Prevalence of chromosomal abnormalities in fetal heart defects, congenital diaphragmatic hernia and non-immune hydrops fetalis based on molecular karyotyping data (experience of the National Center)
Pak V.S., Lyushnina D.G., Naberezhnev Yu.I., Bokerija E.L., Zaretskaya N.V., Bolshakova A.S., Barkov I.Yu., Tetruashvili N.K., Trofimov D.Yu.
The prevalence of congenital fetal malformations is about 3–4%. According to the World Health Organization, about 240,000 children with congenital malformations die each year before 28 days of life, and 170,000 die before the age of 5 years. The management of patients with malformations represents a financial and human resource challenge to the healthcare system, and a significant proportion of these patients require palliative care. Therefore, the search for the causes of congenital malformations at the antenatal stage has been a priority.
Objective: To evaluate the role of chromosomal abnormalities in the development of fetal heart defects, congenital diaphragmatic hernia, and non-immune fetal hydrops.
Materials and methods: The prospective study was conducted between 2018 and 2024 and included 155 pregnant women: 61 with fetal cardiac malformation, 57 with fetal congenital diaphragmatic hernia, and 37 with non-immune fetal hydrops. The study was carried out at the National Medical Research Centre for Obstetrics, Gynecology and Perinatology in Moscow. All patients underwent invasive prenatal diagnostic procedures at different gestational ages. Fetal DNA was examined using SNP oligonucleotide chromosome microarray analysis on CytoScan Optima microarrays (Thermo Fisher Scientific, USA).
Results: The analysis of clinical and anamnestic data of the patients from the three groups did not reveal any statistically significant differences. The analysis of pregnancy complications and outcomes showed that pregnancy in the group of patients with fetal congenital diaphragmatic hernia was more often complicated by threatened preterm labor and anemia, p<0.001 and p=0.002, respectively, while patients with non-immune fetal hydrops were significantly more likely to experience antenatal fetal death, p<0.001. The study revealed that the prevalence of chromosomal abnormalities in fetuses of pregnant women of the study groups was 19.4% (30/155), including 11.6% (18/155) of cases with pathogenic copy number variations.
Conclusion: Conventional karyotyping would classify these patients as fetuses without chromosomal abnormalities. Given these findings, chromosomal microarray analysis is recommended as a first-line test in the genetic study of fetuses with congenital malformations and non-immune fetal hydrops. Since a number of fetal malformations tend to be detected late, it is recommended to perform invasive prenatal diagnostic procedures at more than 22 weeks gestation in order to provide complete perinatal counseling to the couple and to enable the family to make a reproductive choice.
Authors’ contributions: Tetruashvili N.K., Bokerija E.L., Trofimov D.Yu. – developing the concept and design of the study;
Pak V.S., Lyushnina D.G., Naberezhnev Yu.I., Zaretskaya N.V., Bolshakova A.S., Barkov I.Yu. – collecting and processing
of the material; Pak V.S., Lyushnina D.G., Naberezhnev Yu.I., Tetruashvili N.K. – writing the text; Pak V.S., Lyushnina D.G. – statistical processing of the data; Tetruashvili N.K., Bokerija E.L., Bolshakova A.S., Barkov I.Yu., Trofimov D.Yu. – editing the article.
Conflicts of interest: The authors declare that there are no conflicts of interest.
Funding: The study was carried out without sponsorship.
Ethical Approval: The study was approved by the Ethical Review Board of the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia.
Patient Consent for Publication: The patients signed informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Pak V.S., Lyushnina D.G., Naberezhnev Yu.I., Bokerija E.L., Zaretskaya N.V., Bolshakova A.S., Barkov I.Yu.,
Tetruashvili N.K., Trofimov D.Yu. Prevalence of chromosomal abnormalities in fetal heart defects, congenital diaphragmatic hernia and non-immune hydrops fetalis based on molecular karyotyping data
(experience of the National Center).
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2024; (12): 33-41 (in Russian)
https://dx.doi.org/10.18565/aig.2024.281
Keywords
The prevalence of fetal congenital malformations is estimated at approximately 3–4% [1, 2]. According to the World Health Organisation, about 240,000 children with congenital malformations die each year under 28 days of life and 170,000 under 5 years of age. Congenital malformations accounted for the first place (about 30%) among all causes of child mortality under 5 years of age in 2019 [3].
