По данным Всемирной организации здравоохранения (ВОЗ), во всем мире анемией страдают 529 млн женщин репродуктивного возраста, из них около 38% – беременные. Распространенность анемии колеблется у женщин от 5,4% в развитых странах до 80% в развивающихся [1, 2]. Около 30% беременных имеют железодефицитную анемию (ЖДА) [3]. По данным ВОЗ (2020), в группу высокого риска железодефицита входят все менструирующие женщины, а с наступлением беременности из-за увеличения потребности в железе (усиленного эритропоэза, роста плаценты и плода и др.) дефицит железа (ДЖ) манифестирует до ЖДА различной степени тяжести [3, 4]. Глобальные цели ВОЗ предполагают сокращение заболеваемости анемией у женщин репродуктивного возраста на 50% к 2025 г. [2]. Ранняя диагностика и профилактика ЖДА, по мнению ВОЗ (2020), связаны с определением ферритина в сыворотке крови как маркера ДЖ и своевременным лечением путем назначения железосодержащих добавок [3]. В российских клинических рекомендациях «Нормальная беременность» (2019) [5], «Аномальные маточные кровотечения» (2021) [6], «Гиперплазия эндометрия» (2021) [7] говорится о необходимости обследования на содержание ферритина. Однако в большинстве лечебно-профилактических учреждений этот анализ не выполняется, что не позволяет осуществить своевременное назначение добавок железа с целью уменьшения частоты ЖДА у женщин репродуктивного возраста. По данным обследования 160 беременных при постановке на диспансерный учет в БУ Ханты-Мансийского автономного округа–Югры «Ханты-Мансийская районная больница» в период 6 месяцев (ноябрь, декабрь, январь, февраль, март, апрель) 2019–2020 гг., у 38 (23,8%) женщин отмечена ЖДА различной степени тяжести. В предродовом периоде ЖДА диагностирована у 50 (31,3%) беременных, что свидетельствует о недостаточных запасах железа в организме. У 129 пациенток, обратившихся в клинику ВРТ «Мать и дитя» (Москва) (в тот же период времени), ЖДА встречалась реже – у 6 (4,65%), так как пациентки были обследованы и получали лечение экстрагенитальных заболеваний до поступления в клинику. Однако ДЖ, установленный на основании снижения уровня ферритина менее 15 мкг/мл (Рекомендации ВОЗ, 2020), был выявлен у трети женщин – 42 (32,56%). В роддоме № 25 (ГКБ №1 г. Москвы) при постановке на учет (в рассматриваемый временной промежуток) 120 беременных ЖДА была выявлена у 10 (8,33%), во II триместре – у 36 (30%), в III триместре – у 74 (61,67%), что наглядно свидетельствует о дефиците депо железа.
Вместе с тем пациентки, которые имели потери беременности в ранние сроки, значительно чаще обращаются по поводу установления причин неразвивающейся беременности или самопроизвольного выкидыша, реабилитации и подготовки к беременности. Учитывая широкую распространенность ЖДА у беременных в РФ, актуальным представлялось определение частоты ДЖ в прегравидарном периоде у жительниц Москвы с ранними потерями беременности в анамнезе.
Материалы и методы
Ретроспективное исследование проведено в течение 6 месяцев (ноябрь, декабрь, январь, февраль, март, апрель) 2019–2020 гг. на базе многопрофильной клиники г. Москвы. Объектом настоящего исследования стали 98 женщин репродуктивного возраста (18–39 лет). Женщины обращались в клинику по поводу потери беременности в ранние сроки. На каждую пациентку заводили статкарту, содержащую анамнез, данные объективного осмотра, заключения смежных специалистов, данные инструментальных и лабораторных исследований (рекомендации ESHRI, 2017) [8]. У всех пациенток исследовали состояние эндометрия (пайпель-биопсия + оценка морфологии эндометрия) [9, 10], оценивались показатели клинического анализа крови: гемоглобин, гематокрит, количество эритроцитов, эритроцитарные индексы MCH (среднее содержание гемоглобина в эритроците), MCV (средний объем эритроцита), MCHC (средняя концентрация гемоглобина в эритроцитах), RBC (распределение эритроцитов по объему). Диагнозы ДЖ и ЖДА были установлены в соответствии с клиническими рекомендациями «Железодефицитная анемия» (2020) [11].
