1) Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology, and Perinatology, Ministry of Health of Russia, Moscow, Russia;
2) Scientific Society “League for Promoting Clinical Trials”, Moscow, Russia;
3) Institute of Clinical Research, Moscow, Russia;
4) D.O. Ott Research Institute of Obstetrics, Gynecology, and Reproductology, Saint Petersburg, Russia;
5) City Center for Hemostatic Pathology, Chelyabinsk, Russia;
6) Republican Clinical Hospital, Ministry of Health of the Republic of Tatarstan, Kazan, Russia;
7) Omsk State Medical University, Ministry of Health of Russia, Omsk, Russia;
8) Kuban State Medical University, Ministry of Health of Russia, Krasnodar, Russia;
9) Voronezh City Clinical Polyclinic No. 1, Voronezh, Russia;
10) Nurmed Medical Center, Kazan, Russia
Objective: To evaluate the effect of ferrous fumarate/folic acid (FF/FA) on the clinical and laboratory signs of anemia in pregnant women in the second trimester.
Materials and methods: The investigation enrolled 160 women at 14 to 20 weeks’ gestation who were diagnosed with anemia and were prescribed FF/FA.
Results: The use of FF/FA caused a significant improvement in clinical and biochemical blood test results reflecting the severity of anemia: an increase in Hb, Ht, RBC, MCV, MCH, MCHC, serum iron, and ferritin, a decrease in the frequency of microcytosis and serum latent iron-binding capacity. After 3 months of therapy, the total study population displayed an increase in mean values of HgB from 102.0 to 117.0 g/l, ferritin from 46.2 to 55.9 µg/l, serum iron from 12.02 to 20.49 µmol/l. The proportion of patients without anemia was 77% (118/153); that with mild and moderate anemia were 20% (31/153), and 2% (3/153), respectively, which shows a significant improvement compared to that at the start of therapy. In the group with iron deficiency anemia (IDA), (Hb<110 g/l, ferritin<30 µg/l), Hb increased from an average of 103.3 to 109.8 g/l, ferritin from 14.4 to 24.3 µg/l, serum iron from 11.87 to 20.53 µmol/l. After 3 months, mild and moderate IDA was present in 30% (28/93) and 3% (3/93) patients, respectively.
Conclusion. The findings are comparable with international and Russian data and can conclude that FF/FA is an effective and safe way to correct IDA in pregnant women and is suitable for long-term use.
Authors’ contributions: Pavlovich S.V., Melikhov O.G. – development of the concept and design of the investigation, writing the text; Pavlovich S.V., Abashova E.I., Chulkov V.S., Nazipova Z.M., Savelyeva I.V., Andreeva M.D., Kudlai Yu.V., Enkova E.V., Khodzhaeva Z.S., Muminova K.T., Sakalo V.A., Gorodnova E.A., Zaripova A.Sh., Melikhov O.G. – material collection and processing, editing; Melikhov O.G. – statistical data processing; Melikhov O.G., Pavlovich S.V. – analysis of literature data.
Conflicts of interest: The authors declare that there are no conflicts of interest.
Fundind: This investigation has not been sponsored by Acino Rus Co.
Patient Consent for Publication: The patients have signed an informed consent form to their participation in the investigation and to the publication of their data.
Authors' Data Sharing Statement: The data supporting the conclusions of this investigation are available upon request from the corresponding author after approval by the research supervisor of the project.
For citation: Pavlovich S.V., Melikhov O.G., Khodzhaeva Z.S., Muminova K.T., Sakalo V.A., Gorodnova E.A., Abashova E.I., Yarmolinskaya M.I., Chulkov V.S., Nazipova Z.M., Savelyeva I.V., Andreeva M.D., Kudlai Yu.V., Enkova E.V., Zaripova A.Sh. Efficacy of a combined drug containing ferrous fumarate and folic acid in anemia in the second trimester of pregnancy: results of a multicenter study.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2023; 2: 108-116 (in Russian)
https://dx.doi.org/10.18565/aig.2023.51
anemia
iron deficiency anemia
iron preparations
folic acid
pregnancy
second trimester
hemoglobin
ferritin
Анемия у беременных остается одним из наиболее часто встречающихся заболеваний как в развитых, так и в развивающихся странах. В соответствии с рекомендациями Всемирной Организации Здравоохранения (ВОЗ) диагноз анемии беременных устанавливается при снижении уровня гемоглобина (Hb) <110 г/л [1]. Железодефицитная анемия (ЖДА) составляет 80% числа всех анемий у взрослых [2]. При постановке диагноза ориентируются также на дополнительные лабораторные признаки (уровень ферритина) и клинические проявления заболевания [3]. По данным ВОЗ, в 2019 г. глобальная распространенность анемии составила 29,9% среди женщин от 25 до 49 лет. Анемия наблюдается у 29,6% небеременных женщин репродуктивного возраста и у 36,5% беременных. В Российской Федерации распространенность анемии беременных составляет 23,4% (7,9–45,1) [4].