Malformations are typically divided into two categories: isolated malformations, which are defined as single organ abnormalities, and multiple malformations, which are characterized by the presence of multiple organ abnormalities. Depending on their location, malformations can be of the skull and brain, face and neck, spinal cord, central nervous system and sensory organs, cardiovascular system, respiratory system, digestive system, musculoskeletal system, urinary system, genital organs, endocrine glands, skin and its appendages [4, 5].
A number of authors propose a classification system based on the timing of the malformation: gametopathies refer to changes that occur before fertilization in germ cells, blastopathies denote pathological changes in the zygote during the first week of development, embryopathies refer to pathological changes occurring from the first to the twelfth week of embryonic development, and fetopathies denote lesions occurring from the twelfth week of embryonic development to birth [6].
According to the European Surveillance of Congenital Anomalies (EUROCAT), the most common malformations are heart and vascular malformations (81.1 per 10,000 cases per year), extremity anomalies (38.11 per 10,000), kidney and urinary tract anomalies (34.83 per 10,000), and genital anomalies (21.74 per 10,000), gastrointestinal anomalies (18.4 per 10,000), etc. [7].
Several authors note that the development of fetal congenital malformations is influenced by such factors as maternal diabetes mellitus [8], late reproductive age, maternal obesity, alcoholism [9], smoking during pregnancy [10, 11], deficiency of trace elements [12, 13], taking medications [14], etc. However, according to foreign authors, congenital malformations have a genetic cause of development, including chromosomal and monogenic pathology, in 26% [15] to 55% [16, 17] of cases.
The management of patients with malformations can be expensive and demanding for the health care system, and a large proportion of these patients will be treated palliatively. Therefore, the search for the causes of congenital malformations at the antenatal stage is a priority task for sonographers, obstetrician-gynecologists, geneticists, neonatologists, and pediatric surgeons. Counselling of these families at the antenatal stage is multidisciplinary in nature and is carried out as part of the perinatal consultation. Genetic testing during pregnancy can improve counselling options and provide parents with the best possible information about the prognosis for the fetus and the baby’s condition after birth.
Invasive prenatal diagnosis can be performed from 11 weeks using chorion biopsy and from 15 weeks using amniocentesis [18, 19]. Late amniocentesis for diagnostic purposes is possible if congenital malformations are detected at more than 24 weeks’ gestation [20]; repeat amniocentesis is recommended when the results of chorion biopsy are unclear [21].
According to the Russian [22] and foreign [23] researchers, the introduction of molecular karyotyping and chromosomal microarray analysis (CMA) methods into clinical practice has made it possible to expand the possibilities of prenatal diagnosis. This is due to the high sensitivity of these methods for detecting pathogenic copy number variations, in contrast to standard cytogenetic tests such as conventional karyotyping or fluorescence in situ hybridization (FISH).
Taking into account the above data, the search for the causes of congenital malformations of the fetus can help to make a timely diagnosis, provide the couple with complete information about the prognosis of life and development of children, about possible complications, and give the family the opportunity to make reproductive decisions.
The aim of the study was to evaluate the role of the genetic factor in the development of fetal heart defects, congenital diaphragmatic hernia, and non-immune fetal hydrops.
Materials and methods
The prospective study was conducted between 2018 and 2024 at the Academician V.I. Kulakov National Medical Research Centre for Obstetrics, Gynecology and Perinatology in Moscow. The study included 155 pregnant women with fetal cardiac malformation, fetal congenital diaphragmatic hernia, and non-immune fetal hydrops.
The presence of heart defect, congenital diaphragmatic hernia and non-immune fetal hydrops was confirmed by sonographers of the Centre at 11 to 30 weeks’ gestation using Voluson E8/E10 Expert equipment (GE Healthcare, USA).
The couples received perinatal consultations from obstetrician-gynecologists, neonatologists, pediatric surgeons, cardiac surgeons, geneticists and psychologists. The patients were provided with complete information on the type of malformation, possibility/impossibility of its correction, prognosis of the child’s life and development. Invasive prenatal diagnosis was also suggested to provide additional information about the presence of fetal chromosomal abnormalities.
Group I included 61 pregnant women with complicated fetal heart defect requiring repeated cardiac surgical interventions after birth. The following heart defects were included in this group: tetralogy of Fallot (17/61), coarctation of the aorta (14/61), transposition of the main arteries (12/61), stenosis or atresia of the pulmonary valve (9/61), hypoplastic left heart syndrome (1/61), double outlet right ventricle (3/61), common arterial trunk (3/61), atrioventricular canal (1/61).
