Серозная карцинома яичника высокой степени злокачественности (CКЯВСЗ) является наиболее распространенной среди карцином яичника (около 70%) и одной из самых частых причин смерти среди злокачественных опухолей у женщин [1, 2]. Известно, что большинство СКЯВСЗ диагностируются на поздних стадиях, когда известные методы лечения не имеют достаточной эффективности. Для опухоли в большей степени характерна устойчивость к химиотерапии и рецидивирование даже после радикального хирургического лечения [3, 4].
Доступные в настоящее время методы скрининговой диагностики не обладают необходимой чувствительностью и специфичностью, что приводит к поздней диагностике в случае спорадических СКЯВСЗ и облигатному показанию к сальпинговариоэктомии у носительниц BRCA-мутации (в западных странах) [5]. Таким образом, разработка методов раннего выявления СКЯВСЗ крайне необходима для улучшения прогноза заболевания и выживаемости пациенток. В последнее десятилетие накоплено множество доказательств того, что предшественниками СКЯВСЗ являются интраэпителиальные поражения маточной трубы [6]. Поэтому именно их своевременное обнаружение в настоящее время лежит в основе разработки методов ранней диагностики и профилактики СКЯВСЗ. К самым ранним изменениям эпителия маточной трубы можно отнести возникновение участков эпителия маточной трубы, состоящих только из секреторных клеток. Большинство авторов в качестве начального этапа канцерогенеза в маточной трубе относят SCOUT (secretory cells outgrowth) (более 30 подряд расположенных секреторных клеток). Однако, на наш взгляд, ограничение в 30 секреторных клеток для начального поражения эпителия маточной трубы не является достаточно оправданным. Есть основания полагать, что и меньший по протяженности непрерывный ряд клеток (не менее 10) является изменением, имеющим диагностическую и биологическую роль. В англоязычной литературе данное изменение получило название secretory cell expansion (SCE) [7, 8].
Таким образом, целью нашего исследования стало изучение возможности диагностики СКЯВСЗ с помощью оценки SCE и SCOUT в зависимости от возраста.
Материал и методы исследования
В исследование включены 287 пациенток, которые находились на лечении в Научном центре акушерства, гинекологии и перинатологии в 2012–2016 гг. по поводу внеяичниковых патологических изменений (лейомиома тела матки, аденомиоз, выпадение матки, n=70), серозных цистаденом/цистаденофибром яичника (n=75), серозных пограничных опухолей яичника (n=73) и СКЯВСЗ (n=69). Для исследования было отобрано 287 маточных труб (маточные трубы на стороне опухолей и по одной маточной трубе от пациенток из группы внеяичниковвх патологических изменений). Для исследования были взяты 2–3 фрагмента фимбриального отдела маточной трубы.
В маточной трубе выявляли SCE (более 10 подряд расположенных секреторных клеток) и SCOUT (более 30 подряд расположенных секреторных клеток). Иммуногистохимическим (ИГХ) методом оценена экспрессия р53, Ki-67 для исключения серозных трубных интраэпителиальных карцином (СТИК) (только в маточных трубах пациенток с СКЯВСЗ). Использовались следующие антитела для постановки реакций: р53 (клон DO-7, RTU, Dako), Ki-67 (клон MIB-1, RTU, Dako). Всего проанализировано 250 ИГХ-препаратов. Оценка экспрессии Ki-67 проводилась в процентах на 3000 клеток. Экспрессия р53 оценивалась следующим образом: отсутствие экспрессии или сильная экспрессия в более, чем 75% атипических клеток расценивалась как соответствующая мутации в гене ТР 53 (мутантный белок р53), слабая экспрессия р53 в эпителии маточной трубы расценивалась как соответствующая отсутствию мутации в данном гене (wild-type белок).
Результаты исследования были подвергнуты статистической обработке с использованием программы IBM SPSS Statistics 20 [9]. Совокупности количественных показателей, распределение которых отличалось от нормального, описывались при помощи значений медианы и нижнего и верхнего квартилей. Для сравнения показателей использовались критерии Краскела–Уоллиса (при множественных сравнениях) и Манна–Уитни (при парных сравнениях). Зависимость показателей SCE и SCOUT от возраста пациенток изучалась с помощью метода парной линейной регрессии с оценкой тесноты корреляционной связи по коэффициенту Пирсона. Для разработки прогностической модели, позволяющей определить риск серозных карцином в зависимости от различных факторов, использовался метод дискриминантного анализа. В качестве основных характеристик полученной модели рассчитывались показатели диагностической эффективности, чувствительности и специфичности.
