Повышение эффективности лечения заболеваний шейки матки (ШМ), ассоциированных с вирусом папилломы человека (ВПЧ), продолжает оставаться актуальным в связи с ростом диагностируемых предраковых и раковых процессов ШМ и отсутствием специфического лечения ВПЧ-инфекции.
Согласно критериям ВОЗ, рекомендациям Центров по контролю и профилактике заболеваний США, а также и Европейским рекомендациям, для лечения CIN 2–3 применяют эксцизионные методы хирургического вмешательства [1, 2]. Однако до настоящего времени не существует единого мнения по тактике ведения пациенток с CIN 1–2 ввиду высоковероятного регресса данной патологии. В связи с этим представляется актуальной разработка оптимальных схем системной и местной фармакологической коррекции предраковых заболеваний шейки матки с помощью препаратов, таргетно воздействующих на патогенез цервикальных дисплазий и механизмы их последующей опухолевой трансформации.
Результаты многочисленных экспериментальных и клинических исследований показали, что субстанция дииндолилметан (ДИМ), являясь веществом с мультитаргетной противоопухолевой активностью, эффективно блокирует молекулярные механизмы патологической пролиферации и малигнизации ВПЧ-инфицированных клеток цервикального эпителия [3–5]. Противоопухолевая активность ДИМ в отношении ВПЧ-инфицированных клеток эпителия ШМ была подтверждена в экспериментах in vitro, in vivo и в клинических исследованиях [6, 7].
С учетом противоопухолевых свойств ДИМ был создан препарат цервикон-ДИМ в лекарственной форме «суппозитории вагинальные». Интравагинальный способ применения препарата позволяет увеличить концентрацию активного вещества в инфицированных тканях шейки матки, а также свести к минимуму отрицательное системное воздействие препарата.
Цель: изучение эффективности и безопасности препарата цервикон-ДИМ в лекарственной форме «суппозитории вагинальные» у пациенток с диагнозом CIN 1–2 в рамках двойного слепого, рандомизированного, плацебо-контролируемого многоцентрового клинического исследования III фазы.
Материал и методы исследования
Исследование проводилось в 17 медицинских учреждениях России. В задачи исследования входили:
Оценка эффективности препарата цервикон-ДИМ в лечении цервикальной интраэпителиальной неоплазии слабой и средней степени (CIN 1–2).
Регистрация нежелательных явлений при интравагинальном применении препарата цервикон-ДИМ.
В исследовании было предусмотрено 2 периода: период скринингового обследования – до 45 дней и период активной терапии (амбулаторное лечение) – 90 дней. В период скрининга, после подписания пациентками формы информированного согласия на участие в исследовании, оценивались критерии включения и исключения в исследовании. На 1-м визите 160 пациенток, отвечающие критериям включения, были рандомизированы в 2 группы по 80 пациенток в каждую. Каждой пациентке присваивался рандомизационный номер методом генерации случайных чисел с использованием статистической программы SPSS Statistics 19.0. Рандомизация производилась слепым централизованным методом.
Исследуемый препарат цервикон-ДИМ назначался пациенткам первой группы в дозе 200 мг/сут (1 суппозиторий 100 мг дважды в сутки). Пациентки второй группы получали плацебо (1 суппозиторий с плацебо дважды в сутки). Выбор дозы препарата цервикон-ДИМ 200 мг/сут и длительность применения были обоснованы результатами ранее проведенного клинического исследования II фазы [7], по данным которого было установлено, что доза в 200 мг/сут является более эффективной.
В исследовании было предусмотрено 3 визита пациенток, во время которых проводилась оценка параметров эффективности и безопасности. Данное исследование было проведено как двойное слепое. Исследователи и пациентки не обладали информацией о приеме в ходе исследования активного препарата или плацебо.
Параметры эффективности: доля пациенток с полной или частичной регрессией CIN 1–2 через 3 месяца после начала применения препарата цервикон-ДИМ на основании результатов гистологического исследования биоптатов шейки матки.
Параметры безопасности: регистрация нежелательных явлений (НЯ) и случаев приема сопутствующих препаратов на всем протяжении исследования.
