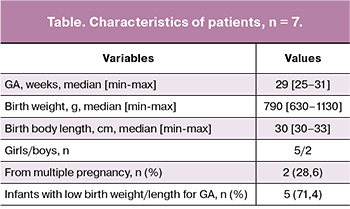
All premature infants with fractures were born before 32 weeks’ gestation, most of them were girls from singleton pregnancies; 5 (71.4%) infants had low birth weight/length for GA (table).
All study participants received a long-term total parenteral nutrition lasting from 9 to 43 days (median 19 days), which is a significant etiopathogenetic factor of MBDP. Doses of calcium for parenteral nutrition were calculated based on calcium blood level. Since parenteral phosphoruspreparations were not available, all children had hypophosphatemia, which could only be corrected with the transition to enteral feeding. Vitamin D was included in a vitamin solution for parenteral nutrition, which was administered from the first day of life in a standard dose (160 IU/kg/day, but not more than 400 IU/day).
Full enteral feeding was achieved on days 20–46 (median 31.5) of life. Four (57.1%) infants were fed with milk mixtures, 1 (14.3%) with breast milk, and 2 (28.6%) received mixed feeding. In 5 (71.4%) premature infants, enteral feeding did not meet their nutritional needs. Due to a history of NEC and/or intolerance to enteral load, 2 children were for a long time (within 14 days) fed with milk formula based on highly hydrolyzed protein, 3 children had late and insufficient breast milk fortification.
 Oral vitamin D was administered to all infants after the transition to full enteral feeding. Doses of vitamin D were determined by the attending physician and ranged from 1000 IU/day to 3000 IU/day, depending on the clinical condition of the child and the findings of laboratory testing and clinical examination.
Oral vitamin D was administered to all infants after the transition to full enteral feeding. Doses of vitamin D were determined by the attending physician and ranged from 1000 IU/day to 3000 IU/day, depending on the clinical condition of the child and the findings of laboratory testing and clinical examination.
NEC (non-surgical stages) was observed in 6 (85.7%) children; BPD was diagnosed in 3 (42.9%) children including one severe and 2 of moderate severity. Cholestasis syndrome due to prolonged parenteral nutrition was detected in 6 (85.7%) premature infants.
In all patients, clinical signs of MBDP were observed from the 2nd month of life. They manifested as characteristic changes in the skull (Fig. 2), rachitic chest deformity, shortening of the limbs and growth restriction.
Bone fractures (femoral, humeral) directly at the time of their occurrence were diagnosed in 4 (57.1%) children between 19 and 76 days of life. Bone radiography was done as an assessment of suspected fractures (pain on palpation, swelling, movement restriction, marked anxiety of the child). In 3 (42.8%) infants, bone fractures were diagnosed belatedly, when they were in the stage of consolidation at the age of 67, 71, and 119 days of life; at the same time, in 2 children, consolidated rib fractures were detected during an MSCT for BPD to assess the lung structures, and in 1 child a hip fracture at the consolidation stage was detected by a planned knee radiography to assess the MBDP stage. By localization, 3 (42.9%), 3 (42.8%), and 2 (28.6%) children had rib, femoral and humeral fractures, respectively.
Several fractures were observed in 2 (28.6%) children: in one case it was a femoral fracture and a fracture of the posterior process of the rib 9, and in the second case - a fracture of the posterior processes of the ribs 5–8. Examples of radiographic images of bone fractures in the study patients are presented in Fig. 3, 4.