Вероятность развития анемии возрастает с увеличением срока беременности вследствие возрастающих потребностей организма в питательных веществах и физиологических изменений в метаболизме. Течение беременности, родового акта и послеродового периода при анемии сопровождается увеличением частоты осложнений. По данным систематического обзора и метаанализа 117 исследований с включением в общей сложности 4 127 430 беременных было показано, что при анемии имеет место существенное увеличение отношения шансов преждевременных родов, преэклампсии, гестационной гипертензии, послеродового кровотечения, перинатальной смертности [5]. Установлена связь между ЖДА во время беременности и увеличением антенатальной смертности [6, 7], что является еще одним серьезным фактором, подчеркивающим необходимость лечения [8].
Недостаточное потребление железа и аномальные маточные кровотечения в предшествующий беременности период являются наиболее значимыми причинами ЖДА. Согласно рекомендациям ВОЗ, беременные для профилактики анемии должны принимать 30–60 мг железа ежедневно [9]. В случае сформировавшейся ЖДА необходимо использовать максимально эффективные и безопасные препараты органического железа [10]. Один из наиболее востребованных препаратов из этой группы – фумарат железа (ФЖ), который хорошо переносится беременными [11, 12].
Дефицит железа и фолиевой кислоты (ФК) во время беременности может усилить окислительный стресс и привести к нарушению внутриутробного развития, преждевременному прерыванию беременности и преэклампсии. Было показано, что препараты железа и ФК снижают уровень окислительного стресса [13].
Недостаточное потребление ФК также может привести к развитию анемии [14]. Фолаты – одни из важнейших синергистов железа. необходимые для поддержки кроветворения [15]. Рандомизированные клинические исследования продемонстрировали, что ФК предотвращает дефекты развития нервной трубки [16]. Прием ФК на этапе планирования беременности и на ее протяжении включен во многие национальные рекомендации [17].
Совместное использование ФЖ, содержащего Fe2+, с ФК у пациенток с ЖДА повышает эффективность терапии [11, 18]. Комбинация хорошо переносится при длительном приеме [19], что значительно повышает ценность применения, так как нежелательные явления (НЯ) на фоне приема препаратов железа встречаются в 30–40% случаев, служат основной причиной ранней отмены, пропуска приема препарата и, как следствие, недостижения целей терапии [20].
В работе Кирилюка А.А. (2020) приведены сравнительные данные по безопасности различных железосодержащих препаратов. ФЖ редко вызывает нежелательные лекарственные реакции (в основном местные). Могут встречаться диспепсия, диарея и боли в животе. Прием ФЖ ассоциировался с меньшим количеством НЯ по сравнению с терапией сульфатом железа и полимальтозными комплексами железа (ПКЖ) [21].
По данным метаанализа, включавшего 11 клинических исследований, ФЖ крайне редко вызывал нежелательные реакции со стороны желудочно-кишечного тракта и тошноту и хорошо переносился беременными. Из 473 беременных, вошедших в метаанализ, всего у 3,38% отмечались НЯ со стороны желудочно-кишечного тракта: тошнота – у 1,2% пациентов (проходила самостоятельно, не требовала отмены препарата), однократная рвота при приеме натощак – у 0,84%, у 0,42% пациентов отмечалась диарея, у 0,84% – запоры, не потребовавшие отмены препарата [22].
Таким образом, комбинированная лекарственная форма органической соли ФЖ и ФК (ФЖ/ФК) является одним из препаратов выбора для использования в профилактике и терапии ЖДА [18].
Недостаток научных данных о том, как ФЖ/ФК влияет на течение анемии у беременных женщин в условиях обычной медицинской практики в Российской Федерации, послужил предпосылкой данного исследования.
Цель исследования: оценить влияние препарата ФЖ/ФК на клинические и лабораторные признаки анемии у беременных во II триместре.
Материалы и методы
Дизайн. Открытое наблюдательное проспективное неинтервенционное исследование в обычной медицинской практике. В проекте приняли участие 7 российских центров.
Исследование проведено в соответствии с Хельсинкской декларацией Всемирной Медицинской Ассоциации [23] и основными положениями Руководства по надлежащей клинической практике Международной конференции по гармонизации в части защиты прав участников исследования и обращения с данными (ICH GCP E6 R2) [24].
Пациентки включались в исследование после постановки диагноза и принятия врачом решения о назначении терапии.
Критерии включения.
1. Период беременности с 14 до 20 недель.
2. Диагноз «анемия беременных».
3. Назначение препарата ФЖ/ФК в соответствии со стандартной практикой медицинского учреждения.
4. Подписанное информированное согласие на обработку персональных данных.
Критерии невключения.
1. Повышенная чувствительность к компонентам препарата ФЖ/ФК.
2. Наличие патогенетических вариантов анемического синдрома, при которых назначение препарата ФЖ/ФК нецелесообразно.
Популяция исследования. Всего в исследование были включены 160 женщин (общая популяция исследования). На визите 2 (через 1 месяц) наблюдались 158 пациенток, 2 были потеряны для наблюдения. На визите 3 (через 3 месяца) данные были доступны для 153 пациенток: после 2-го визита 2 пациентки были потеряны для наблюдения, 2 отказались от продолжения исследования, у 1 пациентки на 2-м визите была достигнута цель лечения, препарат отменен.
