Глобальный рост антибиотикорезистентности приводит к уменьшению доступного арсенала средств для терапии инфекционных заболеваний. Прежде всего снижается эффективность наиболее распространенных препаратов; скорость процесса коррелирует с уровнем потребления антибиотиков населением. По данным Robertson J. et al. (2021) [1], в 2018 г. общее потребление системных антибактериальных препаратов (ATC J01) варьировалось от 8,9 до 34,1 установленных суточных доз (defined daily doses, DDD) на 1000 человек в день, лидировала группа пенициллинов. В работе Klein E.Y. et al. (2020) осуществлен анализ глобального потребления антибактериальных препаратов в период 2000–2015 гг. с использованием классификации ВОЗ «AWaRe» (три группы антибактериальных препаратов: Access (доступные), Watch (поднадзорные), Reserve (резервные)). Результаты обнаружили максимальный рост потребления на душу населения антибиотиков группы Watch (на 90,9%; с 3,3 до 6,3 DDD на 1000 человек в день) в сравнении с ростом на 26,2% в группе Access (с 8,4 до 10,6 DDD на 1000 человек в день); средний мировой уровень потребления в 2015 г. составил 17,2 DDD на 1000 человек в день [2]. Пандемия COVID-19 в 2019 г. послужила пусковым фактором беспрецедентного роста потребления антибактериальных средств. Доля пациентов с COVID-19, получавших антибиотики, по данным метаанализа Langford B.J. et al. (2021), составила 74,6% (95% ДИ 68,3–80,0%), при этом бактериальная коинфекция была подтверждена всего у 8,6% [3]. Влияние пандемии на рост потребления наиболее распространенных антибактериальных препаратов было проанализировано в работе Al-Azzam S. et al. (2021). Максимальный рост потребления был обнаружен для антибиотиков группы линкозамидов – на 106%, макролидов – на 57% (азитромицина – на 74%), карбапенемов – на 52%, III поколения цефалоспоринов – на 19% [4]. Приведенные данные указывают на формирование базиса для драматического роста антибиотикорезистентности в отношении указанных групп препаратов, что свидетельствует о целесообразности поиска эффективных альтернатив. Число новых антибиотиков в ряду регистрируемых препаратов составляет минимальную долю (по данным FDA: 2018 г. – 8,4%; 2019 г. – 8,33%) [5], в связи с чем актуальной тенденцией является пересмотр параметров эффективности и безопасности ранее открытых антибактериальных средств, минимально использовавшихся в течение последних десятилетий в широкой клинической практике [6]. Хлорамфеникол, антибиотик широкого спектра действия, был впервые выделен в 1947 г. из культуры актиномицетов Streptomyces venezuelae, химический синтез был осуществлен в 1949 г. [7]. Впервые публикации, посвященные хлорамфениколу и его терапевтическим возможностям, появились в базе данных PubMed в 1948 г., число публикаций достигло пика (246) в 1952 г., что отражало активное применение дешевого и высокоэффективного антибиотика в мировой практике, второй пик публикационной активности наблюдался в 1960-е гг. (112 публикаций в 1966 г.) и был обусловлен возникновением сообщений о гематотоксическом и миелосупрессивном действии препарата, синдроме «серого ребенка» [8]. Активная дискуссия в отношении высоких рисков побочных эффектов привела к резкому ограничению системного применения хлорамфеникола. В настоящее время хлорамфеникол применяется преимущественно местно, системное введение используется в качестве резервной альтернативы при ведении пациентов с тяжелыми резистентными инфекциями, тем не менее, показательными являются результаты национального опроса, включавшего стационары Израиля, по итогам которого 88,9% респондентов отметили, что применение хлорамфеникола оправдано в условиях высоких рисков антибиотикорезистентности [9]. Топический хлорамфеникол широко применяется в офтальмологии и оториноларингологии, стандартные лекарственные формы включают глазные капли, реже мази. Хлорамфеникол лидировал в группе антибактериальных препаратов для применения в офтальмологии по данным анализа числа регистрационных удостоверений, выданных Агентством по регулированию лекарственных средств и товаров медицинского назначения в Великобритании в период с 2001 по 2018 гг. (30 из 48) [10]. По данным Du H.C. et al. (2014), глазные капли и мази, содержащие хлорамфеникол, в 2007–2008 гг. составляли соответственно 68 и 48% от общего объема назначений данных лекарственных форм в офтальмологии [11]. Эффективное применение топического хлорамфеникола в офтальмологии и оториноларингологии предполагает возможность расширения показаний для местного применения данного препарата, в частности, в гинекологической сфере. Впервые системное применение хлорамфеникола в гинекологии было отмечено практически сразу после выхода препарата на рынок, в 50-х гг. ХХ в., что сопровождалось достаточно большим числом публикаций [12–15]. После возникновения сообщений о тяжелых побочных эффектах его системное применение в данной области было остановлено. В настоящее время возрождается интерес к интравагинальному пути введения хлорамфеникола, что требует полноценного обеспечения врачей информацией об эффективности и безопасности данного антибиотика.
Фармакодинамические характеристики хлорамфеникола
Хлорамфеникол является D-трео-изомером небольшой молекулы, состоящей из р-нитробензольного кольца, соединенного с дихлорацетильным хвостом через 2-амино-1,3-пропандиоловую группу. Бактериостатическая активность широкого спектра является результатом ингибирования синтеза белка на рибосомах, осуществляемого D-трео-изомером при взаимодействии с каталитическим центром пептидил-трансферазы [6, 16]. Дополнительные механизмы действия хлорамфеникола включают нарушение ряда функции рибосом, включая терминацию [17], точность трансляции [18] и биогенез 50S субъединиц [19], а также способность вызывать дефекты рибосомальной сборки путем ингибирования биосинтеза собственных белков рибосом [20]. Помимо антибактериальной активности хлорамфеникола, есть данные, свидетельствующие о его потенциальном противогрибковом действии. В работе Joseph M.R. et al. (2015) in vitro было продемонстрировано ингибирование грибкового роста в 73,3% случаев (всего исследовалось 30 штаммов грибов); для каспофунгина этот параметр составил 83,3%, для кетоконазола – 70% [21].
