Clinical observation of an infant with epidermolytic ichthyosis and congenital lung
Epidermolytic ichthyosis is a rare congenital keratinopathic ichthyosis characterized by a blistering phenotype at birth, which progressively becomes hyperkeratotic. The disease has an estimated prevalence of 1:200,000–300,000 newborns. It is caused by mutations in the genes encoding epidermal suprabasal keratins 1 (KRT1; 12q13.13) and 10 (KRT10; 17q21-q23), which damage intermediate keratin filaments in the suprabasal keratinocytes. Bullous (epidermolytic) ichthyoses are manifested by symptoms, such as erythroderma at birth, skin erosions and blisters, hyperkeratosis, and increased susceptibility to infections.Ryumina I.I., Marycheva N.M., Dinov B.A., Dorofeeva E.I., Perepyolkina A.E., Orlovskaya I.V., Narogan M.V., Koronets K.A., Burov A.A., Trofimov D.Yu., Vasilyev G.S., Bychenko V.G., Kozlova A.V., Filippova E.A., Zubkov V.V., Degtyarev D.N.
Case report. The paper describes a clinical case of epidermolytic ichthyosis and congenital lung malformation in an infant. It presents the clinical manifestations of epidermolytic ichthyosis, its current diagnostic methods, and specific features of therapy.
Conclusion. The described clinical case is unique due to the concurrence of two congenital diseases in one infant, affecting different body systems, and these associations require further investigation.
Keywords
Epidermolytic ichthyosis (erythroderma ichthyosiform bullosa, epidermolytic hyperkeratosis, ichthyosis bullosa, ichthyosis epidermolytica, bullous ichthyosiform erythroderma, epidermolytic generalized hyperkeratosis, bullous ichthyosiform hyperkeratosis, congenital universal akantokeratoliz) is a rare congenital keratinopathic ichthyosis, which is characterized by a blistering phenotype at birth with progression to a hyperkeratic phenotype. The mode of inheritance is autosomal dominant, the prevalence is estimated from 1: 200.000 to 1: 300.000 newborn, men and women are affected equally often [1, 2]. The disease is caused by mutations in the genes encoding epidermal suprabasal keratins 1 (KRT1; 12q13.13) and 10 (KRT10; 17q21-q23), which damage intermediate keratin filaments in suprabasal keratinocytes. There is a definite correlation between genotype and phenotype, the position of the mutation of gene may affect the severity of the phenotype [3]. Bullous (epidermolytic; keratinopathic) ichthyosis is manifested by the symptoms, such as erythroderma at birth, erosion, blisters on the skin, needle-shaped hyperkeratosis, increased susceptibility to superinfections, pronounced hyperkeratosis (brown/ dirty gray/brown-black colour of the skin folds); distinctive foul odour [4]. The mutations in the KRT1 gene provide involvement of the skin on the palms of the hands and soles of the feet.
In this pathology, histological examination manifestsshows intercellular dissociation, degenerative changes in the spinous layer of epidermis leading to the destruction of intercellular bridges (areas of acantholysis) [5]. Electron microscopy reveals large zones of the perinuclear cytoplasmic reticulum, the mass of ribosomes and mitochondria in the cells of the granular and the upper part of the spinous layers, thickening of tonofilaments, vertical orientation of epidermal cells in the stratum corneum, keratinization defects [2].
The clinical symptoms of the disease in newborns should be differentiated from toxic epidermal necrolysis, hereditary epidermolysis bullosa, pigment incontinence, congenital herpes simplex [1].
The article presents the clinical observation of the baby with epidermolytic ichthyosis and congenital lung malformation, who was born in the Perinatal Center.
