An innovative model of endotracheal catheter (FETO-balloon) for fetal endoscopic tracheal occlusion in severe congenital diaphragmatic hernia
The paper presents the data available in the literature on the etiology and pathogenesis of congenital fetal malformations, treatments for congenital diaphragmatic hernias, and fetoscopic endotracheal occlusion (FETO) procedure. It describes the first Russian model of a fetal endoscopic tracheal occlusion catheter (FETO balloon) designed at the Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology, and Perinatology, Ministry of Health of Russia. The novel model consists of a 10-cm distal part with a balloon and a 50-cm proximal one with a channel for administration of saline solution into the cuff of the inflatable balloon with the possibility of separating the distal part from the proximal one. The patient selection criteria for the use of the endotracheal catheter (FETO balloon) are singleton pregnancy, confirmed diagnosis of severe or extremely severe fetal diaphragmatic hernia, and a patient’s signed informed consent form. The prerequisite is the prevention of respiratory distress syndrome before FETO or/and when the signs of threatening preterm birth appear at 24 to 34 weeks’ gestation. The delivery period is 37–38 weeks. Caesarean section is a method for delivery; EXIT is a procedure for balloon removal.Schneiderman M.G., Burov A.A., Naberezhnev Yu.I., Podurovskaya Yu.L., Tetruashvili N.K., Shmakov R.G., Sukhikh G.T.
Conclusion. The introduction of the first innovative Russian model of an endotracheal catheter for fetal endoscopic tracheal occlusion in severe congenital diaphragmatic hernia will be able to avoid repeated fetoscopic interventions aimed at antenatal balloon removal, thereby lowering the risk of intraoperative maternal and fetal complications.
Keywords
Congenital fetal malformations (CFMs) are a problem of both medical and social significance, as these malformations occupy one of the first places among infant deaths and disabilities since childhood [1]. CFMs refer to the group of medico-social perinatal pathology, as they result from the high level of infant mortality and infant disability [1]. Congenital diaphragmatic hernia (CDH), when the abdominal organs move to the chest of the fetus during antenatal development, continues to remain one of the least studied problems in the large group of congenital malformations. CDH is detected in every tenth newborn with CFM, its incidence ranges from 1:2000 to 1:5000 of live births [2–4]. The mortality rate in the group of newborns with CDH continues to remain at a high level and it ranges from 35 to 80% depending on the capabilities of the medical institution [4, 5]. In the leading medical institutions of the European Union and the United States, this indicator is 50% [6].
The death of a newborn with CDH is mainly caused by resistance to the treatment of pulmonary hypertension, primarily due to hypoplasia of the lungs.
The development of CDH may result from chromosomal defects, genetic syndromes, and/or non-genetic factors [7, 8].
Chromosomal defects include trisomy 18, tetrasomy 12p (Pallister–Killian syndrome), Fryns syndrome.
Genetic syndromes associated with CHD are Apert syndrome, CHARGE syndrome, Coffin–Siris syndrome, Gorlin–Goltz syndrome, Perlman syndrome, Swyer syndrome, Cornelia de Lange syndrome, Goldenhar syndrome, Beckwith–Wiedemann syndrome, Simpson–Golabi–Bemel syndrome, Donnai–Barrow syndrome, Matthew–Wood syndrome, Jarcho–Levin syndrome, Fraser syndrome, Stickler syndrome, Pierre Robin syndrome. The literature also describes partial trisomy of chromosome 5, partial trisomy of chromosome 20 and polyploidy [7, 8].
The non-genetic theory of CDH development is based on the inability of the pleuroperitoneal canal to close normally under the influence of environmental factors which affect the differentiation of mesenchymal cells during the formation of the diaphragm.
There can be different types of diaphragmatic hernias depending on their locations:
- posterolateral hernia (Bochdalek hernia) – 75–95% of cases;
- right-sided hernia – 10–15% of cases;
- parasternal hernia (Morgagni hernia) – 1–2% of cases;
- bilateral hernia – 1–2% of cases.