Фармакокинетические характеристики хлорамфеникола
Лекарственные формы хлорамфеникола для системного введения включают эфиры (сукцинат и пальмитат). Эфиры являются пролекарствами, превращающимися в активный хлорамфеникол путем гидролиза. Хлорамфеникол быстро и достаточно полно всасывается при приеме внутрь, создавая высокие концентрации во многих органах, тканях и жидкостях организма, включая спинномозговую жидкость и грудное молоко, проникает через плаценту. Период полувыведения составляет до 3,5 ч, препарат метаболизируется в печени путем глюкуронидации и выводится преимущественно почками [22]. При нарушении функции почек период полувыведения составляет 3–4 ч, при тяжелых нарушениях функции печени – 4,6–11,6 ч. Период полувыведения у детей в возрасте от 1 месяца до 16 лет составляет 3–6,5 ч, у детей в возрасте от 1 до 2 дней – 24 ч, характеризуется высокой вариабельностью [23]. Почечная экскреция хлорамфеникола составляет от 3 до 25%, хлорамфеникола сукцината – от 7 до 45% [24].
Спектр активности хлорамфеникола
Хлорамфеникол обладает широким спектром действия. Высокая эффективность хлорамфеникола в отношении полирезистентной грамотрицательной микрофлоры подтверждается результатами работы Sood S. et al. (2016) [25]. В исследование было включено 650 изолятов грамотрицательных бактерий, из них 75% – полирезистентные (устойчивость минимум к трем различным классам обычно используемых противомикробных препаратов (пенициллины, цефалоспорины, аминогликозиды, фторхинолоны, ингибиторзащищенные β-лактамы и карбапенемы)). В результате обнаружено, что 68% от общего числа изолятов полирезистентных грамотрицательных бактерий были чувствительны к хлорамфениколу. 100% чувствительность была отмечена для Morganella morganii, Empedobacter brevis и Burkholderia cepacia.
Параметры высокой активности хлорамфеникола в отношении Bacteroides spp. в сравнении с рядом других антибактериальных препаратов приведены в работе Cordero-Laurent E. et al. (2012). 93 из 100 проанализированных штаммов были устойчивы к одному или более из 12 антибиотиков. Резистентность к амоксициллину составила 91%, к тикарциллину и пиперациллину – около 70%. Согласно результатам исследования, все штаммы были чувствительны к хлорамфениколу, имипенему, также высокая активность была продемонстрирована для метронидазола [26].
Современные параметры чувствительности грамотрицательной микрофлоры по отношению к хлорамфениколу были продемонстрированы в исследовании ARMOR. Доля чувствительных штаммов H. influenzae составила 99,5% [27].
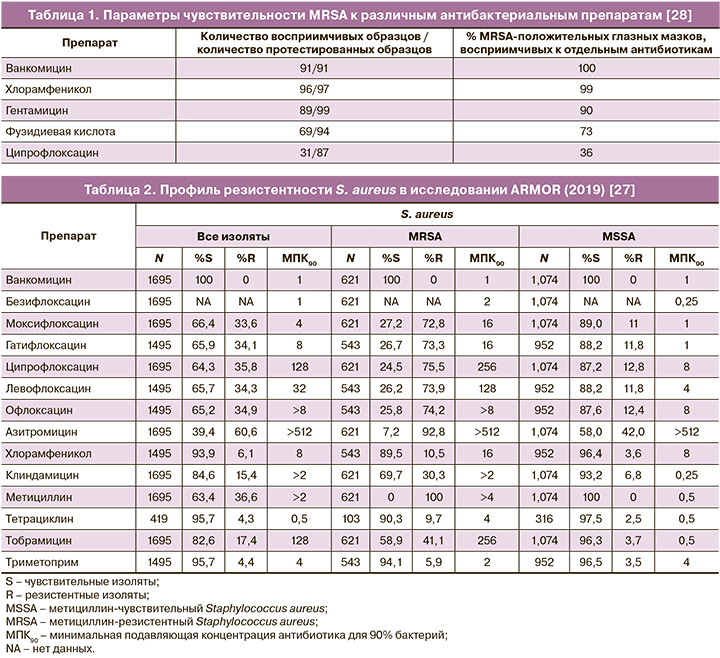
Профиль активности хлорамфеникола в отношении грамположительной микрофлоры также свидетельствует о высокой эффективности в отношении резистентных штаммов. Работа Croghan C.et al. (2018) включает данные о чувствительности изолятов метициллин-резистентного Staphylococcus aureus (MRSA), полученных от пациентов с офтальмологическими инфекциями (100 образцов мазков, содержащих MRSA, период получения образцов – с 03.02.2013 по 28.11.2015). Препаратами – лидерами по уровню чувствительности были ванкомицин (100%) и хлорамфеникол (99%) (табл. 1) [28].
В работе Fayyaz М. et al. (2013) оценивалась чувствительность in vitro 174 изолятов MRSA к хлорамфениколу. Обнаружено, что 75,86% изолятов были чувствительны к хлорамфениколу (минимальная подавляющая концентрация (МПК) ≤8 мкг/мл), 38 (21,84%) были устойчивыми (МПК ≥32 мкг/мл), 4 (2,30%) имели показатели МПК, свидетельствующие о промежуточном диапазоне (МПК ~16 мкг/мл) [29].