Результаты и обсуждение
Все женщины проживали в городе (100%), большинство имели высшее образование (94,9%) и работали в тех или иных сферах (78,6%) (табл. 1).

Анализируя семейное положение, было установлено, что 84 (85,7%) пациентки имели постоянного полового партнера, чуть больше половины из них состояли в зарегистрированном браке (43,9%). Одинокими на момент обследования оказались 14 (14,3%) пациенток (табл. 2).
Средний возраст дебюта менструальной функции обследованных женщин составлял 13,14 года. Минимальный возраст менархе – 10 лет, максимальный – 16,5 года. Средняя продолжительность менструального кровотечения составляла 4,62 дня. Средняя продолжительность менструального цикла – 29,12 дня (табл. 3).
Более половины пациенток (62/98, 63,26%) оценивали интенсивность менструаций как умеренные, каждая третья (34/98, 34,7%) считала их обильными. У 2 (2,04%) пациенток менструации были скудными. Болезненными свои менструации считали 46 (46,9%) опрошенных (табл. 4).
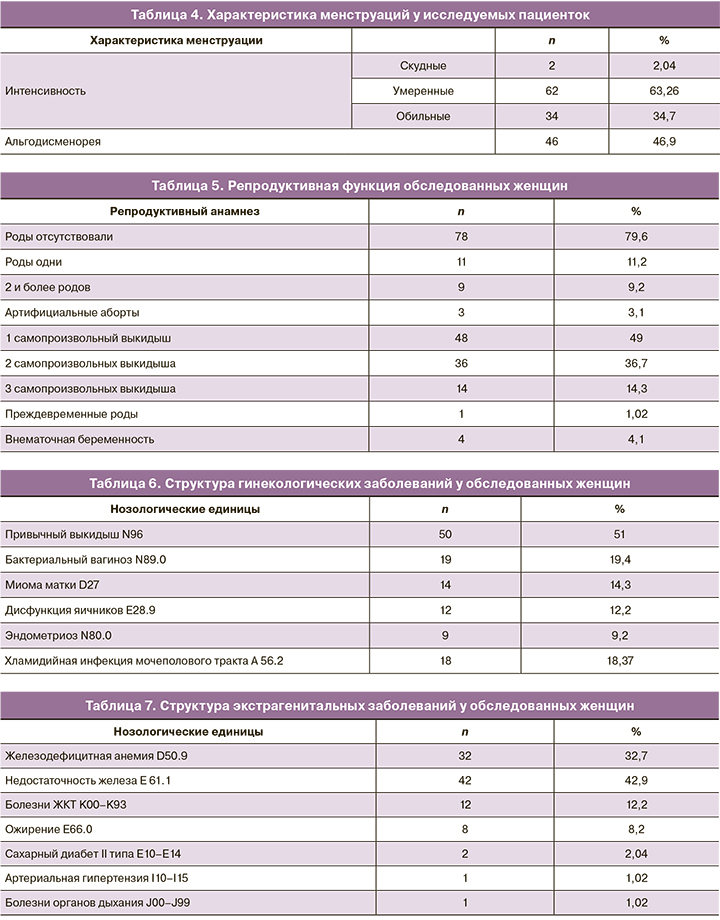
По данным анамнеза, у 78 (79,6%) пациенток роды отсутствовали. У 20 (20,4%) пациенток 1 и более беременностей завершились родами, при этом у 1 (1,02%) женщины произошли преждевременные роды. Примерно у половины (48/98, 49%) пациенток в анамнезе было одно самопроизвольное прерывание беременности, у трети женщин (36/98, 36,7%) произошло 2 самопроизвольных выкидыша и у 14 (14,3%) пациенток – 3. У 4 (4,1%) пациенток в анамнезе была внематочная беременность (табл. 5).
