Вирус папилломы человека (ВПЧ) высокого онкогенного риска является главным этиологическим фактором развития неоплазий и рака шейки матки (РШМ) [1]. Известно, что РШМ занимает одну из лидирующих позиций в структуре онкологических заболеваний и смертности у женщин, что является важной медицинской и социальной проблемой. По данным ВОЗ, ежегодно в мире выявляются 493,2 тысячи новых случаев РШМ [2].
Известно, что в большинстве случаев ВПЧ способно самостоятельно элиминироваться из организма. При персистенции ВПЧ в течение 3 лет у 27% женщин развиваются цервикальные интраэпителиальные неоплазии (ЦИН) тяжелой степени [3]. Это обуславливает важность диагностики ВПЧ-ассоциированных заболеваний шейки матки на ранних этапах их развития [4]. В связи с этим особое значение приобретают различные исследования и анализ молекулярного состава биологических жидкостей (цервиковагинальная жидкость, фоликулярная жидкость, кровь, моча и др.) и тканей современными точными и высокоспецифичными методами, в частности, методом масс-спектрометрии (МC). МС – это метод качественного и количественного определения веществ в пробе. Масс-спектрометрия позволяет изучать молекулярные механизмы патогенеза неоплазий и злокачественных заболеваний репродуктивной системы и выявлять изменения на ранних этапах [5].
Диагностический потенциал протеомного состава цервиковагинальной жидкости (ЦВЖ) обусловлен тем, что помимо продуктов жизнедеятельности микроорганизмов, населяющих нижний отдел женского генитального тракта, ЦВЖ включает в себя отслоившиеся эпителиальные клетки, шеечную слизь, секрет желез эндометрия и маточных труб, воду, муцин, белки, углеводы и неорганические вещества [6]. ЦВЖ имеет огромное значение в поддержании гомеостаза и в иммунной защите женского полового тракта. В виду простоты забора материала для исследования и благодаря изобилию молекулярного состава, ЦВЖ представляет большой интерес для изучения. Ранние исследования доказали роль ЦВЖ в защите организма от вируса иммунодефицита человека (ВИЧ) в связи с наличием в ее составе ряда белков и пептидов, в частности кальгранулина А/В, лизоцима, человеческого нейтрофильного пептида, елафина и некоторых гистонов [7, 8]. Белковый состав ЦВЖ подвергается изменению при различных заболеваниях генитального тракта женщины, в том числе при поражении эпителия шейки матки, ассоциированные с папилломовирусной инфекцией.
 Целью данной работы была характеристика изменений протеомного состава цервиковагинальной жидкости при ВПЧ-ассоциированных поражениях шейки матки различной степени тяжести у пациенток репродуктивного возраста.
Целью данной работы была характеристика изменений протеомного состава цервиковагинальной жидкости при ВПЧ-ассоциированных поражениях шейки матки различной степени тяжести у пациенток репродуктивного возраста.
Материал и методы исследования
В одномоментное проспективное исследование было включено 30 женщин, в возрасте от 21 до 45 лет (средний возраст 30,5 года), обратившихся в научно-поликлиническое отделение ФГБУ Научный центр акушерства, гинекологии и перинатологии им. академика В.И. Кулакова Минздрава России, по поводу наличия патологии шейки матки.
Критерии включения: ВПЧ-инфекция, ВПЧ-ассоциированные заболевания шейки матки, регулярный менструальный цикл, подписанное информированное согласие на участие в исследовании.
Критерии исключения: наличие хронических заболеваний в стадии обострения, прием гормональной терапии, беременность, период лактации, наличие инфекций, передающихся половым путем, нарушение функции почек, печени, легких в стадии декомпенсации.
Комплексное обследование женщин включало: сбор клинико-анамнестических данных, определение гинекологического статуса, типирование, цитологическое исследование, расширенная кольпоскопия, протеомное исследование цервиковагинальной жидкости.Для оценки кольпоскопической картины применяли единую Международную кольпоскопическую классификацию, одобренную на 14-м Всемирном конгрессе IFCPC в Рио-де-Жанейро (2011 г.). Использовалась цитологическая оценка мазков с шейки матки по системе Бетесда [9].
Участники исследования дали добровольное письменное согласие на его проведение, подробности проводимого исследования были им разъяснены.