Также абсолютная эффективность хлорамфеникола в отношении MRSA была продемонстрирована в более ранней работе исследователей, анализировавших активность данного препарата против изолятов золотистого стафилококка, выделенного у пациентов с глазными инфекциями (n=548). 17 изолятов (3%) были MRSA-положительными и обнаружили полную чувствительность к хлорамфениколу; при этом все изоляты, полученные от пациентов старше 50 лет, были охарактеризованы, как резистентные по отношению к офлоксацину [30].
Согласно данным исследования ARMOR 2019 г. (антибиотикорезистентность среди возбудителей глазных инфекций, исследование включало 1695 изолятов S. aureus, 1475 изолятов коагулазонегативных стафилококков, 474 – S. pneumoniae, 586 – H. influenzae и 599 – P. aeruginosa), уровень резистентности S. aureus в отношении хлорамфеникола был минимальным – 6,1%, в то время как для азитромицина, ципрофлоксацина и метициллина показатели резистентности составили 60,6, 35,8 и 36,6% соответственно, для клиндамицина уровень резистентности составил 15,4% (табл. 2). Резистентность MRSA к хлорамфениколу составила всего 10,5%. Коагулазонегативные стафилококки были практически абсолютно чувствительны к хлорамфениколу (резистентность – 1,2%) (табл. 3). Среди изолятов пневмококка резистентность к хлорамфениколу составила всего 2,5% [27].

Согласно данным Mendes C.M.F. et al. (2001), хлорамфеникол обладал достаточно высокой активностью в отношении β-гемолитического стрептококка: среди изолятов, выделенных из дыхательных путей (n=67), 92% были чувствительны, среди изолятов, выделенных с кожных покровов (n=48), – 88%. Анализ S. pneumoniae (n=26, изоляты из дыхательных путей) обнаружил 100% чувствительность к хлорамфениколу и 92% – к клиндамицину [31].
По данным исследования ПеГАС (2014–2017), уровень резистентности S. pyogenes (792 изолятов, 14 российских городов) к хлорамфениколу составил 6,1%, к тетрациклину – 17,2%, уровень резистентности к 14- и 15-членным макролидам варьировался от 12,1 до 17,2% [32].
Интересны результаты оценки динамики показателей чувствительности микрофлоры, типичной для офтальмологических инфекций, полученные за 15-летний период. В опубликованной в 2004 г. работе Chalita M.R. et al. оценивалась активность антибиотиков против Staphylococcus aureus, коагулазонегативных стафилококков, Streptococcus spр. и Pseudomonas spр., полученных из образцов конъюнктивы (n=4585) и роговицы (n=3779) от пациентов, наблюдавшихся в Федеральном университете Сан-Паулу в период с 1985 по 2000 гг. Хлорамфеникол имел высокую активность против S. aureus (чувствительность 85% для изолятов из роговицы и 92% – из конъюнктивы), динамика чувствительности коагулазонегативных стафилококков обнаружила рост с 87 до 88,5%, аналогично в случае Streptococcus sрp. – рост с 95 до 96% [33].
В лечении ряда инфекционных заболеваний, в особенности при поражении слизистых оболочек, большое значение приобретает способность антибактериального препарата преодолевать и разрушать биопленки. В исследовании Liaqat I. et al. (2009) изучались in vitro способность к образованию биопленок изолятов Klebsiella spр., Pseudomonas aeruginosa, Achromobacter spр., Klebsiella pneumoniae и Bacillus pumilus и эффективность воздействия антибиотиков. Все протестированные в исследовании изоляты имели высокую способность к образованию биопленок. Сравнение эффективности воздействия хлорамфеникола и тетрациклина обнаружило значительное сокращение образования биопленок в случае первого препарата (p<0,01) [34].
Способность хлорамфеникола нарушать биопленки в случае офтальмологических инфекций была продемонстрирована в исследовании Drago L. et al. (2019). Результаты показали, что хлорамфеникол имел большую активность, чем тобрамицин, в отношении грамположительных бактерий; его применение способствовало предотвращению образования бактериальных биопленок. Хлорамфеникол имел большую активность против S. aureus по сравнению с тобрамицином и меньшую против P. aeruginosa, тем не менее, хлорамфеникол мог значительно снижать бактериальную адгезию P. aeruginosa [35].
Традиционно сохраняется высокая чувствительность хлорамфеникола в отношении такого специфического возбудителя, как сальмонелла. Patil N. et al. (2019) анализировали 251 изолят Salmonella (192 (76,5%) – S. typhi, 59 (23,5%) – S. paratyphi A). 94,4% (237/251) изолятов были чувствительны к хлорамфениколу и только 3,6% (9/251) – к офлоксацину. Средняя величина минимальной подавляющей концентрации для хлорамфеникола составила 3,89±6,94 мкг/мл [36]. По данным отечественной работы, где анализировались параметры антибиотикорезистентности S. typhi (299 изолятов, период 2005–2018 гг., территория Российской Федерации), было обнаружено, что доля чувствительных к хлорамфениколу изолятов составила 97,3%, при этом чувствительность к фторхинолонам составила всего 10,4% [37].