Clinical observation
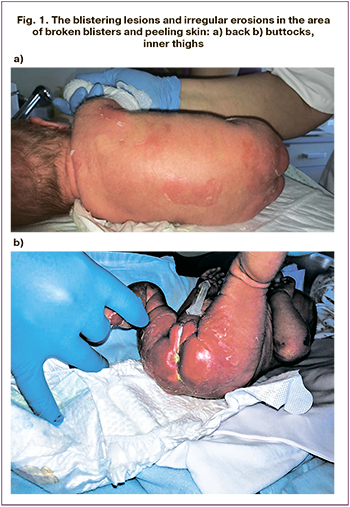 The female baby was born full-term from the 3rd pregnancy of a 29 years old woman. The pregnancy course was complicated by respiratory viral infection with fever up to 39.40 C, edema and proteinuria. This was the second term labor in woman, who underwent cesarian section at 38 weeks. A female baby was born with the body weight of 2,850 g and a length of 51 cm. The Apgar score was 8/8 points. On examination, the blistering lesions and irregular erosions in the area of broken blisters and peeling skin were noted (Fig. 1). A differential diagnosis was made between infectious skin diseases and hereditary pathology (epidermolysis bullosa, ichthyosis). There were no signs of infectious toxicosis in the baby. Microbiology tests (cultures from non-sterile loci, blood, blister contents, blood PCR, throat and rectal swabs to detect gram-negative and gram-positive bacterial flora), excluded infectious origin of the skin lesions. Antenatally, there were no signs of congenital lung disease.
The female baby was born full-term from the 3rd pregnancy of a 29 years old woman. The pregnancy course was complicated by respiratory viral infection with fever up to 39.40 C, edema and proteinuria. This was the second term labor in woman, who underwent cesarian section at 38 weeks. A female baby was born with the body weight of 2,850 g and a length of 51 cm. The Apgar score was 8/8 points. On examination, the blistering lesions and irregular erosions in the area of broken blisters and peeling skin were noted (Fig. 1). A differential diagnosis was made between infectious skin diseases and hereditary pathology (epidermolysis bullosa, ichthyosis). There were no signs of infectious toxicosis in the baby. Microbiology tests (cultures from non-sterile loci, blood, blister contents, blood PCR, throat and rectal swabs to detect gram-negative and gram-positive bacterial flora), excluded infectious origin of the skin lesions. Antenatally, there were no signs of congenital lung disease.
The baby was examined by a dermatologist. Based on the clinical picture, the diagnosis of epidermolytic ichthyosis was stated. Whole exome sequencing confirmed the diagnosis – mutations in the KRT10 gene were found.
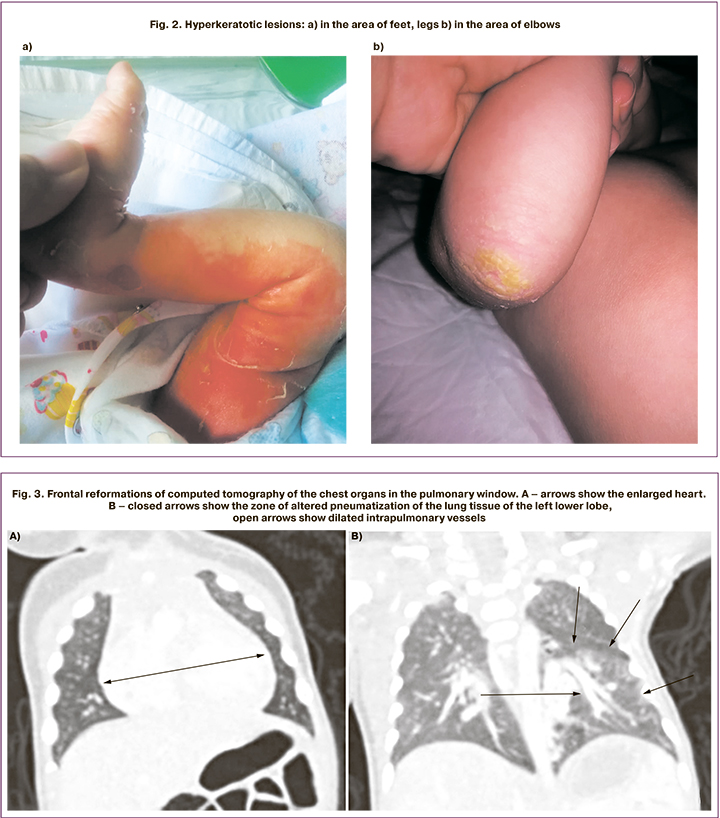