Typical dynamics of blood biochemical parameters were marked hypophosphatemia in the first month of life, accompanied by an episodic increase in the blood concentration of total and/or ionized calcium. Then, after a successful start of enteral feeding, the dynamics changed: the phosphate level approached normal values or returned to normal, and calcium often decreased. Against this background, the level of alkaline phosphatase in most children gradually increased, reaching maximum values at the time of the fracture. Hypophosphatemia was noted in all children; its minimum values were very low: 0.14–0.30 mmol/l. Episodes of hypercalcemia were noted in 5 (71.4%) children, while the maximum blood level of total calcium reached 3.78 mmol/l. Hypocalcemia at 1.5–2 months of life was detected in all children; the minimum level was 1.4 mmol/l. In 5 (71.4%) patients the concentration of alkaline phosphatase increased to 1254 - 2531 U/l (with reference values of 50–360 U/l). Typical changes in serum levels of phosphate, total calcium, and alkaline phosphatase are presented in Fig. 5.
In 5 children, blood levels of 25 (OH) D were analyzed in 57–77 days of life. Vitamin D deficiency (9.6 ng / ml) was observed in 1 child, deficiency (12.7–28.7 ng/ml) in 3 children, and normal level (42.8 ng/ml) in 1 child. These findings are insufficient to conduct a full analysis. However, it can be noted that vitamin D levels in our patients varied significantly.
Of the drugs that adversely affect bone mineralization, caffeine was used most frequently and continuously (loading dose of 20 mg/kg, maintenance dose 5–10 mg/kg/day); all patients received caffeine from 3 to 11 weeks (median 5.5). Furosemide was administered to only one child for 4 days. Also, only one child received a course of dexamethasone for 8 days in a total dose of 0.85 mg/kg.
Phenobarbital was not used.

Discussion
Our study investigated MBDP-associated bone fractures in extremely premature babies weighing less than 1,500 g at birth. The bone fracture rate in this group was 2.1%. The incidence of fractures in children with ELBW and VLBW was 6.25% and 0.4%, respectively. As in other studies, in our study, most of the MBDP-associated bone fractures were found in ELBW infants [5, 6, 11, 12]. It should be noted that the prevalence of MBDP and related fractures is currently declining. In studies published in 1980–90s, the incidence of fractures reached 10.5% in children with a birth weight less than 1500 g [13] and 17% of ELBW infants [14]; in 2009–2013 the incidence of fractures in ELBW infants was 7–10% [6, 7]. Recent studies reported bone fracture rate of 0.6% in children weighing less than 1500g [2] and 2.5% of ELBW infants [11]. Reduction in the incidence of osteopenia of prematurity has been associated with progressive advances in enteral feeding of premature babies: fortification of breast milk, improved enteral nutrition formulas, the more rapid increase in enteral feeding, which also has occurred in our practice [15, 16].
Contrary to data reported by Viswanathan S. at all. [6], in our study, fractures were more common in girls, which, however, may be attributed to small sample size. Also, many children in our study (71.4%) had intrauterine growth restriction. Fetal developmental delay, which often occurs due to pre-eclampsia and placental insufficiency, has been recognized as a significant risk factor for MBDP [2, 3, 17].
Prolonged parenteral nutrition has been recognized as a significant risk factor for MBDP. According to available literature, total parenteral nutrition for more than 4 weeks is associated with MBDP [2, 3, 12, 17]. Given the absence of intravenous phosphorus preparations in our country, the duration of total parenteral nutrition for more than 9 days confers a very high-risk factor for MBDP and bone fractures, especially in ELBW infants. The NEC, the treatment of which requires prolonged parenteral nutrition, is very often associated with MBDP. According to our data, MBDP–associated bone fractures occurred concomitantly with NEC (non-surgical stages), BPD (severe and of moderate severity), and cholestasis in 85.7%, 42.9%, and 85.7% of infants, respectively. BPD, which is associated with malnutrition in neonates, the use of methylxanthines, loop diuretics, and especially glucocorticosteroids, is MBDP risk factors in some studies. Neonatal cholestasis syndrome has also been recognized as one of the most significant risk factors for MBDP due to the negative effect on vitamin D absorption, the inhibitory effect of bilirubin and bile acids on osteoblast function, and possibly due to the fact that it is a marker of the severity of the child’s condition and the duration of parenteral nutrition [2, 12, 18].