Был проведен дополнительный анализ в группе пациенток (n=98) с ЖДА (снижение уровней Hb менее 110 г/л и ферритина менее 30 мкг/л) для визитов 1 и 3. На визите 3 данные были доступны для 93 пациенток: 2 пациентки потеряны для наблюдения, 2 пациентки отказались от продолжения исследования ранее 3-го визита, у 1 пациентки на 2-м визите была достигнута цель лечения, препарат отменен.
Исследуемое лекарственное средство. Препарат ФЖ/ФК («Ферретаб комп.») назначали внутрь по 50 мг в день (в пересчете на Fe2+ 1 капсула содержит 50 мг Fe2+). При необходимости врач мог увеличить дозу до 100–150 мг (2–3 капсулы) в сутки. Препарат назначался в соответствии с инструкцией по медицинскому применению лекарственного препарата, одобренной Министерством здравоохранения Российской Федерации.
Исходно в общей популяции исследования (все пациентки, включенные в проект) 58% (92/160) пациенток принимали по 1 капсуле исследуемого препарата (эквивалент 50 мг железа + 0,5 мг ФК), 41% (66/160) – 2 капсулы (эквивалент 100 мг железа + 1,0 мг ФК), 1% (2/160) – 3 капсулы (эквивалент 150 мг железа + 1,5 мг ФК).
В группе с ЖДА (Hb<110 г/л, ферритин<30 мкг/л, n=98) на 1-м визите 44% (43/98) пациенток была назначена 1 капсула препарата, 54% (53/98) – 2 капсулы, 2% (2/98) – 3 капсулы. Дальнейшие корректировки дозы на визите 2 и визите 3 представлены в таблице 1.
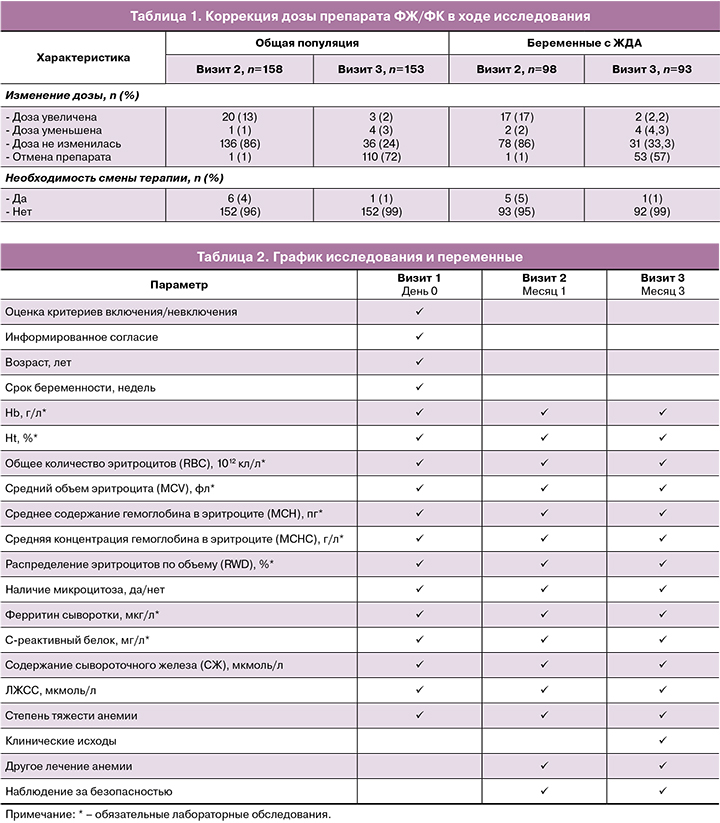
Процедуры исследования и переменные. Контрольные визиты назначались через 1 и 3 месяца. Изучаемые переменные представлены в таблице 2.
Результаты
Характеристика исследуемой популяции
В общей популяции исследования (n=160) средний возраст составил 29 лет (от 18 до 47), средний срок беременности на момент включения – 17 недель (от 14 до 20).
В группе пациенток (n=98) с ЖДА средний возраст составил 31 год (от 19 до 47), средний срок беременности на момент включения в исследование – 17 недель (от 14 до 20).
Эффективность
Результаты лабораторных исследований представлены в таблице 3.
Общая динамика анализа крови. На фоне приема препарата ФЖ/ФК в общей популяции исследования отмечалось значительное улучшение клинического анализа крови и биохимических показателей, отражающих тяжесть анемии: повышение Hb (+14,21 г/л), Ht (+3,7%), RBC (+0,37 ×1012 кл/л), MCV (+1,6 фл), MCH (+2,07 пг), MCHC (+10,3 г/л), СЖ (+8,47 мкмоль/л), ферритина (+9,7 мкг/л) и общей железосвязывающей способности сыворотки (ОЖСС) (10,75 мкмоль/л), а также снижение частоты микроцитоза (на 15%) и латентной железосвязывающей способности сыворотки (ЛЖСС) (-8,1 мкмоль/л) (табл. 3).
