Carboxytherapy of genitourinary syndrome of menopause
Genitourinary syndrome of menopause is one of the challenging problems encountered by gynecologists. Management of this patient category is a complex, lengthy and complicated endeavor. Aim. To investigate the clinical effectiveness of ultrasound-guided carboxytherapy as a conservative treatment for genitourinary syndrome of menopause. Materials and methods. The present prospective clinical study comprised 35 postmenopausal women with mean age 52.9 (3.4) years, duration of postmenopause 3.27 (2.4) years, and mean duration of genitourinary syndrome of menopause 2.9 (0.9) years. Carboxytherapy was performed using a VENUSIAN CO2 Therapy device delivered in four (3.5) 5 min weekly sessions. Ultrasonography was carried out using DC-8, Resona-7 (Mindray, China), Logiq E9 (GE, USA) ultrasound scanners with linear 7.5–15 MHz probes. The analyzed parameters included the vaginal health index (VHI), vaginal epithelium maturation index (VEMI), and the results of the VSQ questionnaire. Results. Compared with baseline values, VHI increased by 30% from 10.4 (5.2) to 17.8 (4.9) (p<0.001); VEMI increased from 53.7 (3.9) to 77.8 (9.9) (p<0.001). Vaginal pH level changed from 6.0 (0.9) to 4.6 (0.7) (p<0.001). VSQ scores improved from 11.7 (4.8) to 5.9 (2.5). Frequency and intensity of vulvovaginal atrophy symptoms reduced by almost 50%, which indicates high effectiveness of carboxytherapy (p<0.001). Conclusion. The study findings suggest that ultrasound-guided carboxytherapy is an effective, acceptable, and safe treatment option for genitourinary syndrome of menopause.Saidova A.S., Sencha A.N., Apolikhina I.A.
Keywords
Genitourinary syndrome of menopause (GSM) is one of the challenging problems encountered by gynecologists. It affects peri- and postmenopausal women, having an adverse impact on their quality of life.
GSM is a chronic complex syndrome of multiple atrophic and dystrophic changes in estrogen-dependent tissues and structures of the lower genitourinary tract (vulva, vagina, bladder, urethra, pelvic ligaments, and pelvic floor muscles) [1, 2].
According to various authors, GSM affects up to 15–57% of midlife and older women. Most commonly, patients report the symptoms of vulvovaginal atrophy (VVA) occurring within the perimenopause/early postmenopause and leading to functional and anatomical changes. Symptoms of VVA include vaginal dryness (27–50%), itching/irritation, (18-25%), dyspareunia (33–41%), recurrent urogenital infectious and pelvic inflammatory diseases (6-8%) [1–7].
Management of this patient category is a complex, lengthy, and complicated endeavor. Treatment aimed at improving symptoms associated with VVA, preventing relapses, and improving the quality of life of menopausal women.
Due to the need to develop new approaches to the treatment of women with GSM, we have proposed ultrasound-guided carboxytherapy as a conservative treatment option for patients with genitourinary syndrome of menopause.
Carboxytherapy, also known as carbon dioxide therapy, is a popular treatment for many diseases. Its therapeutic effect is associated with improved microcirculation, angiogenesis, vasodilation, and, as a consequence, better tissue oxygenation.
Stimulation of the pelvic floor blood flow contributes to the vaginal microflora normalization in patients with vaginal dryness. Carboxytherapy slows down physiological aging without the risks associated with hormone replacement therapy, thereby improving the patient’s quality of life [8–11]. Its safety profile allows it to be used as an alternative prevention and treatment option in patients who cannot use other treatment modalities [7, 9, 11].
