Clinical course of pregnancy, childbirth and perinatal outcomes in women with intrahepatic cholestasis of pregnancy
Background. The risk of adverse perinatal outcomes is significantly high in pregnant women with intrahepatic cholestasis of pregnancy.Cemortan M.I., Sagaidac I.V.
Aim. Assessment and analysis of the characteristics of the course of pregnancy and delivery, the incidence and structure of perinatal morbidity among women with intrahepatic cholestasis of pregnancy.
Materials and methods. The study was based on a retrospective case-control study, which included 205 women aged 17–43 years. They were divided into 2 groups depending on the complications of intrahepatic cholestasis of pregnancy Statistical data were processed using IBM SPSS Statistics V21.0 software.
Results. The women, whose pregnancies were complicated by intrahepatic cholestasis of pregnancy, had an increased incidence of iron deficiency anemia, cesarean sections and needed longer hospital stay. The assessment of perinatal outcomes showed high rates of respiratory distress syndrome in neonates born to mothers with intrahepatic cholestasis of pregnancy.
Conclusion. The study revealed that intrahepatic cholestasis of pregnancy has adverse impact on perinatal outcomes, showed rising rates of preterm birth, neonatal respiratory distress syndrome and cesarean section. This correlates with the data in the published scientific literature.
Keywords
Intrahepatic cholestasis of pregnancy (ICP) also known as holestasis of pregnancy is a transient liver condition. In the European region the incidence rate is 0.5–1 % [1]. The onset of ICP is characterized by the appearance of pruritus, often localized on the palms of the hands and the soles of the feet, which tend to spread throughout the body [1]. Clinical manifestations of ICP are rare symptoms, such as yellowness of the skin, intense urine color, pallor of the stool and steatorrhea. In most cases, the first symptoms of ICP appear at the end of the second trimester of pregnancy, however, there are cases of early onset of the studied pathology – from the 11th week of pregnancy [2]. At the same time, the issue regarding ICP remains controversial: whether the clinical symptoms occur first, or the changes in liver function test (LFT) and bile acids [1, 3].
In most cases, ICP has a favorable maternal prognosis, the clinical symptoms disappear spontaneously right after birth, and LFT results return to normal values 2–4 weeks after giving birth [1, 3]. However, a number of cases have been described, when high values of LFT persisted for a long time after childbirth. [1, 4, 5]. When in postpartum period, pruritus and high levels of liver enzymes persist for a long time, the woman needs to be examined for chronic liver disease, given that this category of patients have a high predisposition to cholelithiasis, cirrhosis and other hepatobiliary disorders [3, 5].
It is difficult to establish the diagnosis of ICP, as not all patients with clinical symptoms of cholestasis in pregnancy have high values of LFT [6]. Thus, the most important diagnostic criterion for ICP is assessment of the level of bile acids in the blood serum, and their concentration more than 10 µmol / L is considered to be a reliable laboratory marker of ICP. However, the study conducted by Glantz A. et al. detected an increased risk of developing unfavorable outcomes for the fetus in case of elevated bile acids above 40 μmol/L in the mother's blood serum [7, 8]. The same study pointed out, that relatively favorable prenatal outcomes were in women with serum bile acids levels below 40 μmol/L. However, even a strict control over the dynamics of the level of bile acids and LFT values in women does not prevent the development of acute distress syndrome and intrauterine fetal death [3].
Currently, the issue regarding the tactics of pregnancy management in patients with ICP remains acute. The goal of pharmacological therapy for ICP is reduction of clinical symptoms in mothers, as well as prevention of respiratory distress syndrome in newborns and antepartum fetal deaths. First-line treatment of ICP is ursodeoxycholic acid, which has been shown to relieve maternal symptoms and improve LFT values in 75% of cases [7]. Also, it was detected that the incidence of respiratory distress syndrome in newborns decreased among women who took ursodeoxycholic acid. A number of other pharmacological agents can be used in treatment of ICP, but there is still no consensus about their effectiveness in relieving pruritus and improving LFT results. Currently, there is published evidence that such drugs as cholestyramine, phenobarbital, dexamethasone can be recommended for treatment of holestasis of pregnancy [1]. At the same time, other researchers noted a limited effect of S-adenosyl-L-methionine and activated carbon for treating ICP [3]. However, symptom relief and improvement of LFT results were not observed in some women who used ursodeoxycholic acid. In these cases, second-line therapy can be recommended, including the above drugs. [3]. In the Guidlines of the Royal College of Obstetricians and Gynaecologists devoted to cholestasis (Obstetric Cholestasis, Green-top Guideline No. 43) it was noted that there are still no sufficient studies on determining the optimal dose of ursodeoxycholic acid, as well as the need for additional use of other drugs for treatment of ICP [9]. Also, until present, there is no consensus regarding the timing of delivery in women with the pathology under study. A number of studies have noted an increased frequency of intrauterine fetal death in women with ICP after 37 weeks of pregnancy. This leads to the need for active management of such patients without continuing the pregnancy beyond this period. [8, 10]. Nevertheless, bearing in mind, that the level of bile acids over 100 µmol/L sharply increases the risk of intrauterine fetal death, an earlier delivery can be recommended, at 35–37 weeks of pregnancy [10]. However, it should be noted that most guidelines for management of patients with ICP recommend an individual approach to treatment and delivery for this category of women, including the risk assessment and possible complications associated with preterm birth and antenatal fetal death. [9].