В структуре гинекологической заболеваемости преобладали пациентки с привычным невынашиванием беременности (50/98, 51%). У 19 (19,4%) пациенток был диагностирован бактериальный вагиноз, у 18 (18,37%) – хламидийная инфекция. Миома матки была выявлена у 14 (14,3%) обследованных. Также было определено, что у 12 (12,2%) пациенток был диагноз дисфункции яичников, у 9 (9,2%) – эндометриоза (табл. 6).
Все пациентки, обратившиеся к гинекологу, были обследованы у смежных специалистов. Наиболее часто из экстрагенитальных заболеваний встречались недостаточность железа (42/98, 42,9%) и ЖДА (32/98, 32,7%), а также болезни желудочно-кишечного тракта (12/98, 12,2%). Четвертое место заняло ожирение – данный диагноз был выставлен 8 (8,2%) пациенткам. У 2 (2,04%) женщин был выявлен сахарный диабет 2-го типа. Артериальная гипертензия и болезни органов дыхания были диагностированы у 1 (1,02%) пациентки (табл. 7).
У всех пациенток в исследуемой когорте была забрана кровь для определения исходных уровней гемоглобина, сывороточного железа и ферритина. Средний уровень гемоглобина составлял 118 г/л. Среднее значение уровня железа сыворотки было 16 мкмоль/л. Среднее значение уровня ферритина – 13 мкг/мл (табл. 8).
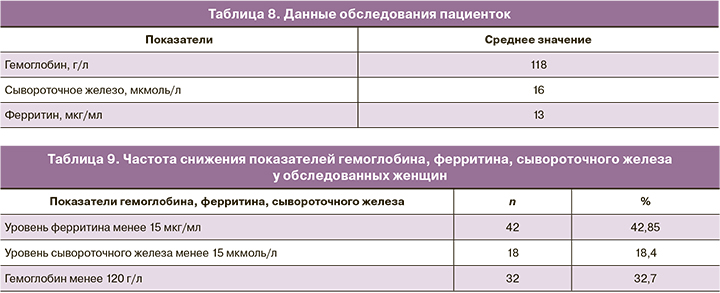
В соответствии с критериями [10] анемия легкой степени была выявлена у 32 (32,7%) обследуемых пациенток, снижение уровня ферритина ниже референcных значений (≤15 мкг/мл) [4] при нормальных показателях гемоглобина отмечено у 42 (42,85%). У 18 женщин уровень железа сыворотки крови составлял менее 15 мкмоль/л (табл. 9).
После обследования и установления отклонений в состоянии репродуктивного здоровья для каждой пациентки составляли план лечения и реабилитации продолжительностью 3–6 месяцев, а также с целью устранения ДЖ и лечения ЖДА назначали фумарат железа в сочетании с фолиевой кислотой (препарат «Ферретаб комп.»). В 1 капсуле препарата содержится 3 мини-таблетки фумарата железа и 1 мини-таблетка фолиевой кислоты, суммарно включающие 50 мг элементного железа (152 мг фумарата железа) и 500 мкг фолиевой кислоты. Высвобождение фолиевой кислоты из мини-таблетки в составе капсулы «Ферретаб комп.» происходит в течение 1–2 ч, а фумарата железа из таблеток пролонгированного высвобождения – в течение нескольких часов, по мере прохождения мини-таблеток с кишечным транзитом [12]. Совместное использование фумарата железа с фолиевой кислотой у пациенток с ДЖ и ЖДА, особенно у женщин на этапе прегравидарной подготовки (нуждающихся в дополнительных дотациях фолатов), повышает эффективность терапии и рекомендовано FIGO (2019) [13].
Совместно с терапевтом женщинам с ДЖ Ферретаб комп. был рекомендован по 1 капсуле 1 раз в день, пациенткам с ЖДА – по 2 капсулы утром.