Результаты исследования
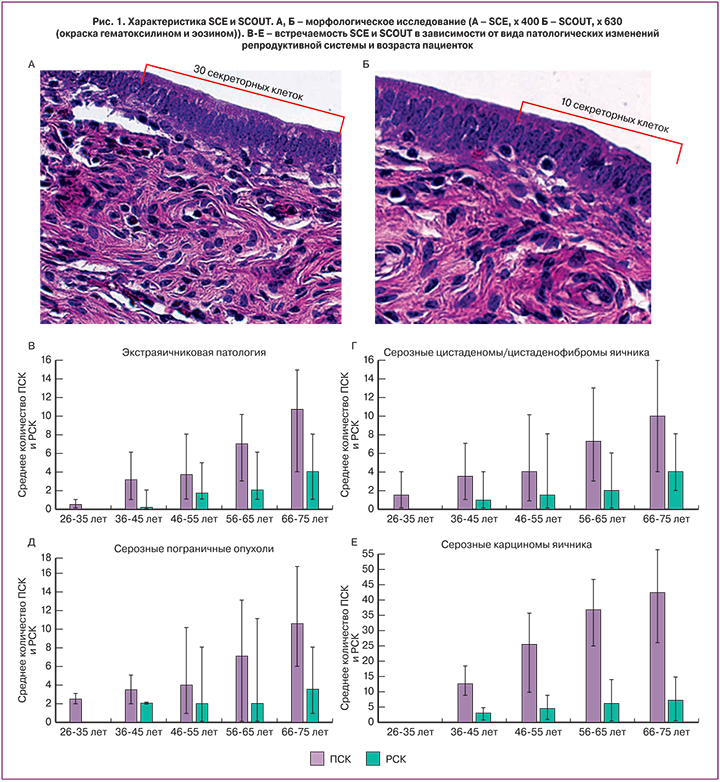
SCE и SCOUT были оценены в маточных трубах пациенток со всеми изучаемыми патологиями репродуктивной системы, которые были разделены на возрастные группы (26–35, 36–45, 46–55, 56–65, 66–75 лет) (рис. 1А и Б).
Количество SCE варьировало от 0 до 57, составляя в среднем 4,96 при внеяичниковых патологических изменениях и 29,3 – при СКЯВСЗ. Такая же тенденция наблюдалась и в отношении SCOUT (от 0 до 15, в среднем – 1,6 при внеяичниковых патологических изменениях и 5,5 – при СКЯВСЗ (рис. 1Г-Ж).
Зависимость количества SCE у пациенток с серозными карциномами яичников от возраста была изучена методом парной линейной регрессии. В результате было получено следующее уравнение (1):
SCE = 0,974 × ВОЗ – 23,84 (1)
где SCE – количество SCE в маточных трубах при серозных карциномах яичника, ВОЗ – возраст женщины (полных лет).
Исходя из значения коэффициента регрессии, увеличение возраста на 1 год сопровождается ростом количества SCE на 0,926. Наблюдаемая зависимость характеризовалась коэффициентом корреляции Пирсона rxy=0,862, что по шкале Чеддока соответствовало ее высокой тесноте. Уровень значимости корреляционной связи составил p<0,001. Согласно коэффициенту детерминации R2, возрастной фактор определяет 74,3% дисперсии показателя количества SCE.
Графически зависимость количества SCE от возраста пациенток при серозных карциномах яичников представлена на рис. 2А.
Зависимость количества SCOUT от возраста может быть описана следующим уравнением парной линейной регрессии (2):
SCOUT = 0,178 × ВОЗ – 4,535 (2)
где SCOUT – количество SCOUT в маточных трубах при серозных карциномах яичника, ВОЗ – возраст женщины (полных лет).
Согласно полученному значению коэффициента регрессии, между показателями отмечалась прямая связь: увеличение возраста женщины на 1 год сопровождается ростом количества SCOUT на 0,178 (p=0,003). Коэффициент корреляции Пирсона составил rxy=0,35, что соответствует умеренной тесноте корреляционной связи по шкале Чеддока. Исходя из значения коэффициента детерминации R2, возрастной фактор определяет 12,2% дисперсии показателя количества SCOUT.