Group II included 57 pregnant women with fetal congenital diaphragmatic hernia. Right-sided diaphragmatic hernia was observed in 14% of fetuses (8/57) and left-sided in 86% (49/57).
Group III included 37 pregnant women with non-immune fetal hydrops, with varying severity of manifestations (hydrothorax, hydropericardium, ascites, anasarca).
Prior to invasive prenatal diagnosis, the patients were examined for contraindications to the intervention. The patients signed an informed consent where the risk of complications, namely miscarriage/preterm labor, rupture of membranes, placental abruption, intrauterine fetal death in less than 1% of cases, was indicated.
The procedure was as follows: 40 ml of amniotic fluid was obtained by transabdominal amniocentesis; then the material was sent to the Laboratory of Molecular Genetic Methods of the Institute of Reproductive Genetics at the Academician V.I. Kulakov National Medical Research Centre for Obstetrics, Gynecology and Perinatology.
Fetal DNA was examined using SNP-oligonucleotide CMA. The study was performed using the GenoScan3000 system on CytoScan Optima microarrays (Thermo Fisher Scientific, USA) according to the manufacturer’s protocol. The thresholds for pathogenic microdeletions were set at a size greater than 1000 kpb, and for microduplications, at a size greater than 2000 kpb. The data were analyzed using the ChAS software (Chromosome Analysis Suite). The generated copy number variation (CNV) was interpreted according to the standards and recommendations of the American Society for Medical Genetics (ACMG) in five categories. The following aspects were evaluated for interpretation: size, location of gene copy number variation, gene composition, description in databases (Clinvar, OMIM, ORPHANET, DECIPHER, DGV), and in the literature. Pathogenic and possibly pathogenic gene copy number variations were reported.
Statistical analysis
Statistical processing of the data was performed using Stattech v. 4.6.1 (Russia) and Statistica for Windows v.10.0 (USA). Categorical data were described with absolute values and percentages. Quantitative variables that had normal distribution were described using arithmetic mean (M) and standard deviations (SD). In the absence of normal distribution, quantitative data were described using median (Me) and lower and upper quartiles (Q1; Q3). Comparison of frequencies in the analysis of multidimensional contingency tables was performed using the Pearson’s chi-square test. The analysis employed a range of statistical methods, including ANOVAs, Kruskal–Wallis test, chi-square test, two-sided Fisher’s exact test, and Z-criterion methods. It is worth noting that end-point correction was applied at 0%. Differences among groups were considered statistically significant using the Bonferroni correction factor for multiple comparisons for the three groups at p<0.017.
Results and discussion
The clinical and anamnestic data of the patients of the study groups were analyzed.
The mean age of pregnant women in the group with fetal heart defect was 31.43 (5.16) years, 21/61 (34.4%) women were older than 35 years. The mean age of patients in the group with fetal congenital diaphragmatic hernia was 31.42 (5.66) years, 20/57 (35%) women were older than 35 years. The mean age of pregnant women with non-immune fetal hydrops was 29.1 (5.42) years and 7/37 (18.9%) women were older than 35 years. There were no statistically significant differences in age in the comparison of three groups (ANOVA, p=0.119), but it is worth noting that the age of the pregnant women was less than 35 years in 30/37 (81.1%) of the pregnancies with non-immune fetal hydrops.
The mean gestational age (median) at detection of fetal heart defect was 21 (3.03) weeks (21 (20; 22)), congenital diaphragmatic hernia was 21 (2.03) weeks (20 (16; 24)), and non-immune fetal hydrops was 20 (5.84) weeks (21 (20; 23)) (Figure).
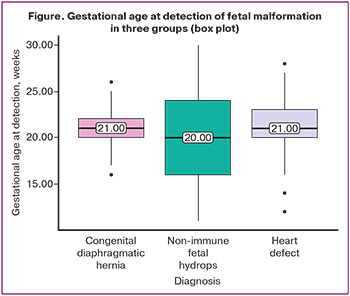
Early diagnosis (before 22 weeks) was made in 90/155 (58%) cases: fetal heart defect was revealed in 37/61 (60.7%) cases, fetal congenital diaphragmatic hernia in 32/57 (57.9%) cases, non-immune fetal hydrops in 21/37 (62.2%) cases respectively.
In the group of pregnant women with fetal heart defect, the share of primiparous women was 25/61 (41%), in the group of pregnant women with fetal congenital diaphragmatic hernia it was 28/57 (49.1%), and in the group of pregnant women with non-immune fetal hydrops it was 12/37 (35.1%) (p=0.362, chi-square test).