У пациенток с ЖДА контроль уровня гемоглобина осуществляли через 4–5 недель. Прирост гемоглобина у 41 (41,84%) женщины с ранними потерями в анамнезе составлял от 9,2 до 11,6 г/л. Нормализация гемоглобина через 4–5 недель была у 29 (29,59%) женщин, у 12 (12,24%) наблюдалась через 8–9 недель. У 2 женщин были побочные эффекты в виде отрыжки и «металлического» привкуса в течение 2 недель, однако они не отменили препарат. 1 пациентка была потеряна для наблюдения. После нормализации гемоглобина пациентки принимали Ферретаб комп. по 1 капсуле до зачатия, в течение 3–4 месяцев. Контроль содержания ферритина осуществляли во время беременности, при постановке на учет. У всех пациенток показатель был выше 30 мкг/мл.
У 42 (42,85%) женщин с недостаточностью железа Ферретаб комп. назначали по 1 капсуле 1 раз в день, контроль содержания ферритина через 3–4 месяца показал, что он достиг нормальных значений, т.е. более 30 мкг/мл. Побочные эффекты зарегистрированы не были.
У 94 пациенток беременность наступила самостоятельно, у 4 были применены вспомогательные репродуктивные технологии. Все женщины родили в срок доношенных детей. В течение беременности ЖДА легкой степени во II–III триместрах была установлена у 4 (4,1%) женщин, и это было связано с кровянистыми выделениями из половых путей (угрожающий выкидыш, предлежание плаценты). Назначение препарата «Ферретаб комп.» по 2 капсулы утром однократно в сутки привело к компенсации уровня гемоглобина у 3 пациенток, у которых беременность была сохранена и кровянистые выделения были кратковременными. У 1 пациентки с повторяющимися кровянистыми выделениями, связанными с предлежанием плаценты, получающей Ферретаб комп. по 2 капсулы утром, компенсации не было, и уровень гемоглобина составлял от 98 до 103 г/л. Роды в 37–38 недель, самопроизвольные, вес новорожденного 3140 г, рост 50 см.
Обсуждение
Около 40% фертильных небеременных женщин имеют уровень ферритина ≤30 мкг/л, т.е. небольшие или отсутствующие запасы и неблагоприятный статус железа (дефицит) в отношении предстоящей беременности в популяции, где ЖДА распространена с частотой 22–24% [13]. Вместе с тем ВОЗ [3] рекомендует с целью установления ДЖ использовать уровень ферритина менее 15 мкг/мл. При использовании этого критерия у женщин с ранними потерями беременности в анамнезе у 42 (42,85%) установлена недостаточность железа и у 32 (32,7%) выявлена ЖДА, т.е. 2/3 женщин – 75 (75,5%) планировали беременность при отсутствии запасов железа в организме. Железодефицит является результатом длительного отрицательного баланса железа, который может быть вызван недостаточным потреблением (из-за недостаточного содержания в рационе или усвоения), повышенной потребностью в этом микроэлементе или хронической потерей из-за кровотечений. Женщины репродуктивного возраста подвергаются более высокому риску развития ДЖ из-за потерь во время менструации [14] и в силу нередких обильных и/или аномальных маточных кровотечений [15–17].
Многочисленные исследования показали, что при дефиците запасов железа анемия манифестирует с ранних сроков беременности и имеет неблагоприятное влияние как на ее течение, так и на рост плода [15]. Низкий уровень гемоглобина у беременной женщины ассоциирован с низкой массой тела плода, преждевременными родами, послеродовым кровотечением, необходимостью переливания крови женщине в родах и послеродовом периоде, высокой перинатальной смертностью, нейрокогнитивными нарушениями у потомства [4, 18, 19]. Поэтому крайне актуальным является вопрос восполнения недостаточности железа и лечения ЖДА у женщин до беременности [3, 14, 19, 20].