График регрессионной функции, описывающей зависимость количества SCE от возраста пациенток при серозных карциномах яичников, представлен на рис. 2Б.
Возможность прогнозирования серозных карцином исходя из показателей экспансии секреторных клеток в маточных трубах с учетом возраста пациенток была изучена с помощью метода дискриминантного анализа. При построении прогностической модели использовался метод шагового отбора, в результате в уравнение дискриминантной функции (3) были включены все исследуемые факторы:
СКЯВСЗ = 0,22×SCE + 0,055×SCOUT – 0,068×ВОЗ + 0,72 (3),
где СКЯВСЗ – дискриминантная функция, характеризующая вероятность серозной карциномы яичника по приведенному ниже алгоритму, SCE – количество SCE в маточных трубах, SCOUT – количество SCOUT в маточных трубах, ВОЗ – возраст пациентки (полных лет).
Исходя из значений коэффициентов при факторных переменных, вероятность СКЯВСЗ увеличивалась с ростом количества SCE и SCOUT, при этом чем в более молодом возрасте отмечался рост экспансии секреторных клеток, тем в большей степени это свидетельствовало о наличии СКЯВСЗ.
Константа дискриминации, разделяющая исследуемых на две группы, определялась как значение функции (3), равноудаленное от центроидов, составивших в группе женщин с серозной карциномой яичника 4,886, а при ее отсутствии – 1,561. Соответственно, константа дискриминации составила 1,663. Таким образом, при значении функции СКЯВСЗ более 1,663 женщина относилась к группе высокого риска серозных карцином яичника, при значении функции менее 1,663 – к группе низкого риска.
Статистическая значимость различий дискриминантной функции в обеих группах подтверждалась значением коэффициента λ Уилкса, составившим 0,115 при уровне значимости p<0,001.
Результаты классификации исследуемых женщин с использованием полученной дискриминантной функции (3) представлены в таблице.
Согласно полученным данным, общий процент верно классифицированных исходных наблюдений (диагностическая эффективность) составил 98,2%. Чувствительность используемой функции составила 92,8%, специфичность – 100,0%, что свидетельствует о большей вероятности ложноотрицательных результатов.
Следует отметить, что 5 женщин, у которых ошибочно было предсказано отсутствие СКЯВСЗ при фактическом его наличии, имели возраст от 40 до 46 лет и были самыми молодыми в группе исследуемых с данным заболеванием. Таким образом, возраст младше 47 лет можно считать ограничением для практического применения прогностической формулы (3).
Учитывая выявленную прямую взаимосвязь риска СКЯВСЗ и количества SCE и SCOUT, мы дополнительно рассмотрели значения показателей экспансии секреторных клеток в зависимости от наличия определенного вида опухоли яичника или внеяичниковых патологических изменений. В связи с тем, что показатели имели распределение, отличное от нормального, результаты были представлены в виде ящичных диаграмм с указанием медиан, нижнего и верхнего квартилей, минимального и максимального значений (рис. 2В).