CDH is usually revealed primarily by ultrasound at the end of the second trimester of pregnancy, when pathognomonic signs of this malformation appear (abnormal image of the chest organs, displacement of the heart and its compression; detection of pathological anechogenic formations which are evidence of the location of the stomach, loops of the small intestine, liver, and spleen in the chest of the fetus) [9].
Currently, the measurement of the fetal lung-to-head ratio (LHR), which was first described by Metkus et al., is the widely used and validated method for predicting neonatal outcome [10]. The real/expected LHR ratio is considered to be the most optimal for assessing the prognosis of newborn survival and therefore it is most frequently used in clinical practice [11]. Mild left-sided CDH has an indicator value of 45%, moderate – 25%, severe and very severe degrees have indicators of up to 25% and less than 15%, respectively. Every degree of severity of CDH correlates with the survival rate of the newborn. Mild and moderate degrees correspond to 95% and 50%, and severe and very severe degrees to 30% and 5%, respectively. Penetration of the liver and/or stomach into the chest significantly reduces the survival rate [12, 13].
Active treatment of CDH began not so long ago, namely, in the middle of the XX century, and approaches to its implementation have significantly changed due to the development of anesthesiology, resuscitation, fetal surgery, and pharmacology [14].
Nowadays, high-frequency oscillatory ventilation and extracorporeal membrane oxygenation (ECMO) are used to treat severe hypoplasia of the lungs in a newborn.
The results of the study on the effectiveness and safety of fetal endoscopic tracheal occlusion are contradictory.
A prospective randomized controlled trial of fetal endoscopic tracheal occlusion for CDH showed no differences in fetal survival after fetal endoscopic tracheal occlusion or standard postnatal surgical correction of diaphragmatic hernia [15]. According to K.D. Wenstrom, there are several reasons why fetal tracheal occlusion cannot result in a better outcome than conventional therapy [16]. First of all, women with detected CDH in the fetus give birth in specialized centers, where newborns receive highly specialized treatment for respiratory diseases, including high-frequency oscillatory ventilation, exogenous surfactant, ECMO, etc. As a result, the current survival rate for all cases of isolated CDH ranging from mild to severe is close to 70% without surgery in the prenatal period, and the survival rate is at least 80% in newborns who do not require ECMO (approximately in 50% of infants with isolated CDH). Moreover, the stability of survival rate and decrease in morbidity after fetal endoscopic tracheal occlusion (FETO) is associated with premature rupture of membranes after intrauterine fetal occlusion. Nevertheless, both M.R. Harrison and K.D. Wenstrom insisted that FETO should be further introduced into general medical practice, including its use in multicenter randomized trials.
The study of R. Ruano proved that fetal endoscopic tracheal occlusion reduces neonatal mortality and accelerates the stabilization of the newborn [17]. It was based on the concept that CDH could be predicted by evaluating the size of the fetal lungs, the degree of liver penetration into the chest, and the degree of development of the lung vessels in the fetus in isolated forms of CDH. These parameters help to classify fetuses with isolated CDH of mild, moderate, severe, or very severe forms. Severe and extremely severe forms of CDH have adverse outcomes and therefore should be managed with innovative therapies such as FETO [14, 18]. Fetal endoscopic tracheal occlusion can usually be performed between 26- and 30-weeks’ gestation. After the procedure, monitoring which is conducted under ultrasound guidance every 2 weeks ensures the structural integrity of the balloon and measures the pulmonary response of the fetus. The balloon is emptied and removed at approximately 34 weeks gestation.
After performing the FETO technique, fetuses and newborns have the following main problems at the present stage:
- dysfunction of the surfactant system in the fetal lungs;
- heart failure;
- pulmonary hypoplasia;
- persistent pulmonary hypertension.
The traditional technique of intrauterine endotracheal balloon occlusion is used for intrauterine fetoscopic correction. The balloon is removed under ultrasound guidance by puncturing the balloon with an 18G spinal needle immediately prior to delivery.