Рекомендации ВОЗ (2016) включают назначение всем менструирующим женщинам 3 месяца в году 30–60 мг железа [21]. FIGO (2019) [13] рекомендует не только добавки железа, но и фолиевой кислоты и йода. К сожалению, данные рекомендации на территории РФ не внедрены, вследствие этого налицо актуальность ранней диагностики недостаточности железа путем определения уровня ферритина [5, 11]. Применение добавок железа нередко сопряжено с побочными эффектами (тошнота, рвота, отрыжка, «металлический» привкус во рту, запор, понос и др.). Ряд авторов также показали, что большие дозы железа резко повышают уровень гепсидина – это нарушает всасывание железа и биодоступность [22], а также увеличивает оксидативный стресс и приводит к повреждению клеток кишечника [23]. Назначение более низких доз (40–80 мг) и отказ от приема 2 раза в день увеличивают фракционную абсорбцию и переносимость препарата [22]. Учитывая, что эпителий кишечника обновляется каждые 6–8 суток и только обновленные клетки способны к всасыванию железа и его дальнейшей транспортировке [24], с целью уменьшения частоты побочных эффектов изучали эффективность прерывистого режима приема препаратов железа [25]. Было показано, что восстановление депо железа и нормализация показателей ферритина эффективнее при непрерывном приеме препарата [25]. Таким образом, был выбран препарат «Ферретаб комп.», содержащий 50 мг элементного железа (152 мг фумарата железа) и 500 мкг фолиевой кислоты. Высвобождение фолиевой кислоты из мини-таблетки в составе капсулы «Ферретаб комп.» происходит в течение 1–2 ч, а фумарата железа из таблеток пролонгированного высвобождения – в течение нескольких часов, по мере прохождения мини-таблеток с кишечным транзитом [12], это также приводит к уменьшению частоты побочных эффектов. В течение 3 месяцев у всех женщин на этапе прегравидарной подготовки отмечено увеличение уровня ферритина до 30 мкг/мл и более, побочные эффекты зарегистрированы не были. У 2/3 из 41 пациентки с ЖДА восстановление гемоглобина произошло в течение 4–5 недель у остальных – в течение 8–9 недель. Одна пациентка была потеряна для наблюдения.
У всех пациенток наступила беременность, у 4 (4,08%) при использовании вспомогательных репродуктивных технологий. Уровень ферритина во время беременности не определяли, ЖДА была установлена у 4 (4,1%). У этих беременных были кровянистые выделения из половых путей (угрожающий выкидыш, предлежание плаценты).
Наши данные подтверждают результаты метаанализа 11 клинических исследований препаратов фумарата железа (в 10 из 11 исследований использовался препарат «Ферретаб комп.»), которые указывают на достоверные ассоциации между приемом препарата «Ферретаб комп.» и снижением встречаемости у пациенток проявлений ЖДА (р=6,9×10-11), гемоглобина менее 110 г/л (p=1,1×10-3), микроцитоза (р=1,2×10-5), уровней ферритина менее 20 мкг/л (р=7,1×10-5) и Fe (сыв.)<20 мкмоль/л (р=5,3×10-7) [26]. Ферретаб комп. является эффективным для терапии железодефицитных состояний и безопасным средством. Метаанализ показал средние эффективные дозировки фумарата железа в составе препарата «Ферретаб комп.». Средняя суммарная доза для достижения Fe (сыв.)>20 мкмоль/л и компенсации ЖДА (гемоглобин более 110 г/л) составила 1,90±0,65 г железа в составе фумарата железа. Средняя суммарная доза для снижения проявлений микроцитоза эритроцитов и достижения уровней ферритина более 20 мкг/л была несколько выше и составила 2,60±1,42 г железа в составе фумарата железа [26].
Заключение
У 2/3 женщин, обратившихся по поводу ранних потерь беременности с целью прегравидарной подготовки, отсутствовали достаточные запасы железа перед предстоящей беременностью. У 42 (42,85%) была установлена недостаточность железа, у 32 (32,7%) выявлена ЖДА. Назначение фумарата железа (50–100 мг элементарного железа в сутки) в сочетании с фолиевой кислотой (500–1000 мкг) привело к компенсации содержания гемоглобина и ферритина. Адекватная обеспеченность беременной железом и фолатами привела к отсутствию ЖДА у женщин, получавших прегравидарную подготовку, за исключением пациенток с кровянистыми выделениями из половых путей (угрожающий выкидыш, предлежание плаценты).
Капсулы «Ферретаб комп.» пролонгированного высвобождения по мере прохождения в кишечнике вызывают минимальное количество побочных эффектов и эффективно восстанавливают уровень ферритина и гемоглобина.