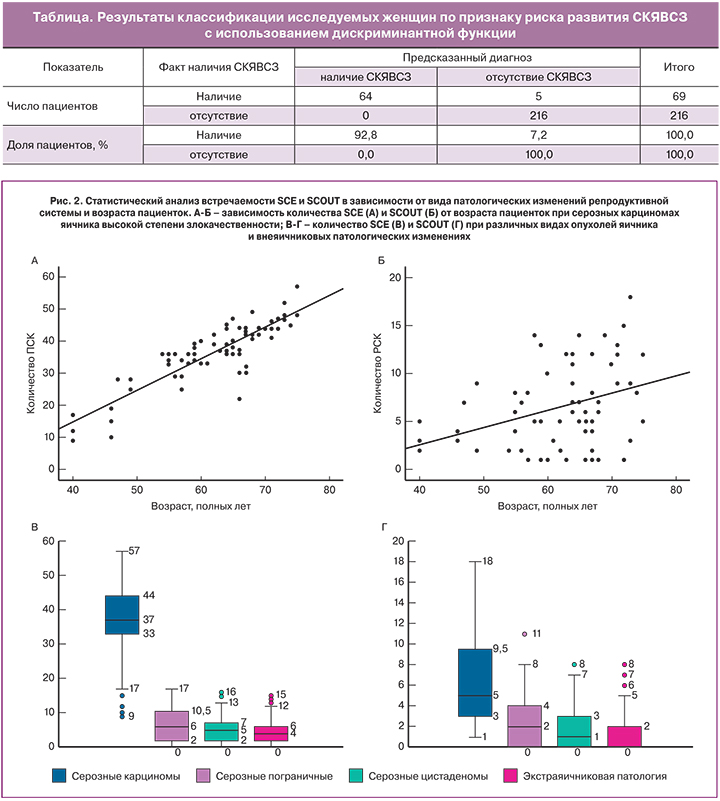
В соответствии с представленными данными, СКЯВСЗ сопровождались существенным ростом количества SCE по сравнению с другими формами патологических изменений. При исключении «выскакивающих» вариантов минимальное количество SCE у пациенток с серозными карциномами составило 17, тогда как среди остальных исследуемых это значение показателя было наивысшим возможным. При сравнении количества SCE в зависимости от формы патологических изменений с помощью критерия Краскела–Уоллиса были установлены статистически значимые различия (p<0,001). Такой же уровень значимости отмечался при проведении парных сравнений количества SCE в группе пациенток с СКЯВСЗ и каждой из групп с прочей патологией с помощью критерия Манна–Уитни.
В случае оценки количества SCE в маточных трубах в зависимости от формы патологических изменений исследуемых была получена диаграмма, отраженная на рис. 2Г.
Как показали результаты проведенного статистического анализа, группа пациенток с СКЯВСЗ также отличались более высоким количеством SCOUT. Медиана показателя при данной опухоли составила 5, значения нижнего и верхнего квартилей – 3 и 9,5 соответственно. Медиана количества SCE при пограничных опухолях яичника составила 2 (интерквартильный размах (ИКР) 0-4), при серозных цистаденомах/цистаденофибромах – 1 (ИКР 0-3), при внеяичниковых патологических изменениях – 0 (ИКР 0-2). Различия показателей в зависимости от типа патологических изменений были статистически значимы (p<0,001); также были существенными различия при парных сравнениях группы пациенток с СКЯВСЗ и пациенток с другими видами патологических изменений (p<0,001).
Обсуждение
Почти 90% тазовых серозных карцином (яичника, маточной трубы, брюшины) возникают спорадически, без предрасполагающих герминогенных мутаций [10]. Таким образом, чаще всего предрасположенность к развитию СКЯВСЗ остается не исследованной, даже при оценке BRCA-статуса. Именно поэтому поиск независимых факторов риска данных опухолей является важнейшей социальной и медицинской задачей для морфологов и онкологов во всем мире. В настоящее время фимбриальный отдел маточной трубы, как полагают, является основным источником развития тазовых карцином, в том числе и СКЯВСЗ. Поэтому наиболее пристально изучается именно состояние эпителия маточной трубы в патогенезе данных опухолей. В эпителии маточной трубы различают три типа клеток – реснитчатые, секреторные и вставочные клетки, причем последние, как полагают, являются предшественниками секреторных и обладают прогениторными свойствами [11]. В ряде работ было показано, что именно в секреторных клетках происходят изменения, дающие начало развития СКЯВСЗ. Так, полагают, на первом этапе происходит экспансия секреторных клеток без изменения их фенотипа, однако в данном участке эпителия отмечают нарушение репарации ДНК, подтверждаемое изменением экспресии γ-H2AX. Затем возникает мутация в гене TP53 с развитием р53-signature, далее происходит накопление мутаций в других генах, изменение морфологии клеток с развитием серозного трубного интраэпителиального поражения и, наконец, возникновение мутаций BRCA1/2 с образованием СТИК [12]. Наиболее ранним секреторным поражением до недавних пор считалось SCOUT, представляющее собой участок эпителия маточной трубы, состоящий из 30 непрерывно расположенных секреторных клеток [13]. Было также показано, что встречаемость SCE при СКЯВСЗ значительно выше, чем при других опухолях яичника, и высказано предположение, что SCOUT может служить потенциальным маркером СКЯВСЗ [14].