Local treatment was carried out (aseptic dressings on the affected skin areas, treatment with emollients). These measures improved the skin condition, with hyperkeratotic areas appearing on the feet, legs, elbows and hands (Fig. 2). On the 6th day of life, the inflammatory changes of the urine (leukocytes covering the entire visual field) were noted. Antibacterial therapy with positive effect was conducted by intravenous bolus injection of Ampisid (75–150 mg/kg/day), and completed by the 12th day of life. On the 14th day of life, the baby’s health condition worsened due to the onset of respiratory disorders, such as tachypnea (70 breaths/min) with subcostal retraction, tachycardia (170 beats/min), and harsh systolic murmurs. Taking into account the detected changes, echocardiographic monitoring was started, showing pulmonary hypertension – with right ventricular pressure as high as 85 mmHg. The enlargement and hypertrophy of the right ventricle and moderate hypertrophy of the left ventricle of the heart were noted. The level of N-Terminal Pro-B-Type Natriuretic Peptide (NT-proBNP) was 38728 pg/mL. The clinical symptoms of respiratory disorders were accompanied by an increase in the number of blood leukocytes up to 19.6 х 109/l. The X-ray showed decreased transparency of the lung fields with an enhancement in the radiographic lung pattern on the right side, and the signs of the right ventricular pressure overload were noted. Antibacterial therapy of neonatal pneumonia was conducted by intravenous drip infusion of Vancomycin (30 mg/kg/day) and Sulperazone (80 mg/kg/day) for 7 days. Considering the clinical signs of heart failure, dilated cardiomyopathy, persisting signs of pulmonary hypertension, therapy with diuretics was conducted – Verospiron (3.5–4.0 mg/kg/day), Furosemide (1–2 mg/kg/day orally). On the 26th day of life, vasodilator therapy with Sildenafil-Cardio (Revatio), 1 mg/kg/day orally), was introduced; however, the circulatory insufficiency remained persistent. Echocardiography exhibited cardiomegaly, and pulmonary hypertension with right ventricular pressure 60 mmHg was documented. On the 33rd day of life, multispiral computed tomography of the thoracic and abdominal cavity with intravenous contrasting was performed. The left lung malformation was detected, including intralobar sequestration of the left lower lobe and blood shunting through an abnormal blood vessel to the pulmonary circulation; the left inferior pulmonary vein dilatation followed by stenosis at its junction to the atrium; hypoplasia of the left pulmonary artery; inferior vena cava thrombosis with partial recanalization and formation of paravertebral plexuses (Fig. 3, 4).