The aim of this study was to assess and analyze the characteristics of the course of pregnancy and childbirth, the incidence and structure of perinatal morbidity among women with intrahepatic cholestasis of pregnancy.
Material and Methods
The observational study was carried out in the Department of Obstetrics and Gynecology of Nicolae Testemiţanu Moldova State University of Medicine and Pharmacy, Institute of Mother and Child (Republic of Moldova) in the period 2018–2019.
The study included 205 women, who were divided into 2 groups. Group A included 55 women whose pregnancies were complicated by ICP (the main group), and group B included 150 women, whose pregnancies were not complicated by ICP (the control group). At the same time, the babies born to mothers in both groups were also included in the study: 70 newborns in group A and 152 newborns in group B.
Statistical analysis
The statistical data were processed using IBM SPSS Statistics V21.0 software. The arithmetic mean (M) and standard deviation (SD) were calculated to describe the numerical indicators. For distribution different from normal distribution, the median (Me), as well as the interquartile range (Q1; Q3) were calculated. To compare categorical variables in the groups, the χ² test was used without Yates' continuity correction, p-value <0.05 was considered statistically significant.
Results
The age of pregnant women included in the study was 17–43 years. Most women were aged less than 35 years (44/55 (80.0%) women in group A and 114/150 (76.0%) women in group B. The average age of women included in group A was 30.3 (5.85) years, in group B – 27.7 (7.29) years.
Comparison of frequency of multiple pregnancy in the studied groups showed that in group A multiple pregnancy was in 13 cases (23,6%), of which there were 11 twin pregnancies (84,6%) and 2 triple pregnancies (15,4%). In the control group, multiple pregnancy was observed in 2 women (1.3%). Twin pregnancies were in both cases (Table 1).
There was a high frequency of iron deficiency anemia in pregnant women in group A compared to pregnant women in group B – 26/55 (47.3%) and 18/150 (12.0%), respectively. It should be noted, that in group A, in 1/55 (1.8%) case the diagnosis of iron deficiency anemia stage II was established, and in group B only stage I was detected.
To assess the characteristics of the course of pregnancy and delivery in women in the studied groups, the duration of hospitalization of pregnant women, the timing and method of delivery, frequency and category of cesarean section, blood loss during childbirth were studied.
The results of the study revealed that pregnant women with ICP stayed in hospital for a longer time. Thus, the duration of hospitalization for women in group A ranged from 2 to 35 days, on average 10 (7; 12) days. In group B, duration of hospital stay was 2–19 days, and average length of stay was 40 (3; 7) days. It should be noted that, 24/55 (43.6%) women in group A required admission to intensive therapy and intensive care units compared to 26/150 (17.3%) women in group B.
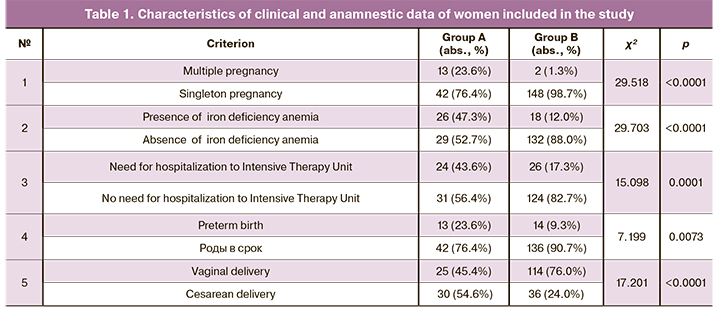
The comparative analysis revealed increased frequency of preterm births among women with ICP (group A), although in both groups, term births prevailed – in 42/55 (76.4%) women in group A and in 136/150 (90.7%) women in group B (Table 1). However, in group A, 2/55 (3.63%) cases of extremely preterm delivery at gestational age 29 and 30 weeks occurred, respectively.