Пробоподготовка образцов для протеомного анализа
Забор ЦВЖ для последующего протеомного анализа осуществлялся путем орошения влагалища и шейки матки 5 мг раствором натрия хлорида 0,9%. Жидкость центрифугировалась при 2000 g в течение 10 мин при 4°C, супернатант замораживали и хранили до анализа при -80°С. Белки растворяли в буфере (0,2 M Tris-HCl, pH 8,5, 2,5 mM EDTA, 8 M мочевина), восстанавливали дитиотреитолом (0,1 М) 45 мин при 39°С, алкилировали йодацетамидом (0,05 М) 45 мин при 20°С и осаждали ледяным ацетоном с трифторуксусной кислотой (0,1%). Центрифугировали при 14000g 15 минут, осадок промывали этанолом, растворяли в 100 мМ аммоний-бикарбонатном буфере и добавляли трипсин в соотношении 1/50. Гидролизовали в течение 16 часов при 37°С, реакцию останавливали добавлением муравьиной кислоты до конечной концентрации 0,5%. Концентрацию белка в ЦВЖ определяли по методу Брэдфорда.
Тандемная хромато-масс-спектрометрия (ВЭЖХ-МС/МС)
Полученная пептидная смесь разделялась на высокоэффективном жидкостном хроматографе Agilent 1100 (Agilent Technologies, USA). Использовалась колонка, содержащая обратнофазный сорбент С18 (диаметр частиц 3 мкм, диаметр пор 100 Å). Для разделения использовался 120 минутный градиент (H20/ACN) от 0–15 мин с 3% ACN; 15–110 мин с 90% ACN; 115–120 мин с 3% ACN при скорости потока 300 нл/мин. Macc-спектрометрический анализ проводился на приборе 7 Тл LTQ-FT Ultra mass spectrometer (Thermo Electron, Bremen, Germany) с электроспрейным источником ионов.
Биоинформационный анализ данных
Полученные данные были проанализированы с помощью программного пакета MaxQuant. Для MS/MS поиска в него включалось до 8 главных пиков в 100 Da окне. Идентификация сформированных списков проводилась по прямой и обратной версии базы данных SwissProt, с максимальным допустимым отклонением от массы предшественника 6 ppm, Карбамидометилированием цистеинов в качестве фиксированной модификации а ацитилированием N-конца белков и окислением метионинов – как вариабельных модификаций. Пептиды идентифицировались минимум по 7 аминокислотам с установленным значением исключения при ложном поиске равным 0,01. В работе использовались только те белки, которые идентифицировались минимум по 2 пептидным фрагментам, причем один из них должен быть уникальным. Для полуколичественного анализа использовался метод «без метки» с дополнительной опцией «match between the runs». Статистический анализ полученных проводился с помощью программного пакета Perseus.

Результаты и обсуждение
Были сформированы 4 группы, в зависимости от результатов цитологического исследования (NILM, ASCUS, LSIL, HSIL): I группа – контрольная – 7 (23%) пациенток с NLIM, ВПЧ-негативные; 23 (77%) – ВПЧ-позитивные, разделены на 3 группы: II группа – 11 (49%) пациенток с ACSUS, III группа – 7 (30%) пациенток с LSIL, IV группа – 5 (21%) пациенток с HSIL. Основная часть обследуемых пациенток были репродуктивного возраста, соматически не отягощены и подходили под все критерии включения в исследование. Возраст менархе в среднем составил ±12,7 года. Средний возраст начала половой жизни ±19,2 года. Общее количество беременностей составило 65, из которых родами завершились 25 (38%), абортами – 40 (62%). У 10 (33%) пациенток было более 4 половых партнеров. Из перенесенных гинекологических заболеваний в анамнезе выявлена высокая частота ИППП (66%). Клинико-анамнестический анализ показал, что приблизительно половина женщин с ВПЧ-инфекцией имели ранний половой дебют (до 18 лет в 48% случаев).
При изучении возрастного распределения выяснилось, что у женщин в возрасте до 30 лет чаще встречались ВПЧ высокоонкогенного типа (52,1%), ВПЧ высокого риска выявлен в 90,9% случаев в группе ASCUS и в 100% – LSIL и HSIL. Наиболее распространенным типами были ВПЧ 16 (34,8%), 31 (17,4%), 52, 58, 56 (13%), 18, 35 (8,7%), остальные типы встречались менее 5%. У 69% пациенток вирусная нагрузка была более 3,2 lоg (в среднем – 5,6 (5,2–6,2 lоg) без существенных различий между группами.
Всем пациенткам была проведена расширенная кольпоскопия. Нормальная кольпоскопическая картина наблюдалась у 7 (23%) пациенток, слабовыраженные и выраженные изменения при кольпоскопии – у 23 (87%) включали наличие ацетобелого эпителия (АБЭ), интенсивность проявления которого коррелировала со степенью тяжести процесса: слабовыраженные изменения выявлялись у 18 (60%), выраженные – у 5 (17%) .