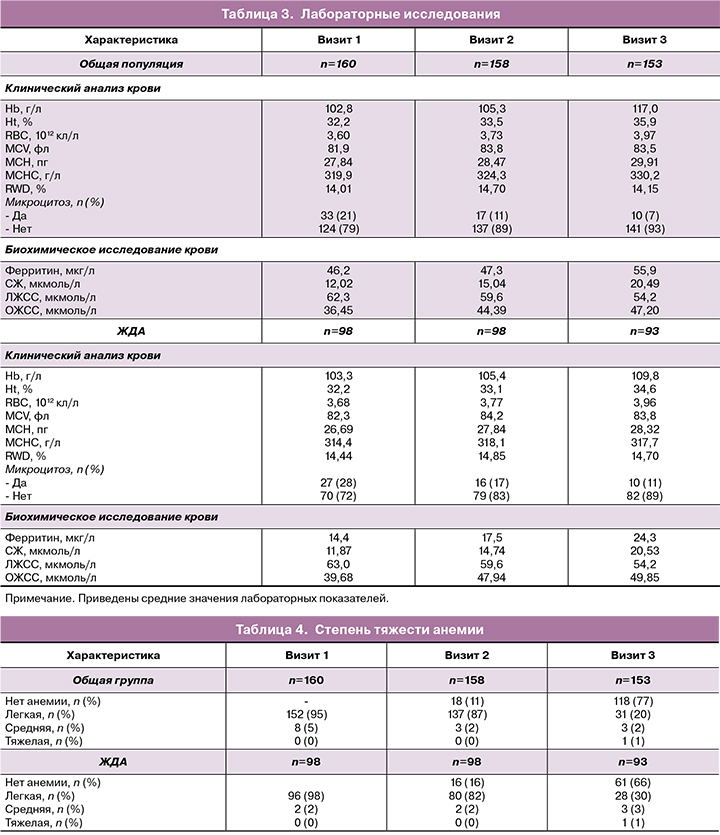
В группе пациенток с ЖДА, так же, как и в общей популяции исследования, отмечалось увеличение уровней Hb, Ht, MCHC (табл. 3). В группе пациенток с ЖДА на визите 1 в среднем назначалась более высокая доза препарата ФЖ/ФК. Доза чаще увеличивалась на последующих визитах (табл. 1).
Динамика тяжести анемии. Степень тяжести анемии определялась в соответствии с Федеральными клиническими рекомендациями Минздрава России «Диагностика, профилактика и лечение железодефицитных состояний у беременных и родильниц», 2013. Легкая степень определялась как снижение Hb до 110–90 г/л, средняя – как снижение Hb от 89 до 70 г/л, тяжелая – снижение менее 70 г/л [25].
В общей популяции исследования исходно (на 1-м визите) 95% (152/160) пациенток имели анемию легкой и 5% (8/160) – средней степени.
После трехмесячного курса терапии (данные доступны для 153 пациенток) анемия отсутствовала у 77% (118/153), анемия легкой степени отмечена всего у 20% (31/153), анемия средней степени – у 2% (3/153), что представляет значительное улучшение по сравнению с визитом 1 и указывает на эффективность терапии. У 1 пациентки во время визита 3 была установлена анемия тяжелой степени.
В группе пациенток (n=98) с ЖДА к визиту 3 анемия была преодолена у 66% (61/93) пациенток, ЖДА легкой степени сохранялась в 30% случаев (28/93), ЖДА средней степени – в 3% (3/93) случаев. Результаты представлены в таблице 4 и на рисунке.

В общей популяции доля пациенток, у которых уровень Hb к 3-му визиту увеличился на 10 г/л и более в сравнении с визитом 1, составила 52% (79/153), на 15 г/л и более – 45% (69/153), на 20 г/л и более – 37% (57/153).
В группе пациенток с исходно низким уровнем ферритина ожидаемо отмечалась тенденция к более медленной динамике в достижении целей лечения. Доля пациенток, у которых к концу исследования содержание Hb увеличилось на 10 г/л и более, составила 27% (25/93), на 15 г/л и более – 18% (17/93), на 20 г/л и более – 12% (11/93). В этой группе к 3-му визиту у 30% (28/93) наблюдалось снижение концентрации как Hb, так и ферритина: Hb оставался на уровне менее 110 г/л, ферритин – менее 30 мкг/л. У 42% (39/93) пациенток уровень Hb повысился до 110 г/л и более, уровень ферритина сохранялся на отметке ниже 30 мкг/л. У 4% (4/93) пациенток наблюдался уровень ферритина 30 мкг/л и более при уровне Hb выше 110 г/л. Таким образом, несмотря на исходно более низкие значения характеризующих анемию параметров крови, к 3-му визиту удалось добиться увеличения уровня Hb и компенсации состояния у 66% пациенток, что говорит об эффективности выбранной тактики лечения.
Данные о лечении после окончания исследования
После окончания исследования препарат был отменен у 110 пациенток: 107 – по причине нормализации лабораторных показателей, 1 женщине – вследствие неэффективности, 2 – по медицинским причинам, связанным с беременностью.
Препарат переносился хорошо и ни у одной пациентки не был отменен по причине плохой переносимости. Продолжение терапии ФЖ/ФК было рекомендовано 43 пациенткам: в той же дозе – 36 женщинам, в большей – 3 пациенткам, в меньшей – 4 пациенткам.