In most premature infants with MBDP, bone fractures are diagnosed at 2–4 months of age during the period of maximal bone demineralization [2, 6]. In our study, one child was found to have a humeral fracture at an earlier age (on the 19th day of life), while in 3 infants, rib and thigh fractures were diagnosed belatedly (between 67 and 119 days of life) when they were in the stage of consolidation. Thus, although bone fractures in premature babies usually occur between the 2nd and the 4th month of the life, the fractures may also develop earlier, which requires a differential diagnosis with a so-called “non-accidental fracture”. Our work also shows the probability of missing fractures and, therefore, of the under-diagnosis of MBDP. Similar findings have also been reported by other authors [2, 5, 7].
In our study, femoral and rib fractures were more common than humeral fractures. A similar pattern was observed in other studies, which may reflect the anatomical location of MBDP-associated fractures at present [2, 6, 7]. In earlier studies of MBDP, rib fractures were also most common, followed in descending rate order by fractures of the radial bone, humeral, elbow, femur fractures, and less frequently fractures of other bones [13].
The most frequently used biochemical markers for MBDP are the serum phosphate and alkaline phosphatase, with a characteristic of MBDP combination of low levels of phosphate and elevated levels of alkaline phosphatase [3, 9, 17, 18]. In our study, all children showed severe hypophosphatemia, reflecting an extreme deficit of phosphorus intake. Phosphorus deficiency has led to the disruption of the hydroxyapatite formation in the bones resulting in fractures due to bone demineralization. Five (71.4%) infants had an increase in alkaline phosphatase; in 2 out of 7 children (28.6%), the level of alkaline phosphatase did not increase during hospitalization. The lack of prognostic value of an isolated increase in serum alkaline phosphatase in MBDP has also been observed in some other studies. On the one hand, the level of alkaline phosphatase tends to increase in the majority of premature babies during the first 5–6 weeks, on the other hand, not all children with MBDP show an increase in this indicator [8, 19].
The current literature has reported the negative effect of some drugs such as caffeine, loop diuretics, glucocorticoids on bone formation in premature babies [3, 4, 20]. Given the small size of our sample, we were not able to assess the effect of these medications on MBDP severity.
Conclusion
Non-traumatic MBDP-associated bone fractures were observed in 2.1% of children with birth weight less than 1500g. Most often this pathology was noted in ELBW infants (6.25%); in VLBW babies, its incidence was 0.4%. Severe demineralization of bones and bone fractures are associated with a combination of factors such as ELBW, intrauterine growth restriction, total parenteral nutrition longer than 9 days (without phosphate preparations), NEC, cholestasis syndrome and sub-optimal enteral feeding. Severe hypophosphatemia, which is often, but not always, develops concurrently with an increase in alkaline phosphatase was a characteristic biochemical feature of MBDP. Femoral and rib fractures were more common than humeral ones. In 42.8% of infants, bone fractures were diagnosed belatedly, when they were in the stage of consolidation, which indicates the likelihood of missed MBDP diagnosis in neonatal practice.
References
- Rustico S.E., Calabria A.C., Garber S.J. Metabolic bone disease of prematurity. J. Clin. Transl. Endocrinol. 2014; 1(3): 85-91.
- Chin L.K., Doan J., Teoh Y.S., Stewart A., Forrest P., Simm P.J. Outcomes of standardised approach to metabolic bone disease of prematurity. J. Paediatr. Child Health. 2018; 54(6): 665-70.
- Nallagonda S., Nallagonda M., Deorukhkar A. Metabolic bone disease of prematurity – an overview. Paediatr. Child. Health. 2017; 27(1): 14-7.
- Moreira A., Jacob R., Lavender L., Escaname E. Metabolic bone disease of prematurity. NeoReviews. 2015; 16( 11): e631-41.
- Patole S., ed. Nutrition for the preterm neonate. A clinical perspective. Springer; 2013.