A combination of multiparametric ultrasound and shear-wave elastography is widely used to assess the elasticity of superficially located organs [12]. This diagnostic imaging modality has been used routinely in clinical practice in the diagnosis of various diseases of the mammary, thyroid, parathyroid, and salivary glands. Contrast-enhanced ultrasound (CEUS) is also effectively used to diagnose a wide range of internal diseases, including those affecting superficially located organs [13]. The latest ultrasound imaging technologies are increasingly used in ultrasound-guided procedures in the treatment of thyroid and parathyroid glands [14]. We have not found published data on the use of ultrasound as a method to control and monitoring carboxytherapy in aesthetic gynecology.
This study aimed to investigate the clinical effectiveness of ultrasound-guided carboxytherapy as a conservative treatment for genitourinary syndrome of menopause.
Material and methods
A prospective clinical study was conducted at the Department of Aesthetic Gynecology and Rehabilitation, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia to investigate the effectiveness of ultrasound-guided carboxytherapy as a conservative treatment for women with VVA symptoms of varying severity and duration.
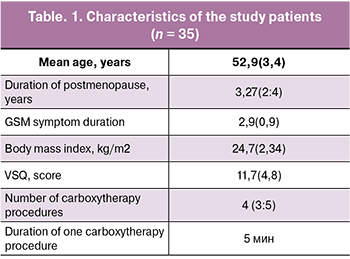 The study comprised 35 postmenopausal women with mean age 52.9 (3.4) years, duration of postmenopause 3.27 (2:4) years, and mean duration of GSM 2.9 (0.9) years. At the time of presentation, none of the patients received systemic and local.
The study comprised 35 postmenopausal women with mean age 52.9 (3.4) years, duration of postmenopause 3.27 (2:4) years, and mean duration of GSM 2.9 (0.9) years. At the time of presentation, none of the patients received systemic and local.
All patients underwent general clinical, gynecological, and ultrasound examinations. Particular attention was paid to detailed medical history taking, specifying symptoms, and duration of GSM symptoms.
The gynecological evaluation included inspection of the external genitalia, vaginal speculum examination, bimanual examination, pH measurement, Valsalva maneuver, and cough test, vaginal epithelium maturation index (VEMI); PAP test, vaginal culture, investigation of the urogenital microflora by a real-time polymerase chain reaction, and pelvic ultrasound.
Also, changes in the vaginal health index (VHI) were assessed over time. The VHI includes scoring of vaginal moisture, vaginal fluid volume, vaginal elasticity, vaginal pH, and vaginal epithelial integrity on a scale of 1 (poorest) to 5 (best). The total scores were interpreted as follows: 20–25 – normal, 15–20 – minor atrophic changes, 15 or less – vaginal atrophy [1, 2].
Carboxytherapy was performed using a VENUSIAN CO2 Therapy device (MBE, Italy) delivered in four (3:5) weekly sessions lasting 5 minutes each.
Manifestations of VVA and their impact on the sexual function and quality of life were examined using the Vulvovaginal Symptoms Questionnaire (VSQ). VSQ is a 21-item written questionnaire with four scales: symptoms, emotions, life impact, and sexual impact. VSQ scores range between 1(yes) and 0 (no). The maximum score is 20. In women who do not report current sexual activity (“no” to question 17), the final four questions related to the sexual impact of vulvar symptoms are omitted [15].
After written informed consent, all 35 patients underwent multiparametric ultrasound. Ultrasound imaging was performed with DC-8, Resona-7 (Mindray, China), Logiq E9 (GE, USA) ultrasound scanners using surface mode with linear 7.5–15 MHz multifrequency probes. Specialized diagnostic ultrasound modes included Ultrasound Elastography, Shear Wave Elastography (SWE), and Contrast (with a low mechanical index MI<0.10). CEUS scans were performed with the intravenous (2.4 ml per scan) sulfur hexafluoride microbubble contrast agent SonoVue (SonoVue, Bracco, Italy).
Regions of interest when scanning the patients included soft tissues of the labia majora, the clitoris, and adjacent areas of the vulvar muscle-aponeurotic layer.