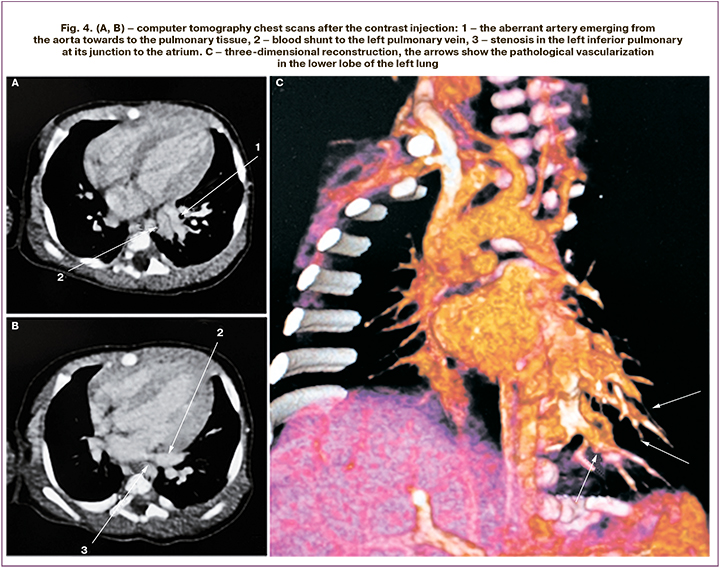
The severity of the baby’s condition was caused by a congenital left lung malformation with vascular shunt from the aorta to the inferior pulmonary veins, which caused pulmonary hypertension, the heart chambers overload and heart failure. Considering the large volume of shunted blood, the signs of pulmonary hypertension, the heart chambers overload and heart failure, the baby was transferred to the Neonatal Surgery Department on the 39th day of life, where thoracoscopic surgery with anomalous vessel ligation of the left lower lung lobe was performed. Postoperatively, the baby’s condition improved progressively. Decrease in the cardiac chambers size, reduction of the pulmonary artery pressure to 40–44 mmHg with systemic pressure 118/66 mmHg were documented. The level of N-terminal pro-brain natriuretic peptide (NT-proBNP) in plasma decreased to 977.8 pg/mL. On the 40th day of life, the baby was transferred to the Department of Pathology of Newborns and Premature Infants, and on the 48th day was discharged home in the satisfactory condition. Echocardiography before the discharge showed an open oval window, slightly thickened myocardial walls in both ventricles, with no further progression, a minor increase in the size of the left chamber of the heart, moderate hypoplasia of the left pulmonary artery; the pump and contractile myocardium functions were sufficient, and there was no evidence of the presence of pulmonary hypertension.
Major clinical diagnosis: Multiple congenital malformations – еpidermolytic ichthyosis; congenital lung malformation (intralobar sequestration of the left lower lobe with pronounced shunt in the blood vessel of pulmonary circulation).
Concomitant diseases: neonatal pneumonia, urinary tract infection.
Complications: Secondary pulmonary hypertension (associated form); dilated cardiomyopathy; circulatory insufficiency, grade 1–2A.
Regular echocardiography and cardiologic surveillance were conducted. The baby is now 11.5 months old, with body weight 7 kg, and height 72 cm, and develops normally for the age. The heart chambers are not enlarged, myocardial contractility is normal, with minor right ventricle hypertrophy.
Discussion
Currently, several syndromic forms including ichthyosis are known: Netherton syndrome, which may include atopy, mental retardation and physical disorder [6]; Refsum disease (family hereditary polyneuric ataxia); Sjögren-Larsson syndrome (mental retardation, gait abnormality, spastic di- or tetraplegia, retinal degeneration, abnormalities of teeth, bones, dermatoglyphic abnormalities, gigantism or dwarfism, skeletal malformations); and Rud's syndrome (seizures and mental retardation) [7].
In this clinical observation, a mutation in the KRT10 gene was detected by exome sequencing. However, this mutation was not detected in the baby’s parents, indicating its pathogenicity. Currently, this investigation is rather laborious and expensive, however, the patients with ichthyosis and their relatives should be advised to undergo genetic counseling in order to obtain more detailed information on the type of inheritance, prognosis and the risk of the condition in the offspring both of the patient and his/her parents. If the mutations responsible for severe forms of ichthyosis are detected, a special counseling during pregnancy planning and management is recommended. It is possible to carry out both preimplantation and prenatal diagnostics with chorion biopsy (at 12 or 20 weeks of gestation) or amniocentesis (starting from the 14th week of gestation) [8, 9]. The delivery, further examination and management of a newborn should be carried out in a Federal or Regional perinatal center.
Conclusion
This clinical observation is unique, due to a combination of two congenital diseases in one baby, affecting different body systems. Such associations require further studies.
References
- Oji V., Tadini G., Akiyama M., Blanchet Bardon C., Bodemer C., Bourrat E. et al. Revised nomenclature and classification of inherited ichthyoses: results of the first ichthyosis consensus conference in soreze 2009. J. Am. Acad. Dermatol. 2010; 63(4): 607-41. https://dx.doi.org/10.1016/j.jaad.2009.11.020.
