Вагинальный путь введения лекарств является привлекательным с двух точек зрения. Во-первых, это возможность быстрой доставки препарата непосредственно в очаг заболевания. В первую очередь речь идет об инфекционных заболеваниях половых путей, когда антибактериальное или противогрибковое средство может быть введено в очаг инфекции, или об атрофии слизистой, когда используется топическое применение эстрогенов.
Во-вторых, особенности влагалищного эпителия (низкая метаболическая активность в отношении ксенобиотиков), относительно благоприятная для кислотоустойчивых молекул водная среда, хорошая васкуляризация органа и попадание абсорбированных веществ в кровоток, минуя печень, создают благоприятные условия для использования препаратов системного действия. Первые попытки интравагинального введения лекарств для получения системного эффекта были предприняты еще в начале прошлого столетия [1]. В середине века при внутривлагалищном введении пенициллина для лечения бактериального кольпита были изучены концентрации препарата в крови. Оказалось, что через 4–6 ч содержание пенициллина достигало терапевтического уровня. Кроме того, на скорость его всасывания влияло состояние слизистой влагалища в разные дни цикла, наличие или отсутствие беременности и время, прошедшее после родов [2].
Для некоторых препаратов впоследствии было показано, что их биодоступность при интравагинальном введении превышает таковую при пероральном приеме, что связано как с большей агрессивностью желудочной среды, так и с эффектом первого прохождения через метаболически активные энтероциты и гепатоциты [3].
В настоящее время существует много систем вагинальной доставки лекарств: растворы, кремы, мази, гели, тампоны, суппозитории, капсулы, пессарии, губки и вагинальные устройства модифицированного высвобождения (например, кольца).
Несмотря на удобство применения, вагинальный путь введения лекарств может быть сопряжен с определенными особенностями, такими как различия в толщине слизистой в разные периоды менструального цикла и в разные периоды жизни, вариации кислотности вагинального секрета, вероятность вытекания лекарственной формы, воздействие на слизистую влагалища и локальный микробиоценоз, вероятный дискомфорт при использовании некоторых лекарственных форм (например, раздражение в месте введения), а также психологические и культурные барьеры.
Анатомические и гистологические особенности влагалища
Влагалище представляет собой канал S-образной формы длиной от 7 до 12 см и шириной до 4 см, образованный мышечной и соединительной тканью. Как правило, передняя и задняя стенки влагалища примыкают друг к другу, при этом длина задней стенки несколько больше из-за анатомических особенностей расположения.
Стенка влагалища состоит из нескольких слоев: неороговевающий многослойный эпителий, собственная пластинка и подслизистый слой, мышечный слой и адвентициальная оболочка (покрывает орган в проксимальной части).
Подслизистый слой богат кровеносными и лимфатическими сосудами. Венозные сплетения влагалища сливаются с маточным, пузырным и ректальным венозными сплетениями. Осуществление оттока через систему внутренних подвздошных вен и частично напрямую через систему нижней полой вены определяет попадание всосавшихся во влагалище веществ в системный кровоток, минуя печень. Слияние венозных сплетений с маточными определяет возможность так называемого первичного прохождения через матку, что является одним из аргументов для попыток доставки некоторых гормональных агентов [4].
Толщина выстилающего влагалище эпителия составляет от 150 до 300 мкм. В разные фазы цикла эпителий содержит от 22 до 46 слоев, 10–15 из которых могут отшелушиваться и сменяться в течение недели [5]. Несмотря на то что вагинальный эпителий по своей природе не является секретирующим, он обычно покрыт тонким слоем жидкости, состоящей из эндометриального, цервикального и вестибулярного секретов, следовых количеств мочи, продуктов апоптоза клеток слизистой и транссудата, количество которого может существенно варьироваться. В целом влагалищный секрет на 90–95% состоит из воды, поэтому может рассматриваться в первую очередь как водная среда растворения лекарств.
Количество и характеристики вагинального секрета зависят от возраста женщины, фазы цикла, гормонального фона, частоты эпизодов сексуального возбуждения и коитусов, общего состояния здоровья. По данным разных авторов, объем вагинального секрета составляет от 2 до 4 мл в сутки, или 0,33 мл/ч.
Показатель pH вагинального секрета у здоровой женщины репродуктивного возраста составляет 3,8–4,5, но может отклоняться под влиянием различных факторов. Например, активная транссудация при сексуальном возбуждении может повышать рН за счет разбавления кислотного секрета. Кроме того, временная нейтрализация кислотности может происходить под влиянием попадания спермы, имеющей слабоосновную реакцию, и после проведения гигиенических процедур с использованием мыла.
Изменение рН в основную сторону характерно при колонизации влагалища болезнетворными бактериями, а также у женщин в постменопаузе, когда по мере истончения слоя эпителия рН среды постепенно нарастает.