Sonographic navigation and control were performed for each patient four times, sequentially in 4 stages:
- Before carboxytherapy procedure;
- During carboxytherapy procedure;
- Immediately after (within 1 hour) carboxytherapy procedure;
- One month after the carboxytherapy procedure.
Ultrasound during the 1st stage of the study included:
- analysis of the area planned for the application of CO2 using gray-scale and color ultrasound imaging modes;
- ultrasound analysis of the elasticity of the affected area with the identification of qualitative and quantitative characteristics of ultrasound elastography;
- analysis of micro-vascularization of soft tissues of the affected area using the contrast agent, determination of qualitative and quantitative parameters of CEUS.
Ultrasound in the 2nd stage, i.e., during the carboxytherapy, was performed to visualize the soft tissue structure of the regions of interest. It aimed to specify the location, control the direction of the needle in various areas of CO2 injection, characterize the volume and distribution of the gas, and analyze the state of the injection area and changes in adjacent tissues.
The volume of ultrasound imaging during the 3 and 4 stages was unchanged and included all characteristics of the multiparametric ultrasound of the 1st stage.
Ultrasound elastography and CEUS were used in addition to the standard methods of gray-scale and color imaging before and after the carboxytherapy to compare the changes in tissue stiffness and elasticity, and analyze the changes in micro-vascularization of the regions of interest.
Ultrasound elastography was performed in two versions:
- compression elastography (CEG);
- shear wave elastography (SWE) to obtain Young’s modulus values.
While performing real-time CEG for 2–5 s after turning on the option, several consecutive static images were displayed on the monitor screen (in the window of interest). Analysis, processing, and superposition of the basic B-mode and the compression image obtained after activating the option resulted in an elastographic image containing the minimum amount of noise and artifacts. As a result of mathematical analysis, the elasticity of the tissues on the screen was displayed in specific colors (color mapping). The devices allowed coloring using blue-green-red colors, shades of red, gray, and a wide range of other colors. In this work, a blue-greenred color pattern was used, in which the “stiff” sites were mapped in blue and the “softer” locations in red, intermediate values were mapped in shades of yellow and green.
Color maps of the regions of interest obtained in the CEG mode at all stages of the study were categorized:
- by the homogeneity of color pattern into homogeneous and heterogeneous;
- by the color pattern into shades of blue, shades of red, and mixed mosaic color.
SWE was performed in the mode for superficially located structures with a range of Young’s modulus values of 0–180 kPa without additional compression while minimizing possible movements of the sensor. The field of view had color patterns according to the stiffness. The areas with the highest and lowest values of Young’s modulus were mapped in red and blue, respectively; intermediate values were mapped from blue to yellow, reflecting increasing stiffness. The process of measuring the stiffness of tissues was carried out automatically with the full (maximum possible) color pattern of the color window; for the measurement, the same window area (Q-Box) was used - three in each color window, which were located in the area of maximum stiffness. The results were displayed on the screen in real-time in units of measure (kPa). The mean value (E-mean), minimum value (E-min), and maximum value (E-max), standard deviation (SD) were displayed on the screen. A mean value of 5 E-mean measurements was included in the analysis.
All obtained color options, color maps, and quantitative indicators of Young’s modulus of soft tissues of the region of interest were recorded in the scanner computer memory for subsequent storage on digital media, analysis, and mathematical processing. Detected changes were analyzed and interpreted based on the totality of echographic findings and the state of adjacent tissues.
Soft tissue micro-vascularization of the region of interest was evaluated based on the CEUS findings. The examination was performed using an aseptic technique required for minimally invasive procedures after obtaining the written consent of each patient. The contrast agent (2.4 ml) was administered intravenously via a 20-G catheter placed in the antecubital, followed by 5 mL 0.9% NaCl saline solution. Simultaneously with the contrast agent injection, the countdown began, and the cine-loop was recorded (at least 90 seconds) and stored in the memory of the ultrasound scanner. At the post-processing stage, CEUS qualitative and quantitative parameters were analyzed using the Contrast QA software. The segments of the cine-loop were taken for analysis from the moment the contrast agent injection until it was completely washed out (the object of interest was scanned during the entire study time, at least 90 s).