Основные критерии включения: наличие письменного информированного согласия об участии в данном исследовании; способность выполнять процедуры, предусмотренные Протоколом исследования; кольпоскопическая картина поражений шейки матки на фоне зоны трансформации I и II типа; возраст 18–45 лет; диагноз цервикальной интраэпителиальной неоплазии 1–2-й степени (CIN 1–2), верифицированный гистологически; согласие использовать барьерный метод контрацепции (презерватив) в течение всего срока исследования.
Критерии исключения: площадь участков дисплазии цервикального эпителия менее 1 см2; необходимость проведения хирургического лечения либо иных хирургических вмешательств на шейке матки; тяжелые сопутствующие заболевания сердечно-сосудистой или нервной систем, психические заболевания, почечная и печеночная недостаточность или иные тяжелые заболевания и/или физические состояния, которые, по мнению исследователя, делают невозможным участие пациентки в исследовании; инфекции мочеполовой системы в фазе активного воспаления; нарушения свертываемости крови; аллергические реакции на йодсодержащие препараты в анамнезе; цервикальная интраэпителиальная неоплазия 3-й степени (CIN 3), либо карцинома ШМ in situ; положительные тесты RW или ВИЧ; злоупотребление алкоголем, наркотическая или лекарственная зависимость; злокачественные новообразования любой локализации; прием какого-либо экспериментального препарата за 30 дней или пять периодов полувыведения (в зависимости от того, какая величина больше) до приема первой дозы исследуемого препарата; беременность, период грудного вскармливания; девственность.
Результаты исследования
По основным демографическим показателям (возраст, рост, масса тела, индекс массы тела) группы исследования не различались, что позволяло объединить их для анализа согласно задачам исследования. Значимость отличий составила 0,250; 0,385; 0,256 и 0,318 соответственно (t-критерий Стьюдента для несвязанных совокупностей).
Значимых различий в характеристиках исходного состояния – данных гинекологического анамнеза (возраст менархе, длительность менструаций, интервал между менструациями, возраст начала половой жизни) выявлено не было (0,995; 0,277 и 0,500, соответственно).
Всем пациенткам проводили стандартное гинекологическое обследование: микроскопия мазка, цитологическое исследование мазков с шейки матки, ВПЧ-тест, кольпоскопия, биопсия с последующим гистологическим исследованием биоптатов шейки матки.
После завершения скрининга из исследования выбыло: из группы плацебо – 2 пациентки, из группы цервикон-ДИМ – 7.
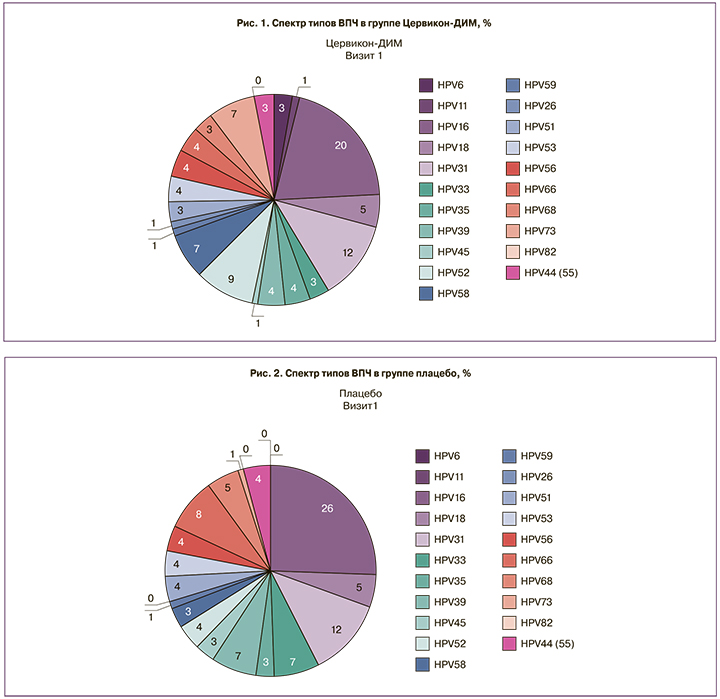
У всех пациенток 1-й и 2-й групп перед исследованием был выявлен вирус папилломы человека (ВПЧ), который определялся методом полимеразно-цепной реакции (ПЦР). Результаты генотипирования показали, что из высокоонкогенных типов ВПЧ превалировал 16-й тип (26%), на втором месте – 18-й тип (8%) и в равных количествах 33-й и 39-й типы (по 7%), из низкоонкогенных – ВПЧ 11-го типа. Результаты представлены на рис. 1 и 2.