More than 30 years ago, M.R. Harrison performed the first fetal surgical intervention [19], and the further improvement of surgical instruments and techniques allowed fetal surgery to become the only chance for the fetus to prevent the progression of CDH. Nowadays, fetoscopic correction of CDH is an invasive, but low traumatic operation that can be performed quickly [20].
The balloon was preferred as an agent providing endotracheal occlusion during intrauterine access. But the most important thing is to choose the material which is the safest for a human and is technically convenient for its development. These characteristics can typically be found in silicone, latex, and polyvinyl chloride, which are most commonly used in the manufacture of medical devices, including implants.
A latex balloon called goldballon is currently used for fetoscopic endotracheal occlusion.
The goldballon is used in the following way: first, the balloon catheter is inserted into the trachea and lowered to the level of the tracheal bifurcation. The balloon inflates and blocks the entrance to the bronchi. After that, the catheter is pulled off the balloon, it is removed and only the inflated balloon remains in the trachea. The accumulation of alveolar fluid in the lungs increases their volume and prevents the compression of the lungs by the abdominal organs; the newborn is subsequently stabilized more quickly.
Two months later, a fetoscopic procedure should be performed and the emptied balloon should be removed by repeated bronchoscopy or the balloon should be punctured with an 18G spinal needle under ultrasound guidance.
Therefore, this procedure is associated with the need for repeated fetoscopic intervention.
This is extremely inconvenient for a pregnant woman who has to return to the clinic for the second time, where a balloon was placed, and undesirable for the fetus that undergoes a repeated fetoscopic procedure with bronchoscopy. Repeated procedure increases the risk of premature birth and complicates the prognosis of nursing a preterm infant with CDH.
The aim of the study is to design a new model of the endotracheal catheter (FETO ballon is the name used in the Russian practice) for developing a new method of innovative fetal endoscopic tracheal occlusion, which will increase the survival rate of infants with CDH and severe lung hypoplasia.
According to this task, a new model of the endotracheal catheter (FETO ballon) was developed at the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology in 2019. This model provides physiological removal of the balloon from the trachea together with the catheter after delivery, reducing all possible risks for women and fetuses (Fig. 1, 2).
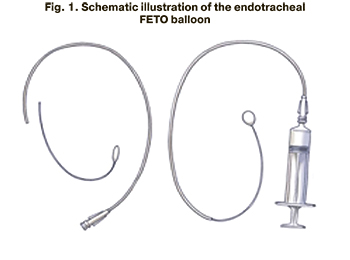
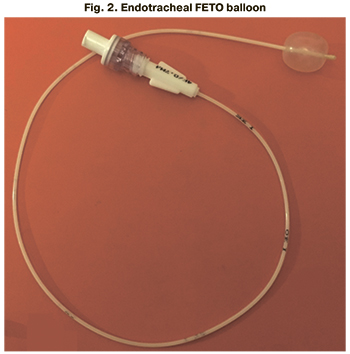
The new model of the endotracheal catheter (ETO ballon) is made of a flexible elastic material, namely polyether block amide PEBAX, and consists of two parts: a short distal section 10 cm long with an inflating balloon at the end and a proximal section 50 cm long with a special channel for administering saline solution into the cuff of the inflatable balloon. Currently, the endotracheal catheter is a prototype balloon that will be registered as a medical device for fetoscopic surgery.
The balloon can be placed at 24–29 weeks gestation, gestational age at delivery is 37–38 weeks, the balloon can be removed using the EXIT method. During the operation, local anesthesia (1% lidocaine 10–20 ml) is used to gain access to the uterine cavity. In order to provide anesthesia of the fetus, its immobilization and prevention of fetal bradycardia, rocuronium bromide (0.6 mg/kg) or pipecuronium bromide (0.4 mg/kg) and fentanyl (10–15 mcg/kg) are injected intramuscularly through a 20G or 22G needle under ultrasound guidance. Tocolysis is maintained with nifedipine 20 mg twice a day or with atosiban (administered using the following scheme: initial dose – 6.75 mg, three-hour infusion – 54 mg, long-term infusion (up to 45 hours) – 270 mg). Cefazoline 2 g intravenously every 8 hours for 24 hours is administered as antibiotic prophylaxis.