Quantitative assessment of changes in contrast enhancement parameters was performed based on the ratio of time and intensity of accumulation and distribution of contrast agent in the region of interest.
Statistical analysis
Statistical analysis was performed using Statistica 13.3. The distribution of continuous variables was tested for normality using the Kolmogorov-Smirnov test. Quantitative variables showing normal distribution were expressed as means (M) and standard deviation (SD); otherwise, the median (Me) and the quartiles Q1 and Q3 were reported. Qualitative variables were summarized as counts (n) and percentages (%).
Changes in continuous variables during treatment were assessed with paired Student’s t-test or nonparametric Wilcoxon sign rank test. Paired proportions were compared with McNemar’s test or the Z-test.
Differences between the groups were considered statistically significant at p<0.05.
Results and discussion
Ultrasound-guided carboxytherapy resulted in positive changes in all clinical, laboratory, and instrumental indicators (Table 2).
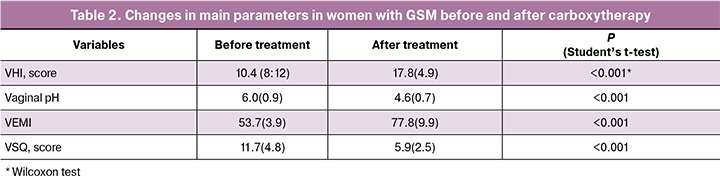
VHI increased by 30% [17.8 (4.9)], compared with baseline [10.4 (8:12)], which was associated with a decrease in atrophic changes (mucosal thinning, fissures) and an increase in vaginal secretion. The pH level after treatment increased from 6.0 (0.9) to 4.6 (0.7) (p <0.001) indicating a normalization of the acid-base balance.
VEMI increased from 53.7 (3.9) at baseline to 77.8 (9.9) after treatment completion. The a66nalysis of mucous membrane cellular composition showed signs of vaginal epithelium restoration, including a decrease in basal, parabasal, intermediate epithelial cells, and a statistically significant increase in surface cells (p <0.001).
Vaginal microbiocenosis before and after the therapy was examined by a real-time polymerase chain reaction (PCR) applied to vaginal smears. The vaginal microflora in postmenopausal women was mainly represented by facultative anaerobes (Enterobacteriaceae, Staphylococcus spp., Streptococcus spp.) and obligate anaerobic bacteria (Eubacterium spp., Clostridocerbium spp., etc.). One month after treatment, there was a statistically significant increase in the proportion of obligate anaerobic bacteria. At the same time, opportunistic pathogens decreased against the background of an increase in the number of lactobacilli, compared with baseline (p < 0.001).
Comparison of scores in responses to the VSQ questionnaire before [11.7 (4.8)] and after [5.9 (2.5)] carboxytherapy showed a decrease in the frequency and intensity of VVA symptoms (dryness, itching, burning, and discomfort) by almost 50%, which indicates the high treatment effectiveness (p < 0.001). Regarding the questions related to the sexual impact of vulvar symptoms, there was a marked reduction or the complete disappearance of dyspareunia.
The sonographic characteristics obtained during carboxytherapy at stages 1, 3, 4 of the study are shown in tables 3-5.