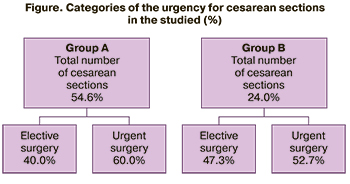 Vaginal births prevailed in group B (114/150 cases) (76.0%). Cesarean sections rate was 54.6 % in group A compared to 24% in group B. In both groups, urgently performed surgeries prevailed to a certain extent (Fig.). At the same time, in the group of patients with ICP these indicators were higher than in group B. Thus, 60.0% of the total number of surgery among women with ICP were performed urgently. The indications for cesarean section in both groups were: premature detachment of a normally positioned placenta, placenta previa, breech presentation of the fetus, uncorrectable weakness of labor, intrauterine growth restriction and deterioration of the pregnant woman’s condition in the studied group.
Vaginal births prevailed in group B (114/150 cases) (76.0%). Cesarean sections rate was 54.6 % in group A compared to 24% in group B. In both groups, urgently performed surgeries prevailed to a certain extent (Fig.). At the same time, in the group of patients with ICP these indicators were higher than in group B. Thus, 60.0% of the total number of surgery among women with ICP were performed urgently. The indications for cesarean section in both groups were: premature detachment of a normally positioned placenta, placenta previa, breech presentation of the fetus, uncorrectable weakness of labor, intrauterine growth restriction and deterioration of the pregnant woman’s condition in the studied group.
Average estimated blood loss during vaginal delivery was comparable in both groups: 308 (15) mL in group A and 318 (13) mL in group B. At the same time, in cases, when cesarean section was performed, the total volume of blood loss increased in the main group (721 (42) mL in group A compared to 626 (19) mL in group B). It should be noted, that in group A, in 4/55 (7,2%) cases the total blood loss was more than 1000 ml., of them massive blood loss (1500 mL) was in 1 case, which necessitated hemostatic hysterectomy. It should be mentioned that there were no cases of massive bleeding in group B. In all cases, the volume of blood loss was less than 1000 mL.
The analysis of the obtained data on perinatal outcomes showed, that all newborns included in the study were born-alive infants. Consequently, no cases of antenatal fetal death were registered. To assess the newborn’s condition, the Apgar score was used at the 1st and 5th minutes after birth. Based on the obtained data, it was found that in most cases infant’s general condition was satisfactory at birth. However, in the main group, 9/70 (12.8%) cases of intrauterine hypoxia (Apgar score of 4-6 points at the 1st minute) were detected compared to 4/152 (2.6%) cases of fetal hypoxia in group B.
The analysis of body weight of babies at birth showed that average weight of newborns in group A was 2883 (718) g compared to 3453 (552) g in group B. It was found, that the percentage of neonates with a birth weight ≥2500 g in group A was higher compared to the control group (18/70 (25.7%) neonates and 12/152 (7.9%) babies, respectively). This is likely due to the increased incidence of preterm births and multiple pregnancies in women with ICP in group A (Table 2).
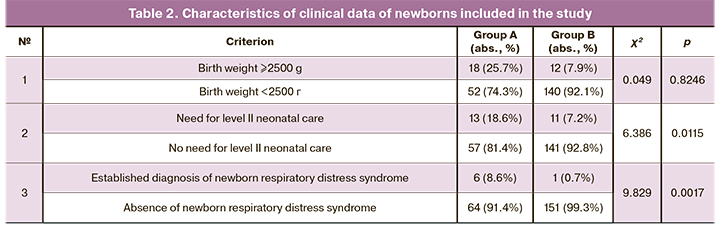
Average length of hospital stay was assessed. It was 4 (4; 8) days in group A and 3 (3; 5) days in group B. The study detected that 12/70 (17.1%) newborns required intensive care and therapy in group A, and 9/152 (5.9%) newborns in group B. 13/70 (18.6%) newborns needed level II neonatal care in group A, and 11/152 (7.2%) newborns in group B.