It was found that overweight was observed in 17/61 (27.9%) patients in the group of pregnant women with fetal heart defect, in 29/57 (50.9%) patients in the group of pregnant women with fetal congenital diaphragmatic hernia, and in 13/37 (35.1%) patients in the group of pregnant women with non-immune fetal hydrops (p=0.0374, chi-square test).
The analysis of clinical and anamnestic data of the patients in the study groups revealed no statistically significant differences in such parameters as age, body mass index, parity, gestational age at the detection of malformation.
The clinical and anamnestic data of pregnant women with fetal malformations are presented in Table 1.
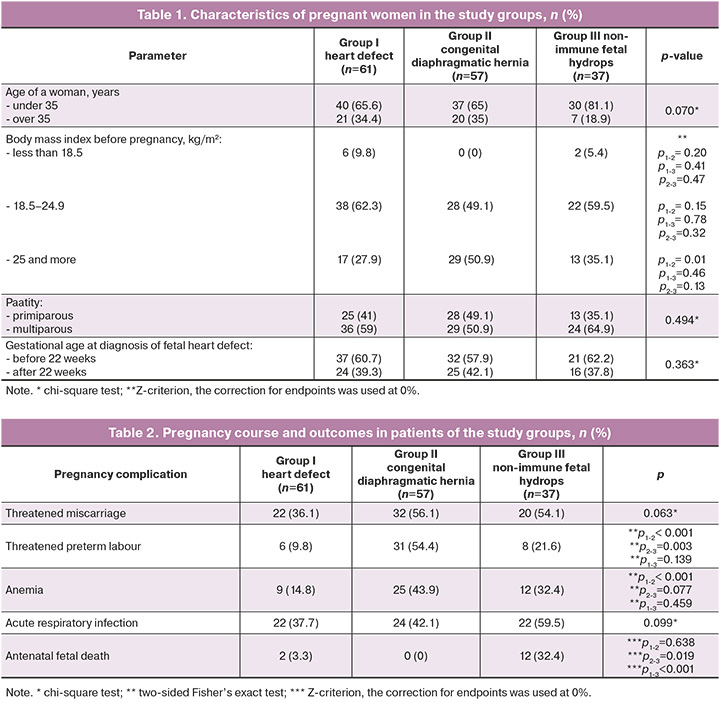
The analysis of pregnancy complications and outcomes in all three groups showed that pregnancy in the group of patients with fetal congenital diaphragmatic hernia was more often complicated by threatened preterm labor and anemia, p<0.001 and p=0.002 respectively, and patients with non-immune fetal hydrops were statistically significantly more likely to experience antenatal fetal death, p<0.001 (Table 2).
The analysis of CMA results in group I found that 17/61 (27.9%) cases of heart defects were associated with chromosomal abnormalities. Chromosomal pathology was represented by aneuploidies in 5/61 (8.2%) cases and pathogenic copy number variations in 9/61 (14.8%) cases (Table 3). The Chinese researchers showed that the diagnostic value of CMA is higher than that of standard karyotyping in the analysis of the chromosome set of fetuses with heart defects: 15.3% and 8.3%, respectively [24, 25]. The authors also note that it is necessary to perform antenatal examination in complicated and combined cases of fetal heart defects.
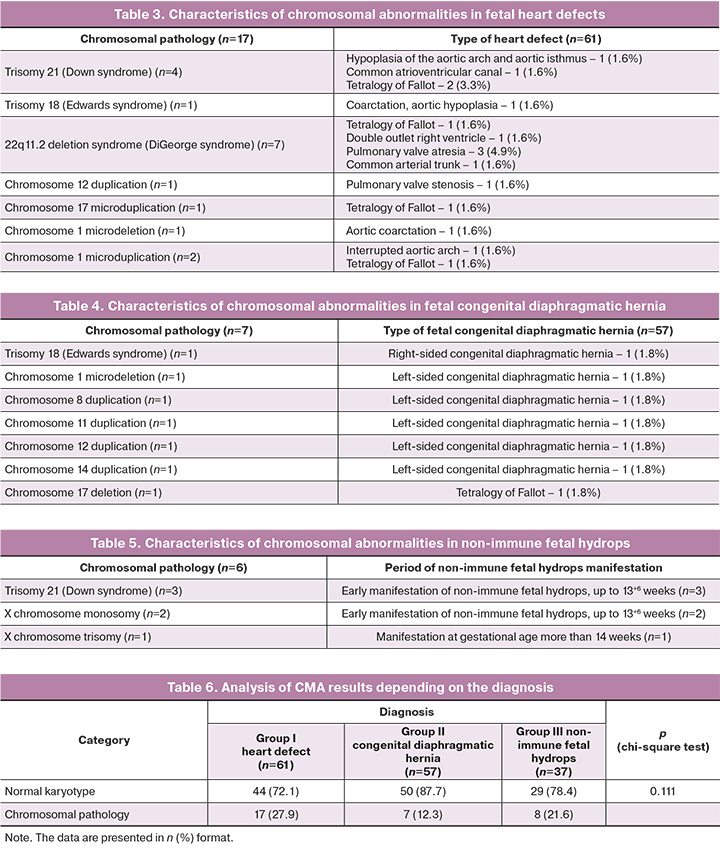
The chromosomal analysis of the group of pregnant women with fetal congenital diaphragmatic hernia revealed that fetal chromosomal abnormality was detected in 7/57 (12.3%) cases, aneuploidy, trisomy 18 was detected in 1/57 (1.8%) case, and pathogenic CNVs were found in 6/57 (10.5%) cases (Table 4).