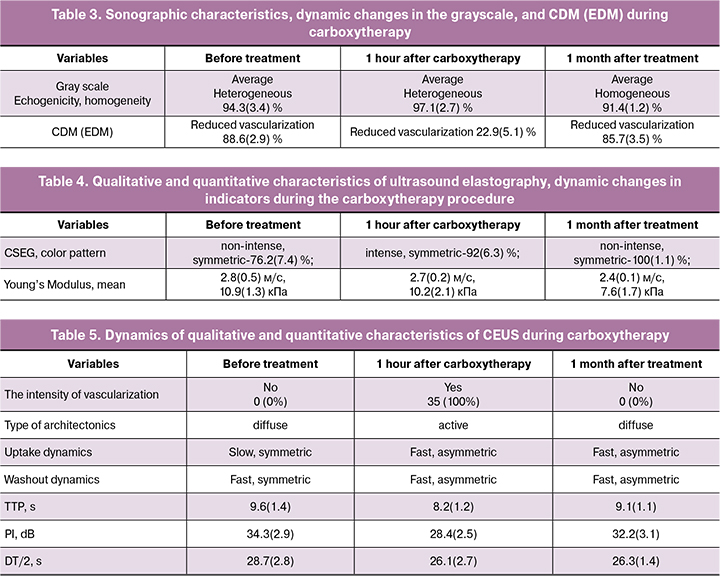
As noted in Table 3, the grayscale indicators (echogenicity, homogeneity) of the soft tissue structure in the region of interest, vascularization of the visualization area in the CDM (EDM) modes during carboxytherapy change only for a short time after the procedure and cannot be used as effectiveness criteria in the long term (> 1 month).
According to the results shown in table 4, there is a change in the stiffness (elasticity) in the region of interest, the color pattern of soft tissues, a decrease in the mean values of Young’s modulus in the region of interest immediately after and one-month post carboxytherapy procedure.
The most essential temporal and spatial characteristics of CEUS were:
- the appearance of vascularization elements (vascular loci) in the affected area after injection of contrast agent;
- the intensity of vascularization;
- vascular architectonics, its type;
- dynamics of contrast agent uptake over time;
- dynamics of contrast agent washout.
During CEUS, the following quantitative characteristics of the time-intensity curve were analyzed:
- TTP (Time To Peak, s): the time when the contrast intensity reaches peak value;
- PI (Peak Intensity, dB): contrast peak intensity;
- Half-life (DT/2, s): the time when the intensity is half the value of the peak intensity;
- The characteristics of CEUS at stages 1, 3, 4 of the study are summarized in Table 5.
As shown in table 5, there were changes in the qualitative and quantitative characteristics of microvascularization in the region of interest immediately after and one-month post carboxytherapy.
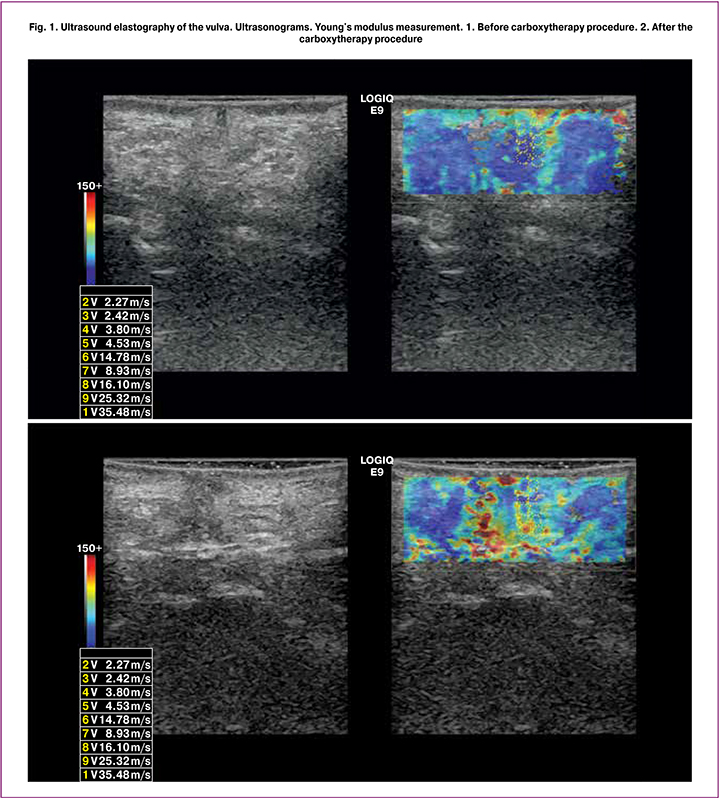
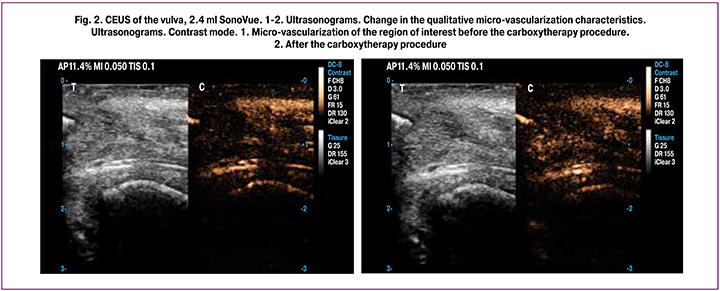
Findings of ultrasound control and monitoring confirmed the primary physiological effects that characterize the dynamics and determine the positive impact of the carboxytherapy (Fig. 1, 2):
- a change in echogenicity and echotexture of the vulvar and vaginal tissues (according to the results of applying the grayscale mode);
- a change in the elasticity of the soft tissues of the region of interest, most likely due to increased gas exchange in tissues, an immunomodulating effect, increased antioxidant cell protection, regenerative properties and tissue repair (Fig. 1);