The technology of using the endotracheal FETO ballon is a sequence of actions:
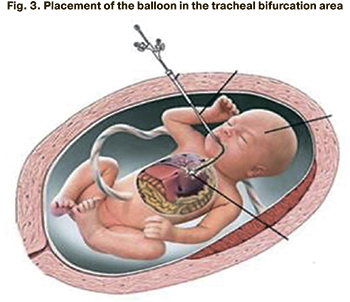
1) the distal end of the balloon is inserted through the oral cavity of the fetus into the trachea to the tracheal bifurcation area under the ultrasound guidance (Fig. 3);
2) the balloon cuff is inflated with a saline solution in the volume of 0.8 ml;
3) the long (proximal) part of the catheter is separated from the short (distal) part using a special conductor; the long part of the catheter is then removed. The inflated balloon with the short part of the catheter remains in the trachea and blocks the entrance to the bronchi. The accumulation of alveolar fluid in the lungs increases their volume and prevents compression of the lungs by the abdominal organs (Fig. 4);
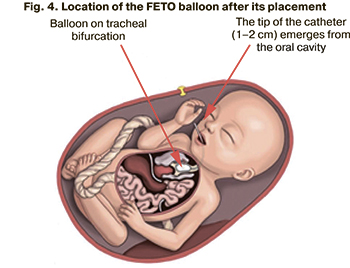
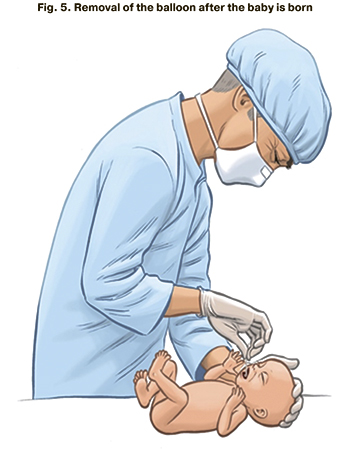
4) short part of the catheter emerges 1–2 cm from the oral cavity of the fetus; after delivery, the balloon is removed from the trachea using the short part of the catheter (Fig. 5).
Conclusion
The introduction of the first innovative Russian model of an endotracheal catheter for fetal endoscopic tracheal occlusion in severe congenital diaphragmatic hernia will be able to avoid repeated fetoscopic interventions aimed at antenatal balloon removal, thereby lowering the risk of intraoperative maternal and fetal complications.
References
- Байбарина Е.Н., Дегтярев Д.Н., Кучеров Ю.И., Жиркова Ю.В., Хаматханова Е.М., Подуровская Ю.Л., Дорофеева Е.И. Совершенствование системы оказания медицинской помощи новорожденным с хирургической патологией. Российский вестник перинатологии и педиатрии. 2011; 2: 12-9. [Baibarina E.N., Degtyarev D.N., Kucherov Yu.I., Zhirkova Yu.V., Khamatkhanova E.M., Podurovskaya Yu.L., Dorofeeva E.I. Improvement of the system of medical care for newborns with surgical pathology. Russian Bulletin of Perinatology and Pediatrics, 2011; 2: 12-9 (in Russian)].
- Satyan L., Payam V. Congenital diaphragmatic hernia: 25 years of shared knowledge; what about survival? J. Pediatr. (Rio J). 2020; 96(5): 527-32.
- Kirby E., Keijzer R. Congenital diaphragmatic hernia: current management strategies from antenatal diagnosis to long-term follow-up. Pediatr. Surg. Int. 2020; 36(4): 415-29. https://dx.doi.org/10.1007/s00383-020-04625-z.