In the group of pregnant women with non-immune fetal hydrops, 6/37 (16.2%) cases showed fetal chromosomal abnormalities, which in all cases were represented by aneuploidies (Table 5).
The comparison of the frequency of chromosomal pathology in three study groups did not reveal any statistically significant differences (Table 6).
Thus, it was determined chromosomal abnormalities were detected in 30/155 (19.4%) cases in pregnant women with fetal malformations, the proportion of aneuploidies (50%) and pathogenic CNVs (50%) were equal.
After the results of genetic examination, the couples were invited to a repeated perinatal consultation with many specialists, where the patients were given full information about the type of chromosomal abnormality detected after invasive prenatal diagnosis. It is evident that the presence of chromosomal pathology in fetuses with congenital malformations has a significant impact on the prognosis of survival at the antenatal stage and after birth; the prognosis for intellectual development is unfavorable, the frequency of successful conservative and surgical treatment is reduced, and the frequency of complications during therapy is increased. Taking into account all the obtained data, families had the right to make reproductive decisions. The patients with fetal heart defect in 13/61 (21.3%) cases terminated the pregnancy due to the presence of fetal chromosomal abnormality. Despite the presence of chromosomal abnormality, couples decided to prolong the pregnancy in 4/61 (6.6%) cases. The patients with fetal congenital diaphragmatic hernia had terminated pregnancy in 10/57 (17.5%) cases: termination of pregnancy in 7/57 (12.3%) cases was associated with fetal chromosomal abnormality, and in 3/57 (5.3%) cases it was connected with the severity of the malformation. The patients with non-immune fetal hydrops had medically indicated termination of pregnancy in 13/37 (35.1%) cases: termination in 8/37 cases (21.6%) was associated with chromosomal abnormality, and in 5/37(13.5%) cases it was connected with the severity of fetal hydrops. No statistically significant differences were found in the frequency of medically indicated pregnancy terminations (Table 7).
Given the late detection of fetal malformations in 65/155 (41.9%) patients in the present study, it is essential to perform a full range of diagnostic procedures, including invasive prenatal diagnosis. The current study revealed that late invasive diagnosis (amniocentesis) does not result in obstetric complications. The researchers Zemet R. et al. confirm that there is no increase in the risk of obstetric complications when amniocentesis is performed after 24 weeks gestation and they also state the high diagnostic value of this type of invasive diagnosis for fetal malformations [26].
The outcome of this study is to determine the prognosis for the offspring in case of a fetal malformation, providing complete clear information about the type of malformation, presence and type of associated chromosomal abnormality in the fetus. The obtained data can help the couple to make a sensible and rational decision.
The optimal approach to perform chromosomal analysis is molecular karyotyping, CMA. In the present study, the diagnostic value of the method increased by 11.6% in the identification of fetal malformations (pathogenic copy number variations were detected in 18/155 cases). The obtained data demonstrate a correlation with the data of foreign researchers, whose studies showed that the CMA method increases the diagnostic value of karyotyping by 12% on average [27, 28]. CMA does not reveal the monogenic cause of fetal malformations, but it should be used as a first-line test in genetic studies.
Conclusion
The study showed that the incidence of chromosomal abnormalities in the fetuses of pregnant women in the study groups was 19.4% (30/155), including 11.6% (18/155) cases of pathogenic copy number variations. Conventional karyotyping would classify these patients as fetuses without chromosomal abnormalities. Given these findings, CMA is recommended as a first-line test in the genetic study of fetuses with congenital malformations and non-immune fetal hydrops.