Кислая реакция среды обусловлена колонизирующими влагалище молочнокислыми бактериями, в основном Lactobacillus spp., метаболизирующими до молочной кислоты моно- и дисахариды, получаемые при расщеплении гликогена, в изобилии содержащегося в десквамированных клетках вагинального эпителия. В свою очередь, молочная кислота замыкает метаболический цикл, превращаясь под воздействием лактатдегидрогеназы в пируват. Последний в процессе глюконеогенеза превращается в глюкозу и в дальнейшем в запасаемый эпителиальными клетками гликоген. Постоянство образуемого ацидофильными бактериями микробиома является одним из существенных факторов, обеспечивающих нормальную функцию влагалища [6].
Другие органические кислоты, например уксусная или янтарная, также могут определять кислотность среды, однако их присутствие обусловлено в большей степени колонизацией влагалища патогенными или условно-патогенными микроорганизмами [7].
Иннервация влагалища такова, что его нижняя четверть имеет высокую чувствительность, в то время как оставшиеся три четверти в большей степени реагируют на растяжение, нежели на тактильные или болевые стимулы. Эта особенность определяет отсутствие дискомфорта при применении вагинальных устройств [8].
Фармакокинетика лекарств при интравагинальном введении
При проведении фармакокинетического моделирования наиболее простым подходом является выбор двухкамерной модели, при котором влагалище может рассматриваться в виде первой камеры с водной средой в объеме, равном объему вагинального секрета. Более точным будет подход, в котором учитывается то, что вводимое лекарство будет перемешиваться с вагинальным секретом и распределяться (растекаться) по стенкам влагалища. При этом площадь всасывающей поверхности составит от 40 до 120 см2 – этот показатель очень вариативен, так как зависит не только от размеров самого органа, но и от выраженности складок слизистой, существенно увеличивающих ее поверхность.
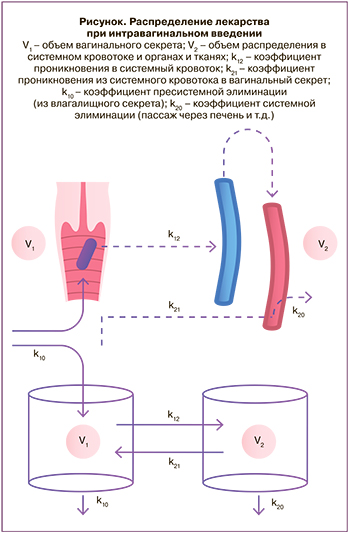 Второй камерой может считаться системный кровоток, центральная камера (рисунок). Проникновение лекарств в центральную камеру зависит от факторов, определяемых свойствами всасывающей поверхности и самой молекулы.
Второй камерой может считаться системный кровоток, центральная камера (рисунок). Проникновение лекарств в центральную камеру зависит от факторов, определяемых свойствами всасывающей поверхности и самой молекулы.
Кроме относительно большой площади поверхности, всасыванию способствуют хорошая васкуляризация органа, а также относительно низкая собственная метаболическая активность (в отличие от энтероцитов тонкого кишечника). Можно предположить, что липофильные молекулы, в особенности небольшого размера, плохо растворяющиеся в водной среде, за счет гидрофобных свойств будут интенсивно проникать через липидный бислой мембран эпителия, двигаясь по градиенту падения концентрации, – это так называемая пассивная абсорбция. В то же время гидрофильные молекулы, хорошо растворяющиеся в водной среде, будут проникать в системный кровоток преимущественно путем водной парацеллюлярной диффузии [9]. Таким образом, при сравнении проникающей способности нужно учитывать растворимость молекулы. Так, при применении глюкокортикостероидных препаратов, преднизолон будет лучше проникать в системный кровоток, чем гидрокортизон, который за счет лучшей водорастворимости будет задерживаться во влагалищном секрете.
С учетом кислой среды необходимо принять во внимание способность молекул диссоциировать в водной среде. Так, согласно уравнению Гендерсона–Гассельбаха, зная константу равновесной диссоциации конкретного вещества и рН среды, можно предположить количество молекул, находящихся в недиссоциированной форме (легче абсорбируются) и в диссоциированной форме (задерживаются в среде). Таким образом, во влагалище будут удерживаться и накапливаться препараты, имеющие основную реакцию, а кислые молекулы будут подвергаться интенсивному всасыванию (феномен ионной, или буферной, ловушки) [10].
Любопытно, что разные стероидные структуры могут демонстрировать различную абсорбцию в зависимости от дня цикла. Так, эстрогены лучше всасываются через более тонкий эпителий в начале цикла, в то время как прогестерон более интенсивно абсорбируется хорошо васкуляризованной слизистой во второй половине цикла.
Несмотря на низкую метаболическую активность клеток вагинального эпителия, базальная мембрана и клетки содержат определенное количество эндо- и экзопептидаз, что, наряду с затрудненным проникновением крупных молекул, определяет нецелесообразность интравагинального введения высокомолекулярных структур (белков) [11].
Кроме указанных факторов, при оценке интенсивности проникновения лекарств в центральную камеру следует учитывать потери как чисто механические (за счет выделения растворенного в секрете препарата наружу), так и метаболические, связанные с активностью влагалищного микробиоценоза.