The study established that the diagnosis of respiratory distress syndrome was detected in 6/70 (8.6%) cases among the babies born to women with ICP, of which in 5/6 (83.3%) cases the neonates needed administration of surfactant. At the same time this diagnosis was established in 1/152 (0.7%) neonate in the control group. The percentage of congenital anomalies during the fetus’s development was evenly distributed in both studied group – 2.8% cases in group A and 2.6% in group B. Developmental anomalies of the fetuses identified during the study were congenital malformations of the cardiovascular system (tetralogy of Fallot, congenital heart defects) and congenital bone deformities.
Discussion
Currently, the etiology and pathogenesis of ICP are not completely studied. It is believed, that risk factors for the ICP are multiple pregnancy, a burdened family anamnesis history, manifestations of intrahepatic cholestasis during previous pregnancies, as well as using hormonal contraception [11]. According to the published data, this pathology is most common in pregnant women with multiple pregnancy compared to singleton pregnancy (20.9% and 4.7%, respectively). This is consistent with the obtained results [12].
ICP poses a significant risk to the fetus of perinatal mortality and reaches 11–20% in untreated cases. Perinatal morbidity and mortality are associated with preterm birth, respiratory distress syndrome, and the presence of meconium in the amniotic fluid [13]. The systematic review of 13 studies found no significant difference in the incidence of intrauterine fetal death in women with ICP compared to the general population. At the same time, there was a higher risk of preterm birth in women with ICP, as well as an increase in the frequency of caesarean section in this group of patients. [14]. Reid R. et al. reported that preterm birth rate in women with ICP was 36%, and Saleh M.M. et al. reported 44% [14]. Compared with these figures, the results of the present study revealed that preterm birth rate was 23.6% in cases complicated by ICP.
Similar to the results of our study, Rosales C. et al. noted an increased incidence of caesarean section in women with ICP up to 36% [13]. This could be attributed to several reasons, including the incidence of preterm birth, possible complications of pregnancy and delivery, including fetal abnormalities detected by cardiotocography, and increased level of meconium amniotic fluid in women with ICP [14].
Meta-analysis showed that in cases of ICP, body weight of babies at birth was lower than in the control group. Moreover, early onset of ICP in the mother was associated with low neonatal birth weight [15]. A number of studies have shown that there is an increased risk of respiratory distress syndrome in the fetus, both in cases of labor induction and in cases of caesarean delivery, regardless of gestational age. There is also some evidence that respiratory distress syndrome in newborns born to mothers with ICP may be a consequence of the pathological process of the disease under study. [3]. According to Arthuis C et al. (2020), as well as the data obtained by us, with ICP, the incidence of respiratory distress syndrome in newborns increases compared to the control group [16].
Conclusion
Thus, ICP is a reversible pathology caused by the changes in liver function associated with pregnancy. Along with the changes in LFT and clinical symptoms in the mother with ICP, a number of complications of pregnancy and delivery are recorded (higher frequency of preterm birth, meconium stained amniotic fluid, fetal distress syndrome), which determine the special clinical significance of the pathology under study. Timely diagnosis and active management of women with ICP can reduce the risks of perinatal complications, both in the mother and in the fetus.
References
- Williamson C., Geenes V. Intrahepatic cholestasis of pregnancy. Obstet. Gynecol. 2014; 124(1): 120-33. https://dx.doi.org/10.1097/AOG.0000000000000346.
- Stulic M., Culafic D., Boricic I., Stojkovic Lalosevic M., Pejic N., Jankovic G. et al. Intrahepatic cholestasis of pregnancy: A case study of the rare onset in the first trimester. Medicina (Kaunas). 2019; 55(8): 454. https://dx.doi.org/10.3390/medicina55080454.
- Smith D.D., Rood K.M. Intrahepatic cholestasis of pregnancy. Clin. Obstet. Gynecol. 2020; 63(1): 134-51. https://dx.doi.org/10.1097/GRF.0000000000000495.
- Manzotti C., Casazza G., Stimac T., Nikolova D., Gluud C. Total serum bile acids or serum bile acid profile, or both, for the diagnosis of intrahepatic cholestasis of pregnancy. Cochrane Database Syst. Rev. 2019; (7): CD012546. https://dx.doi.org/10.1002/14651858.CD012546.pub2.
- Hämäläinen S.T., Turunen K., Mattila K.J., Kosunen E., Sumanen M. Long-term survival after intrahepatic cholestasis of pregnancy: A follow-up of 571 mothers. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019; 240: 109-12. https://dx.doi.org/10.1016/j.ejogrb.2019.06.008.