Данные о безопасности
На визитах 2 и 3 собиралась информация о НЯ, связанных, по мнению врача, с приемом препарата ФЖ/ФК (нежелательных лекарственных реакциях), не описанных в инструкции по медицинскому применению препарата. Подобных лекарственных реакций не было зарегистрировано ни у одной пациентки.
Обсуждение
Результаты исследования согласуются с результатами других исследований, проводившихся в разных странах [26–28] и подтверждающих эффективность ФЖ, а также возможность длительного приема [20]. В 2003 г. Mehta B.C. et al. был представлен отчет о 27 пациентках с ЖДА, которые не отвечали на введение ПКЖ в течение периода от 4 до 52 недель, но ответили на введение ФЖ в течение 4–13 недель [26].
Аналогичные данные были получены Ruiz-Arguelles C.J. et al. (2007). Среди 240 пациентов с ЖДА, получавших перорально ПКЖ, 75 (31%) не ответили на лечение, средний уровень Hb после окончания терапии составлял 103 г/л. После назначения ФЖ в течение периода от 1 до 14 месяцев уровень Hb повысился в среднем до 125 г/л [27].
В слепое рандомизированное исследование, проведенное в Бангладеш (2001), были включены 280 небеременных с дефицитом железа. В исследовании оценивалась эффективность профилактики ЖДА с помощью ФЖ/ФК (200 мг ФЖ еженедельно) в сравнении с плацебо в течение 24 недель. Концентрация Hb увеличилась в среднем на 5,52 г/л, что позволило исследователям сделать вывод об эффективности профилактики ЖДА [28].
Метаанализ, проведенный И.Ю. Торшиным и соавт. (2015), показал достоверную связь между приемом ФЖ/ФК в средней дозе 61 мг/сут в расчете на Fe2+ в течение в среднем 32 суток и снижением проявлений ЖДА (Hb менее 110 г/л, микроцитоз, ферритин менее 20 мкг/л, СЖ менее 20 мкмоль/л). Средняя суммарная доза для достижения концентрации СЖ более 20 мкмоль/л и компенсации ЖДА составила 1,9 г железа [21]. Средняя суммарная доза для снижения проявлений микроцитоза и достижения уровня ферритина более 20 мкг/л составила 2,6 г железа.
В исследовании Баева О.Р. (2011) назначение препарата ФЖ/ФК (Ферретаб комп.) 86 беременным женщинам в 14–18 недель беременности способствовало профилактике не только анемии во время беременности и ряда гестационных осложнений, но и осложнений родов и послеродовой анемии [29].
Оптимальным критерием оценки эффективности терапии ЖДА вне беременности является прирост Hb на 10–20 г/л за 2–4 недели. Оптимальных значений временного интервала восстановления Hb до нормальных значений до настоящего времени не разработано, но очевидно, что нормализация уровня Hb является только первым этапом терапии и после нормализации данного показателя лечение должно продолжаться в течение нескольких недель или месяцев для восстановления запасов железа [3].
Таким образом, результаты данного клинического исследования сопоставимы с результатами исследований, представленных в международных и российских публикациях, и позволяют сделать вывод, что препараты ФЖ/ФК являются эффективным и безопасным способом лечения анемии у беременных и подходят для длительного приема.
Заключение
1) На фоне приема препарата ФЖ/ФК у беременных с анемией отмечалась нормализация параметров клинического и биохимического анализов крови, отражающих тяжесть анемии: повышение уровней Hb, Ht, RBC, MCV, MCH, MCHC, СЖ, ферритина, а также снижение частоты выявления микроцитоза и ЛЖСС.
2) По окончании исследования нормализация параметров крови и устранение клинических признаков анемии наблюдались у 77% (118/153) беременных и у 66% (61/93) беременных со сниженным уровнем ферритина, что свидетельствует об эффективности выбранной терапии. Части пациенток (43 из 153 из общей популяции исследования) понадобилось продолжение терапии свыше 3 месяцев.
Таким образом, результаты исследования позволяют сделать вывод об эффективности и безопасности препарата «Ферретаб комп.» (железа фумарат в комбинации с фолиевой кислотой) для коррекции анемии у беременных и его рекомендации к применению. Назначение препарата требует индивидуального подбора дозы для каждой пациентки и ее коррекции в ходе лечения и соответствует действующим клиническим рекомендациям МЗ РФ «Железодефицитная анемия».
- WHO. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. World Health Organization; 2011. Available at: https://apps.who.int/iris/handle/10665/85839]
- Министерство здравоохранения Российской Федерации. Железодефицитная анемия. Клинические рекомендации. М.; 2021. [Ministry of Health of the Russian Federation. Iron deficiency anemia. Clinical guidelines. Мoscow; 2021. (in Russian)].
- Pavord S., Daru J., Prasannan N., Robinson S., Stanworth S., Girling J.; BBSH Committee. UK guidelines on the management of iron deficiency in pregnancy. Br. J. Haematol. 2020; 188(6): 819-30. https://dx.doi.org/10.1111/bjh.16221.