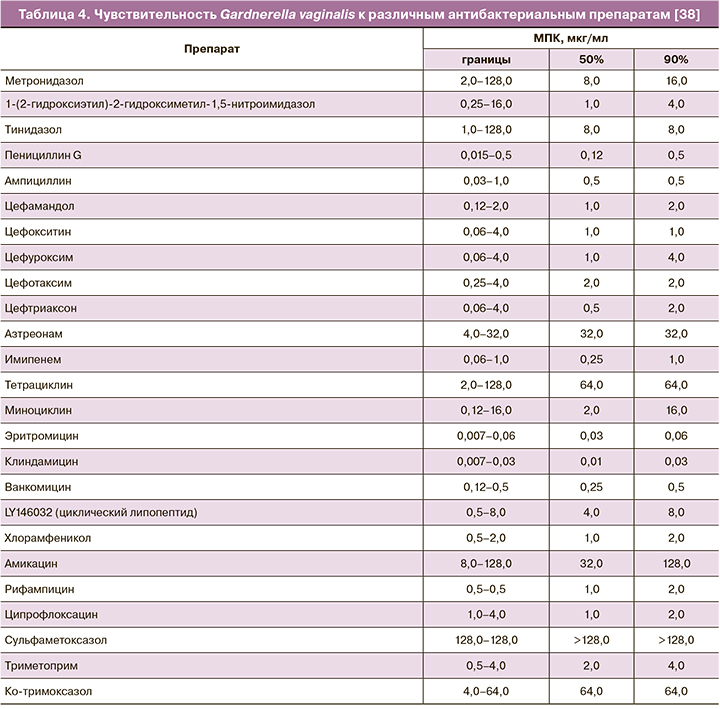
Эффективность хлорамфеникола в отношении такого микроорганизма, как Gardnerella vaginalis, являющегося одним из доминирующих возбудителей бактериального вагиноза, лежит в основе возможности его местного применения в гинекологии. В работе Kharsany A.B. et al. (1993) была продемонстрирована чувствительность 93 штаммов Gardnerella vaginalis к 25 антибактериальным препаратам (определялась МПК). Результаты обнаружили, что все штаммы были чувствительны к пенициллину (МПК90 – 0,5 мкг/мл), ампициллину (MПК90 – 0,5 мкг/мл), эритромицину (MПК90 – 0,06 мкг/мл), клиндамицину (MПК90 – 0,03 мкг/мл), ванкомицину (MПК90 – 0,5 мкг/мл) и хлорамфениколу (MПК90 – 2,0 мкг/мл) (табл. 4) [38].
Более поздняя работа de Souza D.M.K. et al. (2016) продемонстрировала 100% уровень чувствительности изолятов Gardnerella vaginalis к хлорамфениколу. В исследование было включено 204 изолята G. vaginalis, полученных от 42 пациенток (37 – с бактериальным вагинозом, 5 – не с бактериальным вагинозом) [39]. Высокий уровень резистентности Gardnerella vaginalis к наиболее распространенным в клинической практике антибиотикам был представлен вработе Ara N.N.R. et al. (2017), включившей в анализ 38 изолятов. Резистентность возбудителя отмечалась в отношении тетрациклина (81,58%), ко-тримоксазола (78,95%), ципрофлоксацина (68,42%), метронидазола (52,63%). При этом 73,68% изолятов продемонстрировали чувствительность к хлорамфениколу [40].
Таким образом, при сравнении результатов приведенных исследований можно сказать, что за 24 года показатели чувствительности Gardnerella vaginalis к хлорамфениколу не претерпели драматического снижения, в отличие от ряда других антибактериальных препаратов, что свидетельствует о возможности эффективного местного применения данного препарата в терапии бактериального вагиноза наряду с другими топическими антибактериальными препаратами, такими как клиндамицин.
Следующая значимая группа возбудителей бактериального вагиноза включает микоплазм и уреаплазм. В отношении данных возбудителей бета-лактамные антибиотики являются неэффективными, равно как и другие антибиотики, чей механизм действия включает в качестве мишени клеточную стенку. Микоплазма не имеет пептидогликана, вследствие чего устойчива к бета-лактамам, фосфомицину, гликопептидам, рифампину, полимиксинам, налидиксовой кислоте, сульфонамидам и триметоприму. В отношении хлорамфеникола исследования in vitro демонстрируют наличие активности против указанных возбудителей, что является дополнительным преимуществом местного применения данного препарата в гинекологии. В исследовании Wang N. (2020) была проведена оценка активности макролидов, фторхинолонов, тетрациклинов, хлорамфеникола и тиамфеникола в отношении изолятов Mycoplasma pneumoniae (n=110), Mycoplasma hominis (n=26) и Ureaplasma spр. (n=51). Результаты обнаружили высокий уровень резистентности M. pneumoniae (97,3%) к 14- и 15-членным макролидам; штаммов, устойчивых к тетрациклинам или хинолонам, не было выявлено. Значения МПК90тестируемых макролидов: ацетилмидекамицин (1 мг/л) < джозамицин (4 мг/л) < мидекамицин (8 мг/л) < азитромицин (16 мг/л) < эритромицин (> 128 мг/л). МПК90 для тиамфеникола и хлорамфеникола были одинаковыми (2 мг/л). 80,8% M. hominis 21 имели резистентность к фторхинолонам. МПК 14- и 15-членных макролидов были высокими в отношении всех изолятов (> 128 мг/л), для 16-членных макролидов величины МПК были значительно ниже (≤4 мг/л). Хлорамфеникол в отношении M. hominis продемонстрировал более низкое значение МПК90 (2 мг/л) в сравнении с тиамфениколом (4 мг/л). Все препараты тетрациклинового ряда обладали значительной активностью против M. hominis. Анализ Ureaplasma spр. обнаружил, что 13,7% изолятов были устойчивыми к хинолонам, всего 2,0% – к тетрациклину. Все изоляты были чувствительны к макролидам, значения MПК90: ацетилмидекамицин (0,25 мг/л) < джозамицин (0,5 мг/л) = мидекамицин < азитромицин (1 мг/л) = эритромицин. Хлорамфеникол продемонстрировал более низкую величину МПК90 (2 мг/л), чем тиамфеникол (4 мг/л). Результаты исследования обнаружили высокую активность in vitro для ацетилмидекамицина, хлорамфеникола и тиамфеникола [41].