- Успенская Ю.Б., Кузнецова И.В., Шептулин А.А. Сложности дифференциальной диагностики внутрипеченочного холестаза беремкенных. Медицинский алфавит. 2018; 3(22): 51. [Uspenskaya Yu.B., Kuznetsova I.V., Sheptulin A.A. Difficulties in differential diagnosis of intrahepatic cholestasis in pregnant women. Medical alphabet. 2018; 3(22): 51. (in Russian)].
- Westbrook R.H., Dusheiko G., Williamson C. Pregnancy and liver disease. J. Hepatol. 2016; 64(4): 933-45. https://dx.doi.org/10.1016/j.jhep.2015.11.030.
- Glantz A., Marschall H.U., Mattsson L.Å. Intrahepatic cholestasis of pregnancy: relationships between bile acid levels and fetal complication rates. Hepatology. 2004; 40(2): 467-74. https://dx.doi.org/10.1002/hep.20336.
- Royal College of Obstetricians and Gynaecologists. Obstetric cholestasis. Green-top guideline no. 43. 2011. Available at: http://www.rcog.org.uk/files/rcog-corp/GTG43obstetriccholestasis.pdf
- Palmer K.R., Xiaohua L., Mol B.W. Management of intrahepatic cholestasis in pregnancy. Lancet. 2019; 393(10174): 853-4. https://dx.doi.org/10.1016/S0140-6736(18)32323-7.
- Geenes V., Williamson C., Chappell L.C. Intrahepatic cholestasis of pregnancy. Obstetrician Gynaecol. 2016; 18(4): 273-81. https://dx.doi.org/10.1111/tog.12308.
- Вороник Ю.Н., Мацюк Я.Р. Холестаз беременных: тиопатогенез, лечение и прогноз (обзор). Вестник Смоленской государственной медицинской академии. 2018; 17(3): 75-82. [Voronik Yu.N., Matsuk Ya.R. Cholestasis of pregnancy: etiology and pathogenesis, treatment and prognosis (review). Bulletin of the Smolensk State Medical Academy. 2018; 3. (in Russian)].
- Medda S., Sengupta S., Palo U. A study of the outcome of pregnancy complicated by obstetric cholestasis. Int. J. Reprod. Contracept. Obstet. Gynecol. 2018; 7(3): 996-1001. https://dx.doi.org/10.18203/2320-1770.ijrcog20180880.
- Mohan M., Antonios A., Konje J., Lindow S., Ahmed Syed M., Akobeng A. Stillbirth and associated perinatal outcomes in obstetric cholestasis: a systematic review and meta-analysis of observational studies. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019; 3: 100026. https://dx.doi.org/10.1016/j.eurox.2019.100026.
- Li L., Chen Y.H., Yang Y.Y., Cong L.E. ffect of intrahepatic cholestasis of pregnancy on neonatal birth weight: a meta-analysis. J. Clin. Res. Pediatr. Endocrinol. 2018; 10(1): 38-43. https://dx.doi.org/10.4274/jcrpe.4930.
- Arthuis C., Diguisto C., Lorphelin H., Dochez V., Simon E., Perrotin F. et al. Perinatal outcomes of intrahepatic cholestasis during pregnancy: An 8-year case-control study. PLoS One. 2020; 15(2): e0228213. https://dx.doi.org/10.1371/journal.pone.0228213.
Received 11.02.2021
Accepted 02.04.2021
About the Authors
Maria I. Cemortan, PhD student, Nicolae Testemitanu State University of Medicine and Pharmacy of the Republic of Moldova, Chisinau, Republic of Moldova.Tel.: +37369672425. E-mail: maria.cemortan@usmf.md. ORCID: 0000-0003-3137-7524. bd. Ştefan cel Mare şi Sfânt, 165, mun. Chișinău, MD-2004, Moldova.
Irina V. Sagaidac, university аssistant, PhD, Nicolae Testemitanu State University of Medicine and Pharmacy of the Republic of Moldova, Chisinau, Republic of Moldova.
E-mail: irina.sagaidac@usmf.md. ORCID: 0000-0003-2491-9612. bd. Ştefan cel Mare şi Sfânt, 165, mun. Chișinău, MD-2004, Moldova.
For citation: Cemortan M.I., Sagaidac I.V. Clinical course of pregnancy, childbirth and perinatal outcomes in women with intrahepatic cholestasis of pregnancy.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 5: 94-99 (in Russian)
https://dx.doi.org/10.18565/aig.2021.5.94-99