 Во всех группах проводился цитологический контроль, результаты которого представлены в табл. 1.
Во всех группах проводился цитологический контроль, результаты которого представлены в табл. 1.
При кольпоскопии у всех пациенток выявлена зона трансформации 1-го и 2-го типа. Слабовыраженные и выраженные изменения при кольпоскопии наблюдались во всех случаях, что позволило всем участницам исследования выполнить биопсию.
Результаты кольпоскопического исследования шейки матки в группах исследования представлены ниже (табл. 2).
Биопсия шейки матки проводилась под кольпоскопическим контролем из наиболее измененных участков шейки матки с помощью биопсийных щипцов. Количество биоптатов составляло не менее 3, размеры биоптатов составляли от 3×3 до 5×5 мм.
Гистологические данные анализа биопсийного материала, проведенного на первом визите, представлены в табл. 3.

Как следует из табл. 3, пациентки распределились по степени тяжести дисплазии равномерно в группе плацебо и цервикон-ДИМ.
После завершения периода терапии пациенткам были повторно выполнены кольпоскопическое исследование и биопсия шейки матки.
Оценка эффективности лечения проводилась по твердой конечной точке – данным гистологического изучения биоптатов шейки матки. При этом повторная биопсия проводилась по тем же правилам, что и исходная. В случае если установить патологически измененный участок шейки матки при кольпоскопии не представлялось возможным (например, в случае нормальной кольпоскопической картины), биопсия бралась из участка, максимально близкого к месту исходной биопсии.
Основной критерий эффективности лечения был рассчитан как доля пациенток с полной или частичной регрессией CIN 1, CIN 2 через 3 месяца после начала применения препарата цервикон-ДИМ. Считалось, что произошла частичная регрессия CIN в случае, если CIN 2-й степени перешла в CIN 1-й степени (табл. 4).
По результатам сравнения эффективности терапии, определяемой по факту нормализации по данным гистологических результатов, в группах исследования через 3 месяца терапии было установлено следующее: в группе терапии цервикон-ДИМ эффективность терапии была значимо выше и составила 87,3% (95% ДИ по методу Клоппера–Пирсона 76,5–94,4%), в группе плацебо – 69,7% (95% ДИ по методу Клоппера–Пирсона 57,1–80,4%) соот-ветственно (р=0,013 – точный критерий Фишера, р=0,015 – критерий χ2).
Гистологическое исследование на 3-м визите выполнено 63 пациенткам из группы цервикон-ДИМ и 66 пациенткам из группы плацебо. Исследование не выполнялось по причине отказа пациентки или выбывания из исследования.
Одной из задач данного исследования была оценка НЯ. В группе плацебо НЯ (зуд, жжение) отмечались у 11,4% пациенток (9 из 79, 95% ДИ 5,3–20,5%); в группе терапии цервикон-ДИМ НЯ (зуд, жжение) отмечались у 18,7% пациенток (15 из 80 включенных в исследование, 95% ДИ 11,9–30,4%).
Значимые различия между группами по количеству пациенток, у которых было отмечено наличие НЯ, не обнаружены (р=0,101 – точный критерий Фишера, р=0,136 – критерий χ2).
Обсуждение
Дисплазия различной степени является предраковым заболеванием, у которого индекс малигнизации (онкологической трансформации) может достигать 50%. Анализ исходов диспластических изменений плоского эпителия шейки матки показал, что при LSIL (плоскоклеточное поражение легкой степени) регрессия наблюдается в 57%, персистенция – в 32%, прогрессия – в 11%, а развитие инвазивного РШМ происходит только в 1% случаев. В то же время при HSIL (плоскоклеточное поражение тяжелой степени) регрессия прослеживается в 32%, а малигнизация происходит более чем в 12% случаев. Согласно другим исследованиям – 91% легких дисплазий, выявленных на Пап-мазках, самопроизвольно регрессируют в течение 36 месяцев и только 3% переходят в тяжелую дисплазию [8, 9].