- WHO. WHO Global Anaemia estimates, 2021 Edition. WHO Global health observatory. 2022. Available at: https:// www.who.int/data/gho/data/themes/topics/anaemia_in_women_and_children
- Jung J., Rahman M.M., Rahman M.S., Swe K.T., Islam M.R., Rahman M.O., Akter S. Effects of hemoglobin levels during pregnancy on adverse maternal and infant outcomes: a systematic review and meta-analysis. Ann. N. Y. Acad. Sci. 2019; 1450: 69-82. https://dx.doi.org/10.1111/nyas.14112.
- Masukume G., Khashan A.S., Kenny L.C., Baker P.N., Nelson G. SCOPE Consortium. Risk factors and birth outcomes of anaemia in early pregnancy in a nulliparous cohort. PLoS One. 2015; 10(4): e0122729. 10.1371/journal.pone.0122729.
- Christian P., Mullany L.C., Hurley K.M., Katz J., Black R.E. Nutrition and maternal, neonatal, and child health. Semin. Perinatol. 2015; 39(5): 361-72. https://dx.doi.org/10.1053/j.semperi.2015.06.009.
- Резолюция Совета экспертов по профилактике и лечению железодефицитной анемии у беременных. Акушерство и гинекология. 2020; 4: 230-2. [Expert council resolution on prevention and treatment of iron deficiency anemia in pregnant women. Obstetrics and Gynecology. 2020; (4): 230-2. https://dx.doi.org/10.18565/aig.2020.4.230-232.
- Всемирная организация здравоохранения. Рекомендации ВОЗ по оказанию дородовой помощи для формирования положительногоопыта беременности. 2017. 196с. Доступно по ссылке: https://www.who.int/ru/publications/i/item/9789241549912. [WHO. WHO guidelines on prenatal care for a positive pregnancy experience. 2017. 196p. Available at: https://www.who.int/ru/publications/i/item/9789241549912 (in Russian)].
- Wang L., Mei Z., Li H., Zhang Y., Liu J., Serdula M.K. Modifying effects of maternal Hb concentration on infant birth weight in women receiving prenatal iron-containing supplements: a randomised controlled trial. Br. J. Nutr. 2016; 115(4): 644-9. https://dx.doi.org/10.1017/S0007114515004870.
- Коротких И.Н., Литвиненко О.В. Железодефицитные состояния беременных и их медикаментозная коррекция. РМЖ. Мать и дитя. 2019; 2(4): 292-5. [Korotkikh I.N., Litvinenko O.V. Iron deficiencies in pregnancy and their pharmacotherapy. Russian Journal of Woman and Child Health. 2019; 2(4): 292-5. (in Russian)]. https://dx.doi.org/10.32364/2618-8430-2019-2-4-292-295.
- Громова О.А., Торшин И.Ю., Тетруашвили Н.К., Гоголева И.В. Систематический анализ фармакологических свойств протеин сукцинилата железа. Эффективная фармакотерапия. 2018; 13: 20-9. [Gromova O.A., Torshin I.Yu., Tetruashvili N.K., Gogoleva I.V. Systematic analysis of ferrous protein succinilate pharmacological properties. Effective Pharmacotherapy. 2018; (13): 20-9. (in Russian)].
- Wahyuwibowo J., Aziz A., Safitri E., Minidian F., Zulaikhah S.T. Iron-folate supplementation during pregnancy for prevent oxidative stress in pregnant rats: Level of MDA, Creatinine, Glucose, Erythrocite, Blood Pressure, Body Weight and Number of Offspring. Pharmacog. J. 2020; 12(1): 186-91.https://dx.doi.org/10.5530/pj.2020.12.28.
- Cawley S., Mullaney L., McKeating A., Farren M., McCartney D., Turner M.J. An analysis of folic acid supplementation in women presenting for antenatal care. J. Public Health (Oxf.). 2016; 38(1): 122-9. https://dx.doi.org/10.1093/pubmed/fdv019.
- Stamm R.A., Houghton L.A. Nutrient intake values for folate during pregnancy and lactation vary widely around the world. Nutrients. 2013; 5(10): 3920-47. https://dx.doi.org/10.3390/nu5103920.
- Roche M.L., Samson K.L.I., Green T.J., Karakochuk C.D., Martinez H.Perspective: weekly iron and folic acid supplementation (WIFAS):a critical review and rationale for inclusion in the essential medicines list to accelerate anemia and neural tube defects reduction. Adv. Nutr. 2021; 12(2): 334-42. https://dx.doi.org/10.1093/advances/nmaa169.
- O'Malley E.G., Cawley S., Kennedy R.A.K., Reynolds C.M.E., Molloy A., Turner M.J. Maternal anaemia and folate intake in early pregnancy. J. Public Health (Oxf.). 2018; 40(3): e296-e302. https://dx.doi.org/10.1093/pubmed/fdy013.
- Громова О.А., Торшин И.Ю., Тетруашвили Н.К., Павлович С.В. Систематический анализ молекулярного синергизма фолиевой кислоты и фумарата железа при железодефицитной анемии. Акушерство и гинекология. 2022; 12: 178-86. [Gromova O.A., Torshin I.Yu., Tetruashvili N.K., Pavlovich S.V. Systematic analysis of molecular synergy between folic acid and ferrous fumarate in iron deficiency anemia. Obstetrics and Gynecology. 2022; (12): 178-86. (in Russian)]. https://dx.doi.org/10.18565/aig.2022.301.