- Адаскевич В.П. Клинические формы и методы лечения ихтиозиформных дерматозов. Медицинские новости. 2005; 5: 4-9. [Adaskevich V.P. Clinical forms and methods of treatment of ichthyosiform dermatoses. Medical news. 2005; 5: 4-9. (in Russian)].
- Müller F.B., Huber M., Kinaciyan T., Hausser I., Schaffrath C., Krieg T. et al. A human keratin 10 knockout causes recessive epidermolytic hyperkeratosis. Hum. Mol. Genet. 2006; 15(7): 1133-41. https://dx.doi.org/10.1093/hmg/ddl028.
- Клинические рекомендации: ихтиоз у детей. М.: Союз педиатров России; 2016: 14-5. [Clinical guidelines: Ichthyosis in children. Union of Pediatricians of Russia. 2016: 14-5. (in Russian)].
- Eskin-Schwartz M. Epidermolytic ichthyosis sine epidermolysis. Am. J. Dermatopathol. 2017; 39(6): 440-4.
- Бакулев А.Л., Каракаева А.В., Куляев К.А., Епифанова А.Ю., Каткова И.О., Слесаренко Н.А., Давтян В.А., Колпакова Н.Н. Клинический случай синдрома Нетертона. Саратовский научно-медицинский журнал. 2013; 9(3): 605-7. [Bakulev A.L., Karakaeva A.V., Kulyaev K.A., Epifanova A.Yu., Katkova I.O., Slesarenko N.A., Davtyan V.A., Kolpakova N.N. Clinical case of Netherton's syndrome. Saratov Journal of Medical Scientific Research. 2013; 9(3): 605-7. (in Russian)].
- Корнеева Л.С., Мельниченко Н.Е. Учебное пособие «Генодерматозы». Благовещенск; 2014. [Korneeva L.S., Melnichenko N.E. Textbook "Genodermatosis"; 2014. (in Russian)].
- Rout P.D., Nair A., Gupta A., Kumar P. Epidermolytic hyperkeratosis: clinical update. Clin. Cosmet. Investig. Dermatol. 2019; 12: 333-44. https://dx.doi.org/10.2147/CCID.S166849.
- Oji V., Preil M.L., Kleinow B., Wehr G., Fischer J., Hennies H.C. et al. S1 guidelines for the diagnosis and treatment of ichthyoses – update. J. Dtsch. Dermatol. Ges. 2017; 15(10): https://dx.doi.org/1053-65. 10.1111/ddg.13340.
Received 26.05.2020
Accepted 27.07.2020
About the Authors
Irina I. Ryumina, MD, Head of the Department of pathology of newborns and premature babies, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov. Tel.: +7(903)770-80-48. E-mail: i_ryumina@oparina4.ru, i.ryumina@mail.ru. ORCID: 0000-0003-1831-887X.4, Oparina str., Moscow, 117997, Russian Federation.
Natalia M. Marycheva, Candidate of Medical Sciences, Dermatologist of Therapeutic Department, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov. 4, Oparina str., Moscow, 117997, Russian Federation.
Borislav A. Dinov, Candidate of Medical Sciences, pediatric cardiologist, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov, senior researcher of the department of pathology of the cardiovascular system in children, Scientific Research Institute of Pediatrics named after Academician Yu.E. Veltischev. 4, Oparina str., Moscow, 117997, Russian Federation; 2, Taldomskaya str., Moscow, Russian Federation.
Elena I. Dorofeeva, Candidate of Medical Sciences, pediatric surgeon of the highest category, Head of clinical work of the Department of surgery, resuscitation and intensive care of newborns, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov. ORCID: 0000-0003-2822-0462.
4, Oparina str., Moscow, 117997, Russian Federation.