LSIL (также известный как CIN 1) признаны как доброкачественные поражения. Что касается CIN 2, то в настоящее время биологическая ее суть непонятна и вызывает ряд вопросов. Несмотря на доказательства различий в клинических течениях CIN 2 и CIN 3, обновленная гистопатологическая классификация Всемирной организации здравоохранения от 2014 года определила эти поражения как единое целое: плоскоклеточное внутриэпителиальное поражение высокой степени (HSIL). В большинстве клинических случаях CIN 2 и CIN 3 подвергаются хирургическому лечению. Однако эксцизионное удаление пораженной части ШМ увеличивает риск преждевременных родов и самопроизвольных выкидышей во 2-м триместре беременности [10–13]. Поскольку это молодые, чаще нерожавшие женщины, важно избегать агрессивного хирургического вмешательства. В последние годы появляются научные данные том, что CIN 2 является неоднозначным гистологическим диагнозом, в ряде исследований продемонстрированы высокие показатели регрессии, особенно у молодых женщин [14].
Так, в проспективном когортном исследовании среди 95 женщин в возрасте от 18 до 23 лет показатель регрессии CIN 2 составлял 63%, тогда как только у 15% женщин произошла прогрессия до CIN 3 в течение трех лет [15].
В другой проспективной когорте из 5052 женщин в возрасте от 18 до 62 лет 40% поражений CIN 2 регрессировали в течение двух лет [16].
В то же время длительное динамическое наблюдение и тактика невмешательства неприемлемы для пациенток с низкой мотивацией посещать врача в условиях отсутствия в стране организованного цитологического скрининга.
В настоящее время известны соединения природного происхождения, блокирующие развитие неопластических процессов в эпителиальных тканях, высокая эффективность которых доказана в экспериментальных и клинических исследованиях [4].
К числу таких соединений принадлежит дииндолилметан – активный компонент препарата цервикон-ДИМ. Дииндолилметан нормализует метаболизм эстрогенов, а также подавляет экспрессию и туморогенную активность вирусных онкобелков в ВПЧ-инфицированных цервикальных клетках [17]. Кроме того, дииндолилметан активирует иммунные функции организма, повышая чувствительность клеток к противовирусному и противоопухолевому действию интерферона, а также избирательно стимулирует апоптоз вирус-инфицированных и трансформированных клеток цервикального эпителия. Таким образом, в основе терапевтического эффекта дииндолилметана лежит его способность ускорять элиминацию ВПЧ-инфицированных и трансформированных клеток шейки матки. Цервикон-ДИМ действует локально и значительно повышает вероятность регрессии CIN 1–2 по сравнению с плацебо.
Результаты клинического исследования показали, что цервикон-ДИМ при интравагинальном введении обладает высокой местной биодоступностью – 72–73% от введенной дозы ДИМ распределяется в тканях влагалища, но низкой системной абсорбцией – лишь 3–4% ДИМ выявляется в системном кровотоке. Полученные результаты исследования продемонстрировали выраженное влияние ДИМ на эрадикацию ВПЧ: при применении ДИМ в дозе 200 мг/сут эрадикация ВПЧ произошла в 70% случаев. Данные о накоплении препарата в организме и возможности его кумуляции в проведенных ранее исследованиях не выявлялись. Таким образом, цервикон-ДИМ характеризуется высоким уровнем безопасности и переносимости, что объясняется его лекарственной формой и зоной воздействия [7].
Заключение
Результаты, полученные в данном исследовании, свидетельствуют о высокой эффективности препарата цервикон-ДИМ в лечении преинвазивных заболеваний шейки матки – CIN 1–2. Курсовое применение данного препарата позволяет избежать деструктивных хирургических вмешательств, особенно у молодых нерожавших женщин, сохраняя у пациенток анатомо-функциональную целостность шейки матки и архитектонику цервикального канала. Это позволяет рекомендовать включение препарата цервикон-ДИМ в комплекс лечения преинвазивных заболеваний шейки матки, особенно у молодых женщин с нереализованной репродуктивной функцией.