Since a number of fetal malformations tend to be detected late, it is recommended to perform invasive prenatal diagnostic procedures at more than 22 weeks gestation in order to provide complete perinatal counseling to the couple and to enable the family to make a reproductive choice.
References
- Логинова Е.В., Гагаев Ч.Г., Зулумян Т.Н., Костин И.Н., Хамошина М.Б., Лебедева М.Г. Прогнозирование врожденных пороков развития плода при сахарном диабете. Акушерство и гинекология: новости, мнения, обучение. 2023; 11(3): 24-9. [Loginova E.V., Gagaev Ch.G., Zulumyan T.N., Kostin I.N., Khamoshina M.B., Lebedeva M.G. Prediction of congenital malformations associated with diabetes mellitus. Obstetrics and Gynecology: News, Opinions, Training. 2023; 11(3): 24-9. (in Russian)]. https://dx.doi.org/10.33029/2303-9698-2023-11-3-24-29.
- Чебан О.С., Ячикова Н.Н. Юридические аспекты прерывания беременности в сроке более 22 недель при наличии аномалий развития плода. Общественное здоровье, экономика и менеджмент в медицине. 2022; 93(2): 63-93. [Cheban O.S., Yachikova N.N. Aspects of termination of pregnancy at more than 22 weeks in the presence of anomalies of fetus development. Public Health, Economy and Management in Medicine. 2022; 93(2): 63-93.(in Russian)]. https://dx.doi.org/10.52556/2587-3873.2022.2(93).10.
- Всемирная организация здравоохранения. Врожденные заболевания. Доступно по: https://www.who.int/ru/news-room/fact-sheets/detail/birth-defects. [WHO. Congenital disorders. Available at: https://www.who.int/ru/news-room/fact-sheets/detail/birth-defects]
- Куандыков Е.У., Альмухамбетова С.К., Жумагул М.Ж., Молдакарызова А.Ж. Врожденные пороки развития: классификация, причины, механизмы возникновения. Вестник КазНМУ. 2018; 1: 469-73. [Kuandykov E.U., Almuhambetova S.K., Zhumagul M.Zh., Moldakaryzova A.Zh. Congenital development defects: classification, reasons, mechanisms risk. Vestnik KazNMU. 2018; (1): 469-73. (in Russian)].
- Lee K.S., Choi Y.J., Cho J., Lee H., Lee H., Park S.J. et al. Environmental and genetic risk factors of congenital anomalies: an umbrella review of systematic reviews and meta-analyses. J. Korean Med. Sci. 2021; 36(28): e183. https://dx.doi.org/10.3346/jkms.2021.36.e183.
- Шабалов Н.П. Неонатология. т. 1. 7-е изд. 2019. 57 с. [Shabalov N.P. Neonatology. vol. 1. 7th ed. 2019. 57 p. (in Russian)].
- European Comission. Prevalence charts and tables. Available at: https://eu-rd-platform.jrc.ec.europa.eu/eurocat/eurocat-data/prevalence_en
- Капустин Р.В., Коптеева Е.В., Алексеенкова Е.Н., Ковальчук-Ковалевская О.В., Рыбачек А.В., Аржанова О.Н., Коган И.Ю. Неонатальные исходы при сахарном диабете у матери: анализ данных исследования DAPSY. Журнал акушерства и женских болезней. 2024; 73(2): 15-26. [Kapustin R.V., Kopteeva E.V., Alekseenkova E.N., Kovalchuk-Kovalevskaya O.V., Rybachek A.V., Arzhanova O.N., Kogan I.Yu. Neonatal outcomes in maternal diabetes: DAPSY analysis. Journal of Obstetrics and Women's Diseases. 2024; 73(2): 15-26. (in Russian)]. https://dx.10.17816/JOWD624553.
- Kurita H., Motoki N., Inaba Y., Misawa Y., Ohira S., Kanai M. et al. Maternal alcohol consumption and risk of offspring with congenital malformation: the Japan environment and children’s study. Pediatr. Res. 2021; 90(2): 479-86. https://dx.doi.org/10.1038/s41390-020-01274-9.
- Finn J., Suhl J., Kancherla V., Conway K.M., Oleson J., Sidhu A. et al. Maternal cigarette smoking and alcohol consumption and congenital diaphragmatic hernia. Birth Defects Res. 2022; 114(13): 746-58. https://dx.doi.org/10.1002/bdr2.2059.