Интересны данные о чувствительности микоплазм и уреаплазм, выделенных у беременных женщин, к такому распространенному антибиотику для местного применения в гинекологии, как клиндамицин. В исследовании Longdoh N. et al. (2018) изоляты U. urealyticum характеризовались абсолютной резистентностью к клиндамицину. Среди изолятов Mycoplasma hominis уровень чувствительности к клиндамицину составил 75%; абсолютная резистентность (100%) была отмечена в отношении макролидов (эритромицин, рокситромицин, кларитромицин), ципрофлоксацина и тетрациклина. В случае коинфекции U. urealyticum и M. hominis отмечалась устойчивость к клиндамицину, эритромицину, рокситромицину, ципрофлоксацину и кларитромицину [42].
Клиническая эффективность хлорамфеникола
Эффективность вагинального применения хлорамфеникола для терапии генитальных инфекций продемонстрирована в рамках исследования, оценивавшего клиническую эффективность комбинированного препарата, включавшего хлорамфеникол 200 мг. В исследование были включены 500 пациенток от 15 до 85 лет с генитальными инфекциями. Применение препарата привело к улучшению субъективной симптоматики у 98% пациенток, уменьшению лейкореи – у 95%. Излечение было достигнуто в большинстве случаев как острых, так и хронических цервико-вагинальных воспалительных состояний. Полученные результаты свидетельствовали об эффективности местного применения препарата при бактериальном вагините, трихомониазе и смешанном бактериально-микотическом вагините, при цервиците и воспалительных процессах в области малого таза [43].
Эффективность и безопасность применения топического хлорамфеникола как компонента комбинированного препарата Таржифорт (ОАО «Авексима», Россия) изучались в российском многоцентровом исследовании, включавшем 360 женщин в возрасте от 18 до 45 лет с диагнозом «острый вагинит», подтвержденным лабораторно [44]. Продолжительность терапии составила 10 дней. Результаты обнаружили, что эффективность российского препарата Таржифорт оказалась не ниже, чем у препарата Тержинан (Laboratoires Bouchara-Recordati, Франция). В частности, отсутствие боли отмечено как у 98,3% пациенток, получавших Таржифорт, так и пациенток, получавших Тержинан. Чувство жжения было устранено у 97,8% пациенток в обеих группах. Зуд более эффективно устранялся у пациенток, получавших Таржифорт (98,9% против 95,4%). Скорость исчезновения симптомов боли и жжения (4,3 и 4,8 дня) в группе Таржифорта статистически значимо превосходила таковую в группе Тержинана (5,6 и 5,8 дня).
Сравнительная эффективность клиндамицина и хлорамфеникола
Сравнение эффективности клиндамицина и хлорамфеникола было осуществлено в ряде клинических исследований, включавших системное назначение препаратов. Сравнительный анализ эффективности и безопасности системных клиндамицина и хлорамфеникола у пациенток с генитальным сепсисом или абсцессами малого таза (n=102, терапия – пенициллин или аналогичный антибиотик плюс аминогликозид, из них 53 дополнительно получили хлорамфеникол и 49 – клиндамицин) обнаружил аналогичные клинические результаты. У 8 (16%) из 49 пациенток, получавших клиндамицин, и у 3 (8%) из 52 пациенток, получавших хлорамфеникол, применение препарата было прекращено из-за побочных эффектов. По результатам исследования авторы сделали вывод об эквивалентной эффективности и безопасности клиндамицина и хлорамфеникола при терапии гинекологических инфекций [45].
Сравнительный анализ эффективности и безопасности хлорамфеникола, клиндамицина и тикарциллина представлен в работе Harding G.K. et al. (1980), демонстрирующей результаты проспективного рандомизированного сравнительного исследования, включавшего 175 пациенток с тяжелым смешанным аэробно-анаэробным интраабдоминальным или генитальным сепсисом. Было сформировано три группы терапии: хлорамфеникол+гентамицин, клиндамицин+гентамицин, тикарциллин+гентамицин. В случае внутрибрюшного сепсиса показатели излечения были следующими: 79% – для клиндамицина, 81% – для хлорамфеникола, 90% – для тикарциллина. В случае генитального сепсиса: 94% – для клиндамицина, 100% – для хлорамфеникола, 92% – для тикарциллина. Авторы сообщают об эквивалентной эффективности клиндамицина, хлорамфеникола и тикарциллина, использованных в комбинации с гентамицином, в терапии интраабдоминального или генитального сепсиса у женщин [46].
Оценка эффективности клиндамицина и хлорамфеникола с пенициллином в терапии пациенток с септическими абортами проводилась в рандомизированном двойном слепом исследовании, представленном Chow A.W. et al. (1977). Ответ на терапию клиндамицином или пенициллином с хлорамфениколом оценивали у 77 пациенток с септическими абортами. Индекс лихорадки и продолжительность госпитализации были одинаковыми для обеих групп терапии, серьезные осложнения значительно чаще отмечались в группе клиндамицина (p<0,05) [47].