Рациональный выбор лекарств при интравагинальном введении
Отличительные особенности действия лекарств при их интравагинальном введении в большей степени реализуются на местном уровне, когда препараты доставляются непосредственно в фокус заболевания. Наиболее типичным примером такого применения является терапия воспалительных заболеваний, вызванных в первую очередь бактериальными и грибковыми агентами.
Типичный выбор лекарства в этом случае подчиняется следующему алгоритму. Для антибактериальных препаратов выбор склоняется в пользу антибиотиков широкого спектра с бактерицидным эффектом. Подобные предпочтения объясняются как необходимостью получения быстрого эффекта, так и эмпирическим характером терапии, когда микробиологическая диагностика затягивается. В случае назначения комбинированного препарата необходимо, чтобы оба антибиотика не только перекрывали максимально широкий спектр возможных возбудителей и дополняли друг друга (повышали шанс «угадать» с выбором препарата), но и обладали бактерицидным действием, так как комбинация бактерицидного антибиотика с бактериостатическим является в большинстве случаев нерациональной (риск остановки размножения бактерий в фазе, когда бактерицидный антибиотик оказывается неэффективен).
Добавление к антибактериальной терапии противогрибкового агента является обоснованным средством эмпирического лечения против одного из наиболее распространенных возбудителей вагинальных инфекций. Кроме того, после применения антибиотиков широкого спектра действия возникает временное окно (в которое грибок может успешно колонизировать влагалище), особенностью которого в ранний период после терапии антибиотиками является низкий уровень колонизации естественной сапрофитной флорой.
Риск грибковой колонизации особенно возрастает в случае одновременного применения топических глюкокортикостероидных препаратов, которые, вероятно, могут приводить к снижению локального неспецифического иммунитета [12]. Иммунодепрессивные препараты способны изменять биологические свойства грибов. Продемонстрировано, что преднизолон может усиливать патогенные и антигенные свойства Candida [13]. Исследования показали, что глюкокортикоиды усиливают адгезию Candida spp. к клеткам вагинального эпителия и способствуют быстрой инвазии [14]. Кроме того, топические глюкокортикоиды могут снижать противогрибковый эффект нистатина [12].
С учетом возможного общетоксического действия многих противогрибковых средств, а также характерных для них выраженных лекарственных взаимодействий выбор в этом случае падает на антимикотики, характеризующиеся минимальной способностью к проникновению в системный кровоток. Большую обеспокоенность вызывает растущая резистентность грибков рода Candida и особенно Candida non-albicans к ряду противогрибковых азолов. В этой связи стоит обратить внимание на антимикотик полиенового ряда нистатин, высокую чувствительность к которому проявляют все представители грибков рода Candida [15].
Симптоматическое лечение воспалительных заболеваний допускает внутривагинальное применение противовоспалительных препаратов: например, нестероидных противовоспалительных средств (НПВС) или глюкокортикоидов. И если подобный путь введения препаратов группы НПВС в большей степени связан с проникновением их в системный кровоток, то использование глюкокортикоидов может быть сопряжено с определенными рисками их местного введения.
Так, при введении во влагалище разовой дозы преднизолона создается довольно высокая концентрация в секрете (даже если сравнивать с парентеральным введением глюкокортикоидов), что влечет за собой реализацию разных эффектов, характерных для группы глюкокортикоидов, как положительных, так и отрицательных. Важно понимать, что основной, противовоспалительный, эффект глюкокортикоидов, реализуется путем снижения местного иммунитета, что может негативно повлиять на скорость эрадикации возбудителей даже при условии более быстрого уменьшения симптоматики. Разные эфиры преднизолона, используемые в местных препаратах (например, преднизолон в виде натрия фосфата или преднизолона натрия метасульфобензоат), не определяют разницы эффектов. Они влияют на растворимость в вагинальном секрете (транссудат) и на проникновение через слизистую (жирорастворимость), что в итоге может способствовать системному действию глюкокортикоида на организм.
Но нельзя сбрасывать со счетов и местное влияние препаратов для интравагинального применения, содержащих кортикостероиды: во-первых, риск оппортунистической инфекции влагалища (в том числе и за счет подавления местного иммунитета), во-вторых, повышение риска вирусного инфицирования, а в-третьих, риск формирования так называемой биопленки, непосредственно связанный с локальным нарушением активности макрофагов.
Заключение
Несмотря на многочисленные особенности, интравагинальное введение является одним из распространенных и широко изучаемых методов доставки лекарств. При назначении препаратов необходимо учитывать различия в их фармакокинетике, наблюдающиеся как в разные периоды жизни пациенток, так и в разные периоды менструального цикла, и связанные с этим возможные колебания системной концентрации лекарств.
Лекарства, применяемые для достижения исключительно местного эффекта, должны характеризоваться минимальной системной абсорбцией и минимальным влиянием на локальный иммунитет и естественный биоценоз влагалища.