- Giblin T.J. Jr, Lee J.F., Rattigan J.P. Ferrous fumarate: a well-tolerated oral iron preparation. Clin. Med. (Northfield). 1962; 69: 2258-60.
- Yismaw A.E., Tulu H.B., Kassie F.Y., Araya B.M. Iron-folic acid adherence and associated factors among pregnant women attending antenatal care at Metema District, Northwest Ethiopia. Front. Public Health. 2022; 10: 978084.https://dx.doi.org/10.3389/fpubh.2022.978084.
- Кирилюк А.А. Железосодержащие лекарственные средства: от клинической фармакологии до фармацевтической помощи (сообщение 1). Вестник фармации. 2020; 3: 81-97. [Kirilyuk A.A. Iron-containing medicines: from clinical pharmacology to pharmaceutical assistance (report 1). Bulletin of Pharmacy. 2020; (3): 81-97. (in Russian)].
- Торшин И.Ю., Громова О.А., Лиманова О.А., Гришина Т.Р.,Башмакова Н.В., Керимкулова Н.В., Серова О.Ф., Крапошина Т.П.,Косенко И.М. Метаанализ клинических исследований по применению фумарата железа с целью профилактики и терапии железодефицитной анемии у беременных. Гинекология. 2015; 17(5): 24-31.[Torshin I.Iu., Gromova O.A., Limanova O.A., Grishina T.R., Bashmakova N.V., Kerimkulova N.V., Serova O.F., Kraposhina T.P., Kosenko I.M. A meta-analysis of clinical studies on the use of iron fumarate for the prevention and treatment of iron deficiency anemia in pregnantwomen. Gynecology. 2015; 17(5): 24-31. (in Russian)].
- Хельсинкская декларация Всемирной медицинской ассоциации. Принята на 18-й Генеральной Ассамблее ВМА, Хельсинки, Финляндия, июнь 1964 г., изменения внесены на 64-й Генеральной Ассамблее ВМА, Форталеза, Бразилия, октябрь 2013 г. [Declaration of Helsinki of the World Medical Association. Adopted at the 18th WMA General Assembly, Helsinki, Finland, June 1964, amended at the 64th WMA General Assembly, Fortaleza, Brazil, October 2013. (in Russian)].
- Национальный стандарт Российской Федерации ГОСТ Р 52379-2005 «Надлежащая клиническая практика». [National Standard of the Russian Federation GOST R 52379-2005 "Good Clinical Practice".(in Russian)].
- Российское общество акушеров-гинекологов, ФГБУ «Научный Центр акушерства, гинекологии и перинатологии им. В.И. Кулакова». Федеральные клинические рекомендации Минздрава России. Диагностика, профилактика и лечение железодефицитных состояний у беременных и родильниц. 2013. 26с. [Russian Society of Obstetricians and Gynecologists, V.I. Kulakov Scientific Center for Obstetrics, Gynecology and Perinatology. Federal Clinical Guidelines of the Ministry of Health of Russia. Diagnosis, prevention and treatment of iron deficiency conditions in pregnant and postpartum women. 2013. 26p. (in Russian)].
- Mehta B.C. Ineffectiveness of iron polymaltose in treatment of iron deficiency anemia. J. Assoc. Physicians India. 2003; 51: 419-21.
- Ruiz-Argüelles G.J., Díaz-Hernández A., Manzano C., Ruiz-Delgado G.J. Ineffectiveness of oral iron hydroxide polymaltose in iron-deficiency anemia. Hematology. 2007; 12(3): 255-6.https://dx.doi.org/10.1080/10245330701214160.
- Gilgen D., Mascie-Taylor C.G. The effect of weekly iron supplementation on anaemia and on iron deficiency among female tea pluckers in Bangladesh. J. Hum. Nutr. Diet. 2001; 14(3): 185-90. https://dx.doi.org/10.1046/j.1365-277x.2001.00291.x.
- Баев О.Р. Профилактика и лечение железодефицитных состояний во время беременности: применение комбинации железа и фолиевой кислоты. Фарматека. 2011; 13: 47-52. [Baev O.R. Prevention and treatment of iron deficiency conditions during pregnancy: use of combination of iron and folic acid. Pharmateca. 2011; (13): 47-52. (in Russian)].
Received 20.02.2023
Accepted 27.02.2023
Stanislav V. Pavlovich, PhD, Academic Secretary, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia; Professor, Department of Obstetrics, Gynecology, Perinatology and Reproductology, I.M. Sechenov First MSMU, Ministry of Health of Russia (Sechenov University), +7(495)438-20-88,
s_pavlovich@oparina4.ru,
https://orcid.org/0000-0002-1313-7079, 117997, Russia, Moscow, Akademika Oparina str., 4.