Более полноценный анализ сравнительной эффективности комбинации хлорамфеникола с пенициллином с другими препаратами в терапии пациенток с септическими абортами представлен в систематическом обзоре, включавшем 3 рандомизированных клинических исследования (n=233). Результаты обзора свидетельствуют о том, что клиндамицин не имел существенных отличий от комбинации хлорамфеникол+пенициллин в отношении снижении температуры у женщин (среднее различие – 12,30, 95% ДИ 25,12–0,52; женщин 77; 1 исследование). Не было различий в продолжительности госпитализации между исследуемыми методиками терапии. Средняя продолжительность пребывания в стационаре для женщин в каждой группе составила 5 дней (0,00; 95% ДИ 0,54–0,54; женщин 77; 1 исследование). В одном исследовании оценивалась эффективность комбинации хлорамфеникол+пенициллин в сравнении с цефалотином и канамицином до и после дилатации и кюретажа. Полученные данные свидетельствуют о том, что эффект пенициллина с хлорамфениколом в отношении лихорадки не отличался от эффекта цефалотина в комбинации с канамицином (2,30; 95% ДИ 17,31–12,71; женщин 56; 1 исследование). Не было значимых различий между пенициллином и хлорамфениколом по сравнению с цефалотином и канамицином по результатам УЗИ во время антибиотикотерапии (1,00; 95% ДИ 13,84–11,84; женщин 56; 1 исследование 1) [48].
Профиль токсических реакций
Широкий спектр антибактериального действия хлорамфеникола, активность в отношении резистентных штаммов возбудителей как грамположительной, так и грамотрицательной микрофлоры делают его привлекательной альтернативой многим распространенным в клинической практике препаратам. Основной преградой на пути применения хлорамфеникола являются сомнения в отношении профиля безопасности, что особенно важно в случае применения препарата в гинекологической практике. Данный раздел приводит имеющиеся в настоящее время результаты клинических исследований, демонстрирующие частоту и риск развития различных побочных эффектов хлорамфеникола при системном и местных путях введения.
Системный хлорамфеникол
Высокий уровень проникновения хлорамфеникола при системном введении в абсолютное большинство органов и тканей, включая костный и головной мозг, имеет в своей основе физико-химические характеристики молекулы: липофильность, низкое количество доноров ОН и NH-связей (3), низкое число акцепторов N и ОН-связей (7), молекулярная масса 323,14 Да [49]. Концентрация обычного хлорамфеникола в тканях мозга и спинномозговой жидкости ниже в сравнении с плазмой, но может значительно повышаться на фоне длительного использования, что может приводить к развитию таких токсических реакций, как митохондриальные невропатии [50].
В случае большинства инфекций наиболее желательными являются пиковые концентрации хлорамфеникола от 10 до 20 мкг/мл (минимальные концентрации от 5 до 12,5 мкг/мл). Терапевтические концентрации зависят от чувствительности конкретного микроорганизма, а также от типа и тяжести инфекции. Концентрационнозависимые токсические реакции отмечаются, как правило, на фоне поддержания стабильных пиковых концентраций в сыворотке ≥25 мкг/мл [51]. Перечень токсических реакций, характерных для системного введения хлорамфеникола, включает реакции гематотоксичности, нейротоксичности, гастротоксичности и гиперчувствительности [52].
Согласно современным представлениям, токсичность хлорамфеникола, прежде всего в отношении костного мозга, имеет в своей основе воздействие на митохондрии: способность обратимо ингибировать синтез митохондриального белка и фермент феррохелатазу, необходимую для образования протогема. Гематотоксичность может быть минимизирована на фоне поддержания концентрации хлорамфеникола на уровне 15–25 мг/л [53]. Среди всех гематотоксических реакций наибольшую опасность представляет апластическая анемия. Частота ее развития составляет менее 0,01% [53], наиболее сильное цито- и генотоксическое действие (разрыв цепочек ДНК) оказывает такой метаболит, как нитрозохлорамфеникол [54]. Анализ влияния приема хлорамфеникола и воздействия других факторов на риск развития апластической анемии в популяции стран Латинской Америки продемонстрировал следующие результаты. Общая заболеваемость составила 1,6 случая на 1 млн в год. У лиц, использовавших хлорамфеникол в предыдущем году, скорректированное отношение рисков развития апластической анемии составило 8,7 (ДИ 0,87–87,93), при этом у лиц, получавших азитромицин, данный параметр был равен 11,02 (ДИ: 1,14–108,02), что свидетельствует о сравнительно низких показателях риска применения хлорамфеникола в отношении развития апластической анемии [55].
Следующий значимый побочный эффект – синдром «серого ребенка». Его возникновение у новорожденных обусловлено отсутствием реакции глюкуронидации (детоксикация фазы II) и недостаточной функциональной емкостью почек для выведения неконъюгированных лекарств, в итоге накопленные токсичные метаболиты хлорамфеникола вызывают синдром, характеризующийся гипотонией, цианозом, гипотермией, сердечно-сосудистым коллапсом, пепельно-серым цветом кожи и рвотой. Синдром «серого ребенка» проявляется, как правило, при приеме хлорамфеникола в дозах более 200 мг/сут [56].
Приведенный перечень побочных эффектов хлорамфеникола является достаточно серьезным. Тем не менее объективный сравнительный анализ различных антибиотиков, включая хлорамфеникол, свидетельствует об отсутствии статистически достоверной разницы в частоте развития побочных эффектов (ОР 0,80; 95% ДИ 0,62–1,02). Результаты получены в рамках систематического обзора и метаанализа 66 рандомизированных клинических исследований [57]. Единственная позиция, характеризовавшаяся значительно большим риском в случае приема хлорамфеникола, принадлежала анемии (ОР 2,80; 95% ДИ 1,65–4,75). В отношении нейтропении различий между хлорамфениколом и другими антибиотиками отмечено не было (ОР 1,36; 95% ДИ 0,88–2,11). Частота возникновения кожной сыпи также не имела отличий (ОР 0,85; 95% ДИ 0,57–1,26). Интересно отметить, что желудочно-кишечные побочные эффекты были значительно менее характерны для хлорамфеникола в сравнении с другими препаратами (ОР 0,67; 95% ДИ 0,46–0,99).