- blood vessels’ vasodilation, increased soft tissue micro-vascularization of the region of interest. These echo signs can be caused, among other things, by an increase in arterial inflow, increased tissue metabolism, stimulation of energy metabolism, and an increase in venous outflow (Fig. 2).
The advantages of ultrasound control include:
- real-time monitoring of the intervention, low timeconsuming procedure;
- allows targeted visualization of the affected area;
- no ionizing radiation, comfort, safety and security for the patient and medical personnel;
- high resolution;
- the ability to capture images on video, thermal film, and digital media (including for accurate dynamic monitoring).
Conclusion
GSM is experienced by many peri- and postmenopausal women and seriously affects their quality of life, working capacity, and sexual function. The success of treatment directly depends on the timely and correct diagnosis of existing disorders. Therefore, using VHI, VEMI, and the VSQ questionnaire is indispensable elements in a comprehensive examination of patients in the menopause.
Taken together, our findings suggest that ultrasoundguided carboxytherapy is an effective treatment modality in the management of GSM. CEUS data demonstrated the improvement of micro-vascularization of the region of interest, arterial inflow, and venous outflow resulting in better tissue oxygenation probably due to stimulation of neovascularization and collagen genesis. During the treatment follow-up, ultrasound elastography showed an increase in the vulvar soft tissue elasticity compared with baseline values, which indicates a positive effect of carboxytherapy and a possible start of angio- and neocollagenesis.
In summary, ultrasound-guided carboxytherapy is an effective, acceptable, and safe treatment option for genitourinary syndrome of menopause due to specific effects of CO2 and the absence of a proliferative influence on the endometrium and mammary glands. This implies the possibility of using carboxytherapy as an alternative treatment modality in patients who are unable to undergo hormone replacement therapy and other treatments.
References
- Аполихина И.А., Горбунова Е.А. Клинико-морфологические аспекты вульвовагинальной атрофии. Медицинский совет. 2014; 9: 110-7. [Apolikhina I.A., Gorbunova E.A. Clinical and morphological aspects of vulvovaginal atrophy. Medical Council/Meditsinskiy sovet. 2014; (9): 110-7. (in Russian)].