- Zhang Q., Zhang Z.C., He X.Y., Liu Z.M., Wei G.H., Liu X. Maternal smoking during pregnancy and the risk of congenital urogenital malformations: A systematic review and meta-analysis. Front. Pediatr. 2022; 10: 973016. https://dx.doi.org/10.3389/fped.2022.973016.
- Wilson R.D., O’Connor D.L. Folic acid and multivitamin supplementation for prevention of folic acid-sensitive congenital anomalies. J. Obstet. Gynaecol. Can. 2022; 44(6): 707-19. https://dx.doi.org/10.1016/j.jogc.2022.04.004.
- Mires S., Caputo M., Overton T., Skerritt C. Maternal micronutrient deficiency and congenital heart disease risk: A systematic review of observational studies. Birth Defects Res. 2022; 114(17): 1079-91. https://dx.doi.org/10.1002/bdr2.2072.
- Vajda F.J.E., O’Brien T.J., Graham J.E., Hitchcock A.A., Lander C.M., Eadie M.J. The contribution of non-drug factors to fetal malformation in anti-seizure-medication-treated pregnancy. Epilepsy Behav. 2021; 118: 107941. https://dx.doi.org/10.1016/j.yebeh.2021.107941.
- Westenius E., Conner P., Pettersson M., Sahlin E., Papadogiannakis N., Lindstrand A. et al. Whole-genome sequencing in prenatally detected congenital malformations: prospective cohort study in clinical setting. Ultrasound Obstet. Gynecol. 2024; 63(5): 658-63. https://dx.doi.org/10.1002/uog.27592.
- Al-Hamed M.H., Kurdi W., Khan R., Tulbah M., AlNemer M., AlSahan N. et al. Prenatal exome sequencing and chromosomal microarray analysis in fetal structural anomalies in a highly consanguineous population reveals a propensity of ciliopathy genes causing multisystem phenotypes. Hum. Genet. 2022; 141(1): 101-26. https://dx.doi.org/10.1007/s00439-021-02406-9.
- Qin Y., Yao Y., Liu N., Wang B., Liu L., Li H. et al. Prenatal whole-exome sequencing for fetal structural anomalies: a retrospective analysis of 145 Chinese cases. BMC Med. Genomics. 2023; 16(1): 262. https://dx.doi.org/10.1186/s12920-023-01697-3.
- Maděrková Tozzi M., Dvořák Jr. V., Klásková E., Šuláková S., Wita M., Hálek J. et al. Screening for congenital defects and genetic diseases of the fetus at University Hospital in Olomouc and sending/ reporting to the National register of reproductive health in the Czech Republic. Ceska Gynekol. 2022; 87(3): 162-72. https://dx.doi.org/10.48095/cccg2022162.
- Qi Q.G., Tuo Y., Liu L.X., Yu C.X., Wu A.N. Amniocentesis and Next Generation Sequencing (NGS)-based Noninvasive Prenatal DNA Testing (NIPT) for prenatal diagnosis of fetal chromosomal disorders. Int. J. Gen. Med. 2021; 14: 1811-7. https://dx.doi.org/10.2147/IJGM.S297585.
- Sharma A., Kaul A. Late amniocentesis: better late than never? A single referral centre experience. Arch. Gynecol. Obstet. 2023; 308(2): 463-70. https://dx.doi.org/10.1007/s00404-022-06662-6.
- Cagino K., Chasen S.T. Is amniocentesis after CVS risky? Am. J. Perinatol. 2024; 41(7): 876-8. https://dx.doi.org/10.1055/a-1787-6785.
- Киевская Ю.К., Шилова Н.В., Канивец И.В., Кудрявцева Е.В., Пьянков Д.В., Коростелев С.А. Метод SNP-однонуклеотидного хромосомного микроматричного анализа в изучении вариаций числа копий ДНК у плодов с расширенной воротниковой зоной. Современные технологии в медицине. 2021; 13(6): 72-7. [Kievskaya J.K., Shilova N.V., Kanivets I.V., Kudryavtseva E.V., Pyankov D.V., Korostelev S.A. Method of SNP-based chromosomal microarray analysis for detecting DNA copy number variations in fetuses with a thickened nuchal fold. Sovremennye Tehnologii v Medicine. 2021; 13(6): 72-7. (in Russian)]. https://dx.doi.org/10.17691/stm2021.13.6.08.