Указанные особенности токсического действия хлорамфеникола предполагают его невозможность применения при беременности. Тем не менее опубликованы данные, свидетельствующие о минимальных рисках системного применения хлорамфеникола при беременности. Масштабное исследование Czeizel A.E. et al. (2000) представляло парный анализ случаев с врожденными аномалиями и подобранным контролем, выполненный на основе базы данных (Венгрия) за период 1980–1996 гг. (из 38 151 беременных женщин, родивших детей без каких-либо дефектов (контрольная группа), 51 (0,13%) получала хлорамфеникол per os, из 22 865 беременных женщин, чьи новорожденные или плоды имели врожденные аномалии, 52 (0,23%) получали per os хлорамфеникол во время беременности). Анализ пары «случай–контроль» не выявил тератогенного потенциала хлорамфеникола при применении в течение 2–3-го месяцев беременности в различных группах врожденных аномалий. Проведенный анализ случаев приема хлорамфеникола не выявил высоких отношений шансов ни для каких врожденных аномалий [58].
Топический хлорамфеникол
Местное применение хлорамфеникола активно используется в клинической практике в терапии офтальмологических, раневых и гинекологических инфекций. Применение хлорамфеникола в виде мази для лечения раневых инфекций характеризуется достаточно высоким профилем безопасности, что подтверждается данными систематического обзора Shen A.Y. et al. (2018), включившего данные MEDLINE, EMBASE и Cochrane Library. Результаты обзора позволили определить, что потенциальные побочные эффекты включали реакции замедленной гиперчувствительности и острый эзофагит, явлений апластической анемии не было отмечено [59].
Основной объем данных о безопасности топического хлорамфеникола получен в области офтальмологии. Во второй половине ХХ в. активно обсуждался вопрос системной токсичности при применении хлорамфеникола в форме глазных капель, прежде всего, возможность подавления функции костного мозга. В работе Walker S. et al. (1998) применялся метод высокоэффективной жидкостной хроматографии (предел минимального обнаружения – 1 мг/л) для определения уровня хлорамфеникола в сыворотке у пациентов (n=40), получавших хлорамфеникол в форме глазных капель (средняя доза спустя 1 неделю лечения – 8,0 мг, 2 недели – 15,3 мг). Результаты обнаружили, что при данном пути введения хлорамфеникол не смог создать достаточных для обнаружения концентраций в крови пациентов, что подтверждает отсутствие риска ингибирования костного мозга при местном применении данного препарата [60]. Приведенные данные поддерживаются более поздними исследованиями, в частности результатами рандомизированного двойного слепого плацебо контролируемого клинического исследования, в рамках которого 163 ребенка с конъюнктивитом получали хлорамфеникол в виде глазных капель и 163 – плацебо: распределение и характер побочных эффектов не имели значительных отличий между группами [61]. В работе McGhee C.N. et al., опубликованной в 1996 г., приведены данные о том, что в Великобритании в Комитет по безопасности лекарственных препаратов с 1966 г. поступило только 11 сообщений (нефатальных) о подозрении на гематологические проблемы, вызванные местным применением хлорамфеникола [62].
Наиболее грозное проявление гематотоксичности хлорамфеникола – апластическая анемия. Обсуждение риска возникновения данного побочного эффекта при местном использовании препарата в офтальмологии в 80-х гг. ХХ в. привело к публикации результатов масштабного проспективного исследования (случай–контроль, период 1980–1995 гг., 67,2 млн человеко-лет), обнаруживших, что частота апластической анемии среди людей, применявших хлорамфеникол (глазные капли и мази), составила 0,36 случая на 1 млн недель лечения против частоты 0,04 случая на 1 млн недель среди лиц, не использовавших препарат [63]. Анализ случаев идиосинкратических и дозозависимых гематотоксических реакций, включая апластическую анемию, был осуществлен в работе Walker S. et al. (1998). Результаты свидетельствовали об отсутствии связи приобретенной апластической анемии и топического хлорамфеникола (лекарственные формы в офтальмологии) [60].
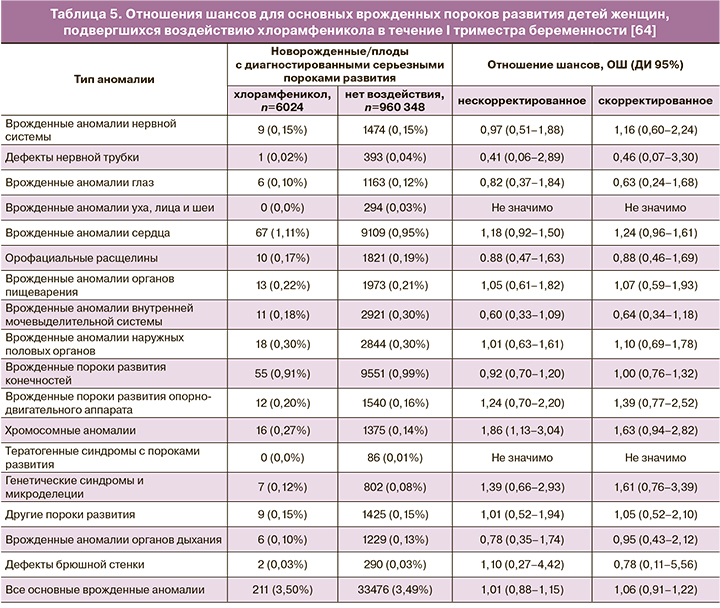
Безопасность топического хлорамфеникола в офтальмологии у беременных подтверждается данными масштабного исследования Thomseth V. et al. (2015). Авторы провели общенациональное когортное исследование, включившее всех женщин, родивших живых детей в период с 1997 по 2011 гг. в Дании (n=966 372), из которых была отобрана популяция получавших, по крайней мере однократно, хлорамфеникол в форме глазных капель или глазной мази в течение первых 84 дней беременности. 6024 женщины подвергались воздействию местного хлорамфеникола в I триместре. Частота врожденных пороков развития составила 3,50% для детей женщин, получавших хлорамфеникол, и 3,49% для не подвергавшихся воздействию. В итоге был сделан вывод, что местное применение хлорамфеникола в I триместре беременности не имело связи с серьезными врожденными пороками развития (скорректированное отношение шансов 1,06; 95% ДИ: 0,91–1,22) или специфическими значительными пороками развития [64]. Подробные данные по влиянию хлорамфеникола на развитие отдельных пороков развития представлены в таблице 5.