Anna E. Perepyolkina, Candidate of Medical Sciences, junior researcher, Department of pathology of newborns and premature babies, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov. 4, Oparina str., Moscow, 117997, Russian Federation.
Irina V. Orlovskaya, Candidate of Medical Sciences, senior researcher, Head of clinical work, Department of pathology of newborns and premature babies, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov. 4, Oparina str., Moscow, 117997, Russian Federation.
Marina V. Narogan, MD, leading researcher of the Department of pathology of newborns and premature babies, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov; professor of the Department of neonatology, I.M. Sechenov First Moscow State Medical University (Sechenov University), Ministry of Health of Russia. Tel.: +7(905)550-16-21. E-mail: m_narogan@oparina4.ru. ORCID: 0000-0002-3160-905X.
4, Oparina str., Moscow, 117997, Russian Federation; 8-2 Trubetskaya str., Moscow, 119991, Russian Federation.
Karina A. Koronets, neonatologist at the Department of pathology of newborns and premature babies, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov. ORCID: 0000-0003-2828-4474. 4, Oparina str., Moscow, 117997, Russian Federation.
Artem A. Burov, Candidate of Medical Sciences, Head of clinical work, anesthesiologist-resuscitator of the Department of surgery, reanimation and intensive care of newborns, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov.
4, Oparina str., Moscow, 117997, Russian Federation.
Dmitry Yu. Trofimov, Doctor of Biological Sciences, Professor of the Russian Academy of Sciences, Director of the Institute of Reproductive Genetics, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov. 4, Oparina str., Moscow, 117997, Russian Federation.
Grigory S. Vasilyev, doctor-geneticist of the Department of clinical genetics, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov. ORCID: 0000-0002-1364-8418. 4, Oparina str., Moscow, 117997, Russian Federation.
Vladimir G. Bychenko, Candidate of Medical Sciences, Head of the Department of radiation diagnostics, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov. 4, Oparina str., Moscow, 117997, Russian Federation.
Alina V. Kozlova, radiologist of the Department of radiation diagnostics, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov. 4, Oparina str., Moscow, 117997, Russian Federation.
Elena A. Filippova, Head of the Department of ultrasound diagnostics in neonatology and pediatrics, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov. E-mail: e_filippova@oparina4.ru. 4, Oparina str., Moscow, 117997, Russian Federation.
Viktor V. Zubkov, MD, Director of the Institute of Neonatology and Pediatrics, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov; professor of the Department of neonatology, I.M. Sechenov First Moscow State Medical University (Sechenov University), Ministry of Health of Russia. E-mail: victor.zubkov@mail.ru. ORCID: 0000-0001-8366-5208.
4, Oparina str., Moscow, 117997, Russian Federation; 8-2 Trubetskaya str., Moscow, 119991, Russian Federation.
Dmitriy N. Degtyarev, MD, Professor, Deputy Director for Research National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov; Head of the Department of neonatology, I.M. Sechenov First Moscow State Medical University (Sechenov University), Ministry of Health of Russia.
E-mail: d_degtiarev@oparina4.ru. orcid: 0000-0001-8975-2425. 4, Oparina str., Moscow, 117997, Russian Federation; 8-2 Trubetskaya str., Moscow,
119991, Russian Federation.
For citation: Ryumina I.I., Marycheva N.M., Dinov B.A., Dorofeeva E.I., Perepyolkina A.E., Orlovskaya I.V., Narogan M.V., Koronets K.A., Burov A.A., Trofimov D.Yu., Vasilyev G.S., Bychenko V.G., Kozlova A.V., Filippova E.A., Zubkov V.V., Degtyarev D.N. Clinical observation of an infant with epidermolytic ichthyosis and congenital lung malformation.
Akusherstvo i Ginekologiya/ Obstetrics and Gynecology. 2020; 8: 194-200 (in Russian).
https://dx.doi.org/10.18565/aig.2020.8.194-200