Однако в ряде работ было показано, что наиболее оправданным является участок эпителия маточной трубы протяженностью в 10 секреторных клеток, поскольку именно такой участок является наименьшим, отличающимся от неизменного эпителия (secretory cell expansion). Нами был проведен анализ зависимости количества участков эпителия маточной трубы, состоящих из секреторных клеток (SCE и SCOUT) от вида патологических изменений репродуктивной системы пациенток и их возраста. Увеличение SCE и SCOUT с возрастом было отмечено для всех исследуемых патологических изменений (p<0,001), хотя количество SCE во всех группах было выше, чем количество SCOUT. При сравнении групп пациенток с различными патологиями было выявлено, что количество SCE и SCOUT в группе пациенток с СКЯВСЗ было значительно выше, чем в остальных группах (p<0,001). Исследования, посвященные особенностям SCOUT, проводились и ранее, но сравнение SCE и SCOUT с учетом возраста и заболевания пациенток не изучалось. Так, C. Quick и соавт. отметили увеличение SCOUT у пациенток с серозными карциномами, однако авторы не смогли отнести данный показатель к независимым факторам риска после применения поправки на возраст пациенток [15]. В нашем исследовании корреляция между возрастом и количеством для SCE была сильнее, чем для SCOUT (коэффициент Пирсона 0,86 и 0,35 соответственно). Хотя статистически значимыми полученные результаты были в обоих случаях. Полученные данные позволяют рассматривать возраст пациенток в качестве независимого фактора риска для развития СКЯВСЗ, хотя и менее существенного, чем мутации в генах BRCA1/2 [16, 17]. Отличия наших данных от полученных в предыдущих исследованиях могут быть объяснены прицельным взятием фимбриального отдела маточной трубы, в котором, как известно предшественники СКЯВСЗ возникают в 5 раз чаще, чем в других отделах [18].
Также на основании полученных результатов нами была построена прогностическая модель (СКЯВСЗ=0,22×SCE+0,055×SCOUT–0,068×ВОЗ+0,72), на основании применения которой было выявлено, что вероятность развития СКЯВСЗ увеличивалась с ростом количества SCE и SCOUT, при этом чем в более молодом возрасте отмечался рост экспансии секреторных клеток, тем в большей степени это свидетельствовало о наличии СКЯВСЗ. Однако вследствие ошибочных результатов, полученных у самых молодых женщин в группе СКЯВСЗ (40–46 лет) мы предлагаем установить нижний возрастной порог для применения данной формулы равный 47 годам.
Кроме того, мы сравнили SCE и SCOUT в качестве прогностического критерия. Было выявлено, что можно выделить минимальное количество SCE, встречающееся при СКЯВСЗ, в то время как для других патологических изменений это самое большое значение SCE. Для SCOUT такое минимальное значение выделить невозможно, что показано на рис. 2В и Г. Таким образом, в качестве изолированного маркера SCE обладает большей эффективностью, чем SCOUT, однако наибольшей эффективностью обладает совместная оценка SCE, SCOUT и возраста пациенток, что и было продемонстрировано в настоящем исследовании с помощью предложенной прогностической формулы. По сравнению с имеющимися в настоящее время способами ранней диагностики рака яичников предложенный нами метод обладает гораздо большей эффективностью [19–21].
На основании полученных в данном исследовании результатов подана заявка на патент № 2016139367 от 07.10.2016.
Заключение
В исследовании было показано, что поражение эпителия маточной трубы, для которого характерно наличие 10 подряд расположенных секреторных клеток, является более эффективным изолированным маркером, чем поражение, для которого характерно более 30 подряд расположенных секреторных клеток. Для обоих поражений характерно увеличение их количества с возрастом; также отмечается большее количество участков с более коротким изменением эпителия вне зависимости от вида патологических изменений репродуктивной системы. Также значительно большее количество обоих видов изменений эпителия маточных труб характерно для пациенток с СКЯВСЗ. На основании полученных данных нами разработана формула, включающая оценку количества измененных участков эпителия маточной трубы и возраст пациенток, на основании применения которой пациентку можно отнести к группе высокого или низкого риска серозных карцином яичника. Общий процент верно классифицированных по предложенной формуле исходных наблюдений (диагностическая эффективность) составил 98,2%, чувствительность – 92,8%, специфичность – 100,0% (возраст младше 47 лет считали ограничением для применения формулы из-за возможных ложноотрицательных результатов).