- Viswanathan S., Khasawneh W., McNelis K., Dykstra C., Amstadt R., Super M. et al. Metabolic bone disease: a continued challenge in extremely low birth weight infants. JPEN J. Parenter. Enteral Nutr. 2013; 38(8): 982-90.
- Smurthwaite D., Wright N.B., Russell S., Emmerson A.J., Mughal M.Z. How common are rib fractures in extremely low birth weight preterm infants? Arch. Dis. Child. Fetal Neonatal Ed. 2009; 94(2): F138-9.
- Gomella T.L., Cunningham M.D., Eyal F.G., eds. Neonatology: management, procedures, on-call problems, diseases, and drugs. 7th ed. McGraw-Hill; 2013.
- Нароган М.В., Рюмина И.И., Степанов А.В. Остеопения (метаболическая болезнь костей) у недоношенных: возможности диагностики, лечения и профилактики. Неонатология. 2014; 3: 77-83. [Narogan M.V., Ryumina I.I., Stepanov A.V. Osteopenia (metabolic bone disease) in prematurity: the possibility of diagnosis, treatment and prevention. Neonatology. 2014; 3: 77-83. (in Russian)]
- Koo W.W., Gupta J.M., Nayanar V.V., Wilkinson M., Posen S. Skeletal changes in preterm infants. Arch. Dis. Child. 1982; 57(6): 447-52.
- Ukarapong S., Venkatarayappa S.K.B., Navarrete C., Berkovitz G. Risk factors of metabolic bone disease of prematurity. Early Hum. Dev. 2017; 112: 29-34.
- Machado A., Rocha G., Silva A.I., Alegrete N., Guimarães H. Bone fractures in a neonatal intensive care unit. Acta Med. Port. 2015; 28(2): 204-8.
- Dabezies J., Warren P.D. Fractures in very low birth weight infants with rickets. Clin. Orthop. Relat. Res. 1997; (335): 233-9.
- Lyon A.J., McIntosh N., Wheeler K., Williams J.E. Radiological rickets in extremely low birthweight infants. Pediatr. Radiol. 1987; 17(1): 56-8.
- Ионов О.В., Балашова Е.Н., Ленюшкина А.А., Киртбая А.Р., Кухарцева М.В., Зубков В.В. Сравнение двух стартовых схем - быстрого и медленного увеличения объема энтерального питания у новорожденных с очень низкой массой тела в условиях отделения реанимации и интенсивной терапии. Неонатология: новости, мнения, обучение. 2015; 4: 73-81. [Ionov O.V., Balashova E.N., Lenyushkina A.A., Kirtbaya A.R., Kukhartseva M.V., Zubkov V.V. Comparison of two starting schemes - fast and slow increase in the volume of enteral nutrition in newborns with very low body weight in the conditions of the intensive care unit and intensive care. Neonatology: news, opinions, training. 2015; 4: 73-81. (in Russian)]
- Грошева Е.В., Ионов О.В., Ленюшкина А.А., Нароган М.В., Рюмина И.И. Энтеральное вскармливание недоношенных детей. В кн.: Иванов Д.О., ред. Клинические рекомендации (протоколы) по неонатологии. СПб.: Информ-Навигатор; 2016: 252-70. [Grosheva E.V., Ionov O.V., Lenyushkina A.A., Narogan M.V., Ryumina I.I. Enteral feeding of premature babies. In book: Ivanov DO, ed. Clinical recommendations (protocols) on neonatology. SPb .: Inform-Navigator; 2016: 252-70. (in Russian)]
- Montaner R.A., Fernández E.C., Calmarza C.P., Rite G.S., Oliván Del Cacho M.J. Risk factors and biochemical markers in metabolic bone disease of premature newborns. Rev. Chil. Pediatr. 2017; 88(4): 487-94.
- Gaio P., Verlato G., Daverio M., Cavicchiolo M.E., Nardo D., Pasinato A. et al. Incidence of metabolic bone disease in preterm infants of birth weight <1250 g and in those suffering from bronchopulmonary dysplasia. Clin. Nutr. ESPEN. 2018; 23: 234-9.