Топическое применение хлорамфеникола в гинекологии характеризуется достаточно высоким профилем безопасности, что подтверждается данными исследований, включавшими в том числе беременных.
Оценка безопасности применения хлорамфеникола при интравагинальном введении у беременных была проведена Harauchi S. et al. (2017) [65]. Исследование включало 37 беременных женщин, получавших 100 мг трансвагинального хлорамфеникола 1 раз в день для терапии бактериального вагиноза. Концентрация хлорамфеникола в плазме варьировалась от 0,043 до 73,1 нг/мл (определение методом жидкостной хроматографии/тандемной масс-спектрометрии не ранее 2-го дня приема). По результатам анализа не было обнаружено корреляции между концентрацией хлорамфеникола в плазме женщин и фоновыми состояниями (число предыдущих родов, гестационный возраст при приеме препарата, клинические и лабораторные параметры), а также параметрами здоровья новорожденного (масса тела при рождении, балл по шкале Апгар, гестационный возраст во время рождения) [65].
Обсуждение
Большинство режимов ведения пациенток с бактериальными вагинитами включают в качестве препаратов выбора клиндамицин и метронидазол [66, 67]. Активность хлорамфеникола в отношении грамотрицательной и грамположительной микрофлоры не уступает указанным препаратам; в случае ряда резистентных возбудителей (MRSA) было отмечено превосходство хлорамфеникола [27]. Широкий спектр действия, низкие показатели резистентности разнообразной микрофлоры в отношении хлорамфеникола предполагают клиническую выгоду его применения. Сравнительный профиль безопасности хлорамфеникола свидетельствует о среднем риске побочных эффектов, сопоставимом в большинстве случаев с таковым для наиболее применяемых системных антибиотиков [57].
Анализируя побочные эффекты клиндамицина и метронидазола, можно отметить, что случаи их системного применения также включают гематологическую сферу: в случае клиндамицина возможно возникновение эозинофилии у 0,1–1% пациентов [68], а также DRESS-синдрома [69–71]; в случае метронидазола – у 0,1–1% пациентов может развиваться лейкопения, у 0,01–0,1% – нейтропения, тромбоцитопения, панцитопения, агранулоцитоз [72]. Результаты исследования Soule A.F. et al. (2018) свидетельствуют о развитии лейкопении у 41,2% пациентов, принимавших метронидазол каждые 8 ч [73]. При применении системного хлорамфеникола наиболее значимым побочным эффектом со стороны гематологической сферы является апластическая анемия, возникающая менее чем у 0,01% пациентов [52]. При топическом (интравагинальном) применении риск возникновения системных побочных эффектов зависит от величины системной абсорбции лекарственного препарата. В случае интравагинального введения клиндамицина и метронидазола ни гематотоксические, ни нейротоксические реакции (характерные для их системного применения) не встречаются в широкой клинической практике, несмотря на достаточно высокий уровень системной абсорбции данных антибиотиков. Интравагинальное применение клиндамицина приводит к системной абсорбции до 4,7% в случае крема [74], 30% – в случае овули [75]. Побочные эффекты при применении интравагинального клиндамицина развиваются у 12% [76]. Интравагинальное применение метронидазола характеризуется уровнем системной биодоступности, равным в среднем 20% [77]. Результаты анализа системной биодоступности вводимого интравагинально хлорамфеникола обнаружили значение, близкое к нулевому [65]. Незначительное содержание хлорамфеникола в плазме (0,043–73,1 нг/мл), обнаруженное в данном исследовании [65], указывает на огромное окно между концентрацией, характерной для интравагинального применения, и концентрацией, способной привести к развитию токсических реакций (≥25 мкг/мл) [51].
Выводы
Опубликованные данные эффективности хлорамфеникола в отношении широкого спектра возбудителей наряду с фармакокинетическими характеристиками, демонстрирующими абсолютно незначительный уровень системной абсорбции препарата при интравагинальном пути введения, делают возможным его применение в лечении бактериальных вагинитов, что расширяет арсенал доступных врачам-гинекологам фармакотерапевтических инструментов.
Лекарственным препаратом для топической терапии неспецифических вагинитов является Таржифорт: комплексное лекарственное средство, содержащее, кроме хлорамфеникола, также метронидазол, натамицин и гидрокортизон. Данные о практически нулевой системной биодоступности хлорамфеникола позволяют клиницисту обоснованно назначать Таржифорт как для лечения вагинитов различной этиологии, так и для профилактики инфекционно-воспалительных осложнений при гинекологических манипуляциях.