- Kosiński P., Wielgoś M. Congenital diaphragmatic hernia: pathogenesis, prenatal diagnosis and management – literature review. Ginekol. Pol. 2017; 88(1): 24-30. https://dx.doi.org/10.563/GP.a2017.0005.
- Демидов В.Н., Машинец Н.В., Подуровская Ю.Л., Буров А.А. Врожденная диафрагмальная грыжа плода – возможности ультразвуковой диагностики и прогнозирование постнатального исхода. Акушерство и гинекология. 2014; 4: 38-45. [Demidov V.N., Mashinets N.V., Podurovskaya Yu.L., Burov A.A. Fetal congenital diaphragmatic hernia: ultrasound diagnosis possibilities and prediction of postnatal outcome. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2014; 4: 38-45. (in Russian)].
- Hollinger L.E., Harting M.T., Lally K.P. Long-term follow-up of congenital diaphragmatic hernia. Semin. Pediatr. Surg. 2017; 26(3): 178-84. https://dx.doi.org/10.1053/j.sempedsurg.2017.04.007.
- Yu L., Herman R.R., Wynn J., Chung W.K. The influence of genetics in congenital diaphragmatic hernia. Semin. Perinatol. 2020; 44(1): 151169. https://dx.doi.org/10.1053/j.semperi.2019.07.008.
- Piersigilli F., Syed M., Lam T.T., Dotta A., Massoud M., Vernocchi P. et al. An omic approach to congenital diaphragmatic hernia: a pilot study of genomic, microRNA, and metabolomic profiling. J. Perinatol. 2020; 40(6): 952-961. https://dx.doi.org/10.1038/s41372-020-0623-3.
- Юдина Е.В. Легкие. В кн.: Медведев М.В., ред. Пренатальная эхография. М.: Реальное Время; 2005: 341-71. [Yudina E.V. Lungs. In: Medvedev M.V., ed. Prenatal echography. M.: Real Time; 2005: 341-71. (in Russian)].
- Metkus A.P., Filly R.A., Stringer M.D., Harrison M.R., Adzick N.S. Sonographic predictors of survival in fetal diaphragmatic hernia. J. Pediatr. Surg. 1996; 31(1): 148-51. discussion 151-2. https://dx.doi.org/10.1016/s0022-3468(96)90338-3.
- Terui K., Nagata K., Hayakawa M., Okuyama H., Amari S., Yokoi A. et al. Novel risk score for fetuses with congenital diaphragmatic hernia based on ultrasound findings. Eur. J. Pediatr. Surg. 2020; 30(1): 51-8. https://dx.doi.org/10.1055/s-0039-1698768.
- Basurto D., Russo F.M., Van der Veeken L., Merwe J.V., Hooper S., Benachi A. et al. Prenatal diagnosis and management of congenital diaphragmatic hernia. Best Pract. Res. Clin. Obstet. Gynaecol. 2019; 58: 93-106. https://dx.doi.org/10.1016/j.bpobgyn.2018.12.010.
- Tanacan A., Orgul G., Aydin E., Kayki G., Celik H.T., Yalcin S. et al. Antenatal management and outcomes of pregnancies with congenital diaphragmatic hernia. J Neonatal Perinatal Med. 2020; 13(3): 323-30. https://dx.doi.org/10.3233/NPM-190266.
- Raffensperger J.G. Congenital diaphragmatic hernia: lessons learned and lost. J. Pediatr. Surg. 2018; 53(8): 1627-31. https://dx.doi.org/10.1016/j.jpedsurg.2018.04.033.
- Sydorak R.M., Harrison M.R. Congenital diaphragmatic hernia: advances in prenatal therapy. Clin. Perinatol. 2003; 30(3): 465-79. https://dx.doi.org/10.1016/s00955108(03)00057-5.
- Wenstrom K.D. Fetal surgery for congenital diaphragmatic hernia. N. Engl. J. Med. 2003; 349(20): 1887-8. https://dx.doi.org/10.1056/NEJMp038151.