- Доброхотова Ю.Э., Ильина И.Ю., Венедиктова М.Г., Морозова К.В., Суворова В.А. Локальная негормональная терапия больных с генитоуринарным менопаузальным синдромом. Российский вестник акушера-гинеколога. 2018; 18(3): 88-94. [Dobrokhotova Yu.E., Ilyina I.Yu., Venediktova M.G., Morozova K.V., Suvorova V.A. Local nonhormonal therapy in patients with genitourinary menopausal syndrome. Russian Bulletin of Obstetrician-Gynecologist/Rossiyskiy vestnik akushera-ginekologa. 2018; 18(3): 88-94. (in Russian)]. https://dx.doi.org/10.17116/rosakush201818288-94.
- Ермакова Е.И., Балан В.Е., Тихомирова Е.В., Лазарева И.Н., Лапина А.В., Панина Е.М. Генитоуринарный менопаузальный синдром: диагностика и принципы лечения (краткие клинические рекомендации). Российский вестник акушера-гинеколога. 2017; 17(6): 89-95.[Ermakova E.I., Balan V.E., Tikhomirova E.V., Lazareva I.N., Lapina A.V., Panina E.M. Genitourinary syndrome of menopause: diagnosis and principles of treatment (brief clinical recommendations). Russian Bulletin of Obstetrician-Gynecologist/Rossiyskiy vestnik akushera-ginekologa. 2017; 17(3): 89-95. (in Russian)]. https://dx.doi.org/10.17116/rosakush201717689-95.
- Оразов М.Р., Хамошина М.Б., Бебнева Т.Н., Поликарпова С.Р. Возможности гидролизата плаценты человека в комплексном лечении симптомов генитоуринарного синдрома в постменопаузе. Гинекология. 2017; 19(1): 27-30. [Orazov M.R., Khamoshina M.B., Bebneva T.N., Policarpova S.R. The possibility of human placenta extract in the treatment of symptoms genitourinary syndrome in postmenopausal women. Ginecology/Ginekologiya. 2017; 19(1): 27-30. (in Russian)].
- Прилепская В.Н. Генитоуринарный менопаузальный синдром: возможности эстриола. Гинекология. 2018; 20(1): 5-8. [Prilepskaya V.N. Genitourinary menopausal syndrome: the potential of estriol. Ginecology/Ginekologiya. 2018; 20(1): 5-8. (in Russian).] https://dx.doi.org/10.26442/2079-5696_20.1.5-8.
- Palmaa F., Volpea A., Villa B., Cagnacci A. Vaginal atrophy of women in postmenopause. Results from a multicentric observational study: The AGATA study. Maturitas. 2016; 83: 40-4. https://dx.doi.org/10.1016/j.maturitas.2015.09.001.
- Sinha A., Ewies A.A. Non-hormonal topical treatment of vulvovaginal atrophy: an up-to-date overview. Climacteric. 2013; 16(3): 305-12. https://dx.doi.org/10.3109/13697137.2012.756466.
- Nach R., Zandifar H., Gupta R., Hamilton J.S. Subcutaneous carboxytherapy injection for aesthetic improvement of scars. Ear Nose Throat J. 2010; 89(2): 64-6.
- Muzi F., Delicato G., D’Andria D., Baffigo G., Tartaglia E., Tati E. et al. Carboxytherapy and platelet rich plasma: a new therapy for trigonitis, abacterial and interstitial cystitis. J. Pharm. Pharmacol. 2015; 3: 405-10. https://dx.doi.org/10.17265/2328-2150/2015.09.001.
- Бунятян Н.Д., Дроговоз С.М., Кононенко А.В., Зеленкова Г., Прокофьев А.Б. Карбокситерапия – одно из инновационных направлений в курортологии. Вопросы курортологии, физиотерапии и лечебной физической культуры. 2018; 95(5): 72-6. [Bunyatyan N.D., Drogovoz S.M., Kononenko A.V., Prokofiev A.B.Carboxytherapy – an innovative trend in resort medicine. Problems of Balneology, Physiotherapy, and Exercise Therapy/Voprosy kurortologii, fizioterapii i lechebnoi fizicheskoi kul’tury. 2018; 95(5): 72-6. (in Russian)]. https://dx.doi.org/10.17116/kurort20189505172.