- Moczulska H., Chrzanowska-Steglinska M., Skoczylas B., Wojda K., Borowiec M., Sieroszewski P. Prenatal karyotype results from 2169 invasive tests. Ginekol. Pol. 2023. https://dx.doi.org/10.5603/GP.a2022.0143.
- Lu Q., Luo L., Zeng B., Luo H., Wang X., Qiu L. et al. Prenatal chromosomal microarray analysis in a large Chinese cohort of fetuses with congenital heart defects: a single center study. Orphanet. J. Rare Dis. 2024; 19(1): 307. https://dx.doi.org/10.1186/s13023-024-03317-4.
- Li M., Ye B., Chen Y., Gao L., Wu Y., Cheng W. Analysis of genetic testing in fetuses with congenital heart disease of single atria and/or single ventricle in a Chinese prenatal cohort. BMC Pediatr. 2023; 23(1): 577. https://dx.doi.org/10.1186/s12887-023-04382-7.
- Zemet R., Maktabi M.A., Tinfow A., Giordano J.L., Heisler T.M., Yan Q. et al. Amniocentesis in pregnancies at or beyond 24 weeks: an international multicenter study. Am. J. Obstet. Gynecol. 2024: S0002-9378(24)00693-8. https://dx.doi.org/10.1016/j.ajog.2024.06.025.
- Xia M., Yang X., Fu J., Teng Z., Lv Y., Yu L. Application of chromosome microarray analysis in prenatal diagnosis. BMC Pregnancy Childbirth. 2020; 20(1): 696. https://dx.doi.org/10.1186/s12884-020-03368-y.
- Mitrakos A., Kosma K., Makrythanasis P., Tzetis M. Prenatal chromosomal microarray analysis: Does increased resolution equal increased yield? Genes (Basel). 2023; 14(8): 1519. https://dx.doi.org/10.3390/genes14081519.
Received 07.11.2024
Accepted 05.12.2024
About the Authors
Viktoriia S. Pak, PhD student, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 4 Acad. Oparin str., Moscow, Russia, 117997, +7(913)897-28-49, v_pak@oparina4.ru, https://orcid.org/0009-0002-1444-9071Daria G. Lyushnina, PhD student, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia,
4 Acad. Oparin str., Moscow, Russia, 117997, +7(906)308-60-78, d_lyushnina@oparina4.ru, https://orcid.org/0009-0004-3160-8737
Yu. I. Naberezhnev, PhD, Head of the Department of Perinatal Care, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 4 Acad. Oparin str., Moscow, Russia, 117997, +7(910)323-12-47, rubick@yandex.ru, https://orcid.org/0000-0003-2547-9735
Ekaterina L. Bokerija, PhD, Researcher at the Department of Patology for Newdorn and Prematurely-Born Children No. 2, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 4 Acad. Oparin str., Moscow, Russia, 117997, +7(495)438-27-05,
e_bokeriya@oparina4.ru, https://orcid.org/0000-0002-8898-9612
Nadezhda V. Zaretskaya, PhD, Head of the Laboratory of Clinical Genetics of the Institute of Reproductive Genetics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 4 Acad. Oparin str., Moscow, Russia, 117997, +7(495)438-24-11,
n_zaretskaya@oparina4.ru, https://orcid.org/0000-0001-6754-3833
Anna S. Bolshakova, MD, Geneticist, Department of Clinical Genetics of the Institute of Reproductive Genetics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 4 Acad. Oparin str., Moscow, Russia, 117997, +7(495)438-24-11, a_bolshakova@oparina4.ru, https://orcid.org/0000-0002-7508-0899
Ilya Yu. Barkov, PhD, Head of the Laboratory of Prenatal DNA Screening of the Institute of Reproductive Genetics Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 4 Acad. Oparin str., Moscow, Russia, 117997, +7(495)438-24-10, i_barkov@oparina4.ru, https://orcid.org/0000-0001-6297-2073
Nana K. Tetruashvili, PhD, Head of the Obstetric Department of Pregnancy Pathology No. 2, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 4 Acad. Oparin str., Moscow, Russia, +7(495)438-14-77, n_tetruashvili@oparina4.ru,
https://orcid.org/0000-0002-9201-2281
Dmitry Yu. Trofimov, PhD, Professor of the RAS, Corresponding Member of the RAS, Director of the Institute of Reproductive Genetics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 4 Acad. Oparin str., Moscow, Russia, 117997, +7(495)438-49-51,
d_trofimov @oparina4.ru, https://orcid.org/0000-0002-1569-8486