Oleg G. Melikhov, PhD, Director, Institute of Clinical Research; Chairman, Scientific Society “League of Clinical Research”, +7(916)695-05-30,
melikhov.oleg@gmail.com, https://orcid.org/0000-0001-9442-7707, 119590, Russia, Moscow, Ulof Palme str., 1.
Zulfiya S. Khodzhaeva, Dr. Med. Sci., Professor, Deputy Director of Obstetrics Institute, Professor at the Department of Obstetrics, Gynecology, Perinatology of the Department of Professional Education, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(495)438-07-88,
zkhodjaeva@mail.ru,
https://orcid.org/0000-0001-8159-3714, 117997, Russia, Moscow, Ac. Oparina str., 4.
Kamilla T. Muminova, PhD, Researcher at the High Risk Pregnancy Department, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(495)438-06-74,
kamika91@mail.ru, https://orcid.org/0000-0003-2708-4366, 117997, Russia, Moscow, Ac. Oparina str., 4.
Viktoriya A. Sakalo, PhD, Junior Researcher at the Department of Pregnancy Pathology, Institute of Obstetrics, doctor at the 1st Obstetric Department of Pregnancy Pathology, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russiaa, +7(929)588-72-08,
v_sakalo@oparina4.ru, https://orcid.org/0000-0002-5870-4655, 117997, Russia, Moscow, Ac. Oparina str., 4.
Elena A. Gorodnova, PhD, MPH (Master of Public Health), Head of Scientific & Clinical Center, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(495)531-44-44,
e_gorodnova@oparina4.ru, Author ID Scopus 6508176604, Author ID 597062, https://orcid.org/0000-0003-39937629,
117997, Russia, Moscow, Ac. Oparina str., 4.
Elena I. Abashova, PhD, Senior Researcher, Department of Gynecology and Endocrinology, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology, +7(812)328-98-20, +7(921)945-90-90,
abashova@yandex.ru, https://orcid.org/0000-0003-2399-3108, SPIN-код: 2133-0310,
199034, Russia, St. Petersburg, Mendeleevskaya line, 3.
Maria I. Yarmolinskaya, Professor of RAS, Dr. Med. Sci., Professor, Head of the Department of Gynecology and Endocrinology, Head of Center “Diagnostics and treatment of endometriosis”, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology; Professor at the Department of Obstetrics and Gynecology, I.I. Mechnikov North-Western State Medical University, Ministry of Health of Russia,
m.yarmolinskaya@gmail.com, https://orcid.org/0000-0002-6551-4147, eLibrary SPIN-код: 3686-3605,
199034, Russia, St. Petersburg, Mendeleevskaya line, 3.
Vasilii S. Chulkov, Dr. Med. Sci., Professor at the Department of Faculty Therapy, South-Ural State Medical University, Ministry of Health of Russia,
vschulkov@rambler.ru,
https://orcid.org/0000-0002-0952-6856, 454092, Russia, Chelyabinsk, Vorovskogo str., 64.
Zulfira M. Nazipova, hematologist, Republican Clinical Hospital, Ministry of Health of the Republic of Tatarstan, +7(905)376-21-36,
zulfira93@mail.ru,
https://orcid.org/0009-0002-3591-522Х, 420064, Russia, Republic of Tatarstan, Kazan, Orenburgsky Trakt str., 138.
Irina V. Savelyeva, Dr. Med. Sci., Professor, Head of the Department of Obstetrics and Gynecology No. 1, Omsk State Medical University, Ministry of Health of Russia, +7(913)654-35-77,
saveljeva_iv_omsk@mail.ru, https://orcid.org/0000-0001-9342-7342, 644099, Russia, Omsk, Lenina str., 12.
Margarita D. Andreeva, Dr. Med. Sci., Associate Professor, Professor at the Department of Obstetrics, Gynecology and Perinatology, Faculty of Postgraduate Education, Kuban State Medical University, Ministry of Health of Russia,
andreeva_md@mail.ru, https://orcid.org/0000-0002-6524-3965, 350063, Russia, Krasnodar, M. Sedina str., 4.
Yulia V. Kudlai, student of the Faculty of Pediatrics, Kuban State Medical University, Ministry of Health of Russia, +7(988)188-78-92,
kudlai.j@yandex.ru,
https://orcid.org/0000-0002-3192-7170, 350063, Russia, Krasnodar, M. Sedina str., 4.
Elena V. Enkova, Dr. Med. Sci., Professor, Head of the Department of Obstetrics and Gynecology No. 2, N.N. Burdenko Voronezh State Medical University,
Ministry of Health of Russia,
enkova@bk.ru, https://orcid.org/0000-0001-8885-1587, 394036, Russia, Voronezh, Studentskaya str., 10.
Aliya Sh. Zaripova, postgraduate student of the Department of Obstetrics and Gynecology named after V.S. Gruzdev, Kazan State Medical University,
Ministry of Health of Russia; Chief Physician of MC "Nurmed", obstetrician-gynecologist, ultrasound diagnostics doctor, +7(917)250-50-70,
zaripovaash@yandex.ru,
https://orcid.org/0000-0002-2701-319X, 420012, Russia, Kazan, Butlerov str., 49.
Corresponding author: Oleg G. Melikhov,
melikhov.oleg@gmail.com