- Zelenková H. Carboxytherapy non-invasive method in dermatology and some other branches of medicine. Acta Scientific Medical Sciences. 2019; 3(5): 42-8.
- Сенча А.Н. Ультразвуковая диагностика. Поверхностно-расположенные органы. М.: Видар; 2015. 512 с. [Sencha A.N. Ul’trazvukovaya diagnostika. Poverkhnostno-raspolozhennye organy. Moscow: Vidar; 2015. 512p.(in Russian)].
- Сенча А.Н. Ультразвуковое исследование щитовидной железы. Шаг за шагом. От простого к сложному. М.: МЕДпресс-информ; 2019. 205 с. [Sencha A.N. Ul’trazvukovoe issledovanie shchitovidnoi zhelezy. Shag za shagom. Ot prostogo k slozhnomu. Moscow: MEDpress-inform; 2019. 205p. (in Russian)].
- Патрунов Ю.Н., Пампутис С.Н., Сенча А.Н., Могутов М.С. Эффективность ультразвук-ассистированной перкутанной лазерной абляции у пациентов с первичным гиперпаратиреозом. Ультразвуковая и функциональная диагностика. 2015; 5 (Приложение – Тезисы VII Съезда Российской ассоциации специалистов ультразвуковой диагностики в медицине. ч.2. Москва, 10-13 ноября 2015 года): 133а. [Patrunov Yu.N., Pamputis S.N.,Sencha A.N., Mogutov M.S. Effektivnost’ ul’trazvuk-assistirovannoi perkutannoi lazernoi ablyatsii u patsientov s pervichnym giperparatireozom. Ul’trazvukovaya i funktsional’naya diagnostika. 2015; 5(Suppl.-Tezisy VII S”ezda Rossiiskoi assotsiatsii spetsialistov ul’trazvukovoi diagnostiki v meditsine. ch.2. Moscow, 10-13 November 2015): 133a.(in Russian)].
- Erekson E.A., Yip S.O., Wedderburn T.S., Martin D.K., Fang-Yong Li., Choi J.N. et al. The Vulvovaginal Symptoms Questionnaire: a questionnaire to measure vulvovaginal symptoms in postmenopausal women. Menopause. 2013; 20(9): 973-9. https://dx.doi.org/10.1097/GME.0b013e318282600b.
Received 04.12.2019
Accepted 07.02.2020
About the Authors
Ayna S. Saidova, Ph.D., Obstetrician-Gynecologist at the Department of Aesthetic Gynecology and Rehabilitation, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia.Tel.: +7(926)206-60-51. E-mail: asekova14@yandex.ru. ORCID.0000-0003-3473-3109. 4 Oparina str., Moscow, 117997, Russian Federation.
Alexandr N. Sencha, Dr.Med.Sci., Head of the Department of Diagnostic Imaging, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia. Tel.: +7(968)844-90-15. E-mail: senchavyatka@mail.ru. ORCID 0000-0002-1188-8872. 4 Oparina str., Moscow, 117997, Russian Federation.
Inna A. Apolikhina, Dr.Med.Sci., Professor, Head of the Department of Aesthetic Gynecology and Rehabilitation, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia; Professor at the Department of Obstetrics, Gynecology, Perinatology and Reproductology, I.M. Sechenov First MSMU of Minzdrav of Russia (Sechenov University).
Tel.: +7(495)735-10-55. E-mail: apolikhina@inbox.ru. ORCID 0000-0002-4581-6295.
8-2 Trubetskaia st., Moscow, 119991, Russian Federation.
For citation: Saidova A.S., Sencha A.N., Apolikhina I.A.
Carboxytherapy of genitourinary syndrome of menopause.
Akusherstvo i Ginekologiya/ Obstetrics and gynecology. 2020; 5: 113-21 (In Russian).
https://dx.doi.org/10.18565/aig.2020.5.113-21