- Tinnion R.J., Embleton N.D. How to use… alkaline phosphatase in neonatology. Arch. Dis. Child. Educ. Pract. Ed. 2012; 97(4): 157-63.
- Ali E., Rockman-Greenberg C., Moffatt M., Narvey M., Reed M., Jiang D. Caffeine is a risk factor for osteopenia of prematurity in preterm infants: a cohort study BMC Pediatr. 2018 18(1) 9.
Received 15.06.2018
Accepted 22.06.2018
About the Authors
Kukhartseva, Marina V., neonatologist, Department of Pathology of Newborns and Premature Babies, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Health of Russia. 117997, Russia, Moscow, Ac. Oparina str. 4; PhD student at the Department of Neonatology, I.M. Sechenov First MSMU of Minzdrav of Russia (Sechenov University). 119991, Russia, Moscow, Trubetskaya str. 8, bld. 2. E-mail: m_kukhartseva@oparina4.ru.Narogan, Marina V., MD, leading researcher, Department of Pathology of Newborns and Premature Babies, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Health of Russia. 117997, Russia, Moscow, Ac. Oparina str. 4; professor, Department of Neonatology,
I.M. Sechenov First MSMU of Minzdrav of Russia (Sechenov University). 119991, Russia, Moscow, Trubetskaya str. 8, bld. 2. E-mail: m_narogan@oparina4.ru
Kozlova, Alina V., radiologist, Department of Radiology, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Health of Russia. 117997, Russia, Moscow, Ac. Oparina str. 4. E-mail: av_kozlova@oparina4.ru.
Ryumina, Irina I., MD, head of the Department of Pathology of Newborns and Premature Babies, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Health of Russia. 117997, Russia, Moscow, Ac. Oparina str. 4. E-mail: i_ryumina@oparina4.ru
Orlovskaya, Irina V., PhD, clinical care supervisor, Department of Pathology of Newborn and Premature Babies, National Medical Research Center for Obstetrics,
Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Health of Russia.
117997, Russia, Moscow, Ac. Oparina str. 4. E-mail: i_orlovskaya@oparina4.ru.
Parnas, Alexandra Yu., 5th year student, Faculty of Pediatrics, N.I. Pirogov RNRMU. 117997, Russia, Moscow, Ostrovityanova str. 1.
Semenova, Olga E., 5th year student, Medical Faculty, I.M. Sechenov First MSMU of Minzdrav of Russia (Sechenov University);
119991, Russia, Moscow, Trubetskaya str. 8, bld. 2.
Zubkov, Viktor V., MD, professor, head of the Department of Neonatology and Pediatrics, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Health of Russia. 117997, Russia, Moscow, Ac. Oparina str. 4; professor, Department of Neonatology, I.M. Sechenov
First MSMU of Minzdrav of Russia (Sechenov University). 119991, Russia, Moscow, Trubetskaya str. 8, bld. 2. E-mail: v_zubkov@oparina4.ru
Degtyarev, Dmitry N., MD, professor, deputy director for research, V National Medical Research Center for Obstetrics, Gynecology and
Perinatology named after Academician V.I. Kulakov Ministry of Health of Russia.
117997, Russia, Moscow Ac. Oparina str. 4; Head of the Department of Neonatology, I.M. Sechenov First MSMU of Minzdrav of Russia (Sechenov University).
E-mail: d_degtiarev@oparina4.ru
For citation: Kukhartseva M.V., Narogan M.V., Kozlova A.V., Ryumina I.I., Orlovskaya I.V., Parnas A.Yu., Semenova O.E., Zubkov V.V., Degtyarev D.N. Clinical and diagnostic aspects of non-traumatic fractures in metabolic bone disease of prematurity.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2019; (3): 106-13. (in Russian)
https://dx.doi.org/10.18565/aig.2019.3.106-113