- Ruano R., Ali R.A., Patel P., Cass D., Olutoye O., Belfort M.A. Fetal endoscopic tracheal occlusion for congenital diaphragmatic hernia: indications, outcomes, and future directions. Obstet. Gynecol. Surv. 2014; 69(3): 147-58. https://dx.doi.org/10.1097/OGX.0000000000000045.
- Kardon G., Ackerman K.G., McCulley D.J., Shen Y., Wynn J., Shang L. et al. Congenital diaphragmatic hernias: from genes to mechanisms to therapies. Dis. Model Mech. 2017; 10(8): 955-70. https://dx.doi.org/10.1242/dmm.028365.
- Harrison M.R., Golbus M.S., Filly R.A., Callen P.W., Katz M., de Lorimier A.A. et al. Fetal surgery for congenital hydronephrosis. N. Engl. J. Med. 1982; 306(10): 591-3. https://dx.doi.org/10.1056/NEJM198203113061006.
- Tsao K., Johnson A. Fetal tracheal occlusion for congenital diaphragmatic hernia. Semin. Perinatol. 2020; 44(1): 151164. https://dx.doi.org/10.1053/j.semperi.2019.07.003.
Received 15.01.2021
Accepted 01.04.2021
About the Authors
Mikhail G. Schneiderman, PhD, obstetrician-gynecologist, Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology, and Perinatology,Ministry of Health of Russia. Tel.: +7(926)245-05-51. E-mail: innamike@lmi.net. 117997, Russia, Moscow, Ac. Oparina str., 4.
Artem A. Burov, PhD, Head of Clinical Affairs, Department of Newborn Surgery, Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology, and Perinatology, Ministry of Health of Russia. Tel.: +7(910)471-69-34. E-mail: burovmd@gmail.com. 117997, Russia, Moscow, Ac. Oparina str., 4.
Yury I. Naberezhnev, PhD, Head of the Department of Organization of Perinatal Care, Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology, and Perinatology, Ministry of Health of Russia. Tel.: +7(910)323-12-47. E-mail: rubick@yandex.ru. 117997, Russia, Moscow, Ac. Oparina str., 4.
Yulia L. Podurovskaya, PhD, Head of Department of Newborn Surgery, Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology, and Perinatology, Ministry of Health of Russia. Tel.: +7(916)107-13-88. 117997, Russia, Moscow, Ac. Oparina str., 4.
Nana K. Тetruashvili, Dr. Med. Sci., Deputy Director, Institute of Obstetrics; Head of the 2nd Obstetrics Department of Pathology of Pregnancy, Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology, and Perinatology, Ministry of Health of Russia. Tel.: +7(495)438-14-77. E-mail: tetrauly@mail.ru.
117997, Russia, Moscow, Ac. Oparina str., 4.
Roman G. Shmakov, Dr. Med. Sci., Professor of RAS, Director of the Institute of Obstetrics, Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology, and Perinatology, Ministry of Health of Russia. Tel.: +7(916)171-06-76. E-mail: mdshmakov@mail.ru. 117997, Russia, Moscow, Ac. Oparina str., 4.
Gennady T. Sukhikh, Dr. Med. Sci., Professor, Academician of RAS, Director of Academician V.I. Kulakov Research Center of Obstetrics, Gynecology, and Perinatology Ministry of Health of Russia. Tel.: +7(495)438-18-00. E-mail: g_sukhikh@oparina4.ru. 117997, Russia, Moscow, Ac. Oparina str., 4.
For citation: Schneiderman M.G., Burov A.A., Naberezhnev Yu.I., Podurovskaya Yu.L., Tetruashvili N.K., Shmakov R.G., Sukhikh G.T. An innovative model of endotracheal catheter (FETO-balloon) for fetal endoscopic tracheal occlusion in severe congenital diaphragmatic hernia.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 5: 174-179 (in Russian)
https://dx.doi.org/10.18565/aig.2021.5.174-179