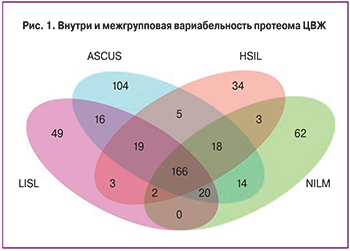
В результате исследования в образцах ЦВЖ было выявлено 515 различных белков. Для определения меж- и внутригрупповых изменений протеомного состава проводился сравнительный анализ усредненных значений интенсивности белков по группам (рис. 1). В дальнейшем был введен критерий, в соответствии с которым потенциальный маркерный белок должен был встречаться как минимум в трех повторах в одной группе. В результате в анализе из 515 белков принимало участие 295. Отдельно проводилась оценка белков, характерных только для определенной группы, и оценка белковой композиции, характерной для всех четырех групп, для выявления достоверно изменяющихся белков.
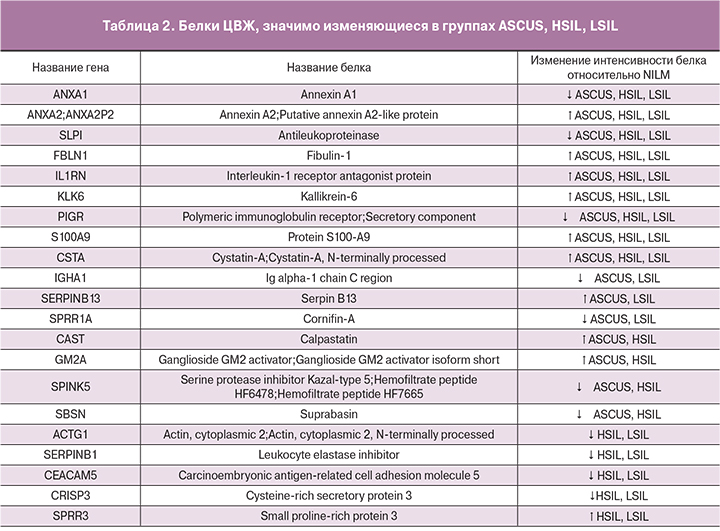
Для поиска потенциальной панели белков-биомаркеров были проанализированы белки, характерные для каждой из групп и белки, встречающиеся только в одной группе. Был введен дополнительный критерий, в соответствии с которым белок должен был присутствовать как минимум у двух пациенток. Из 295 белков уникальными для группы ASCUS являются 12 белков, в том числе белок LRG1, отвечающий за регуляцию пролиферации эндотелиальных клеток; белок C4BPA, который на фоне персистенции ВПЧ дополнительно угнетает работу системы комплемента; белки-шапероны P4HB и HSPA8, которые характеризуют ранние изменения, ассоциированные с ВПЧ-инфекцией, в том числе проникновении вируса в клетку и его транскрипцию [10]; белки PRDX5 и YWHAE, связанные с нарушением регуляции апоптоза и усилением пролиферации атипических клеток. Также среди уникальной панели белков для группы ASCUS был выявлен белок ACTN4, считающийся в настоящее время одним из потенциальных маркеров неоплазий шейки матки [4]. Было показано, что повышение активности ACTN4 происходит при злокачественных трансформациях, и связано со снижением прочности межклеточных связей [11, 12].
Однако, несмотря на то, что в подобных исследованиях ACTN4 выявлялся при различных типах неоплазий, в нашем исследовании белок присутствовал только в группе ASCUS. Для групп HSIL специфичными оказались 2 белка, среди которых представляет интерес белок CPM, принимающий участие, как в деградации внеклеточных белков, так и в контроле активности белковых гормонов и факторов роста. Для группы контроля специфичным оказался белок MUC16, который обеспечивает синтез муцина, дополнительно защищающего клетки от вирусной инвазии (табл. 1).
С учетом вариабельности протеомного состава было решено сузить спектр анализируемых белков для выявления изменений среди белков, присутствующих во всех трех группах минимум в трех повторах. В результате 128 белков встречались во всех группах. Проанализировав данную выборку с использованием базы данных Gene Ontology, было показано, что 66 белков участвуют в метаболических процессах, причем большая их часть – в протеолизе, 29 белков ассоциированы с биологической регуляцией, 30 – с иммунными процессами и 26 стресс-белков, генез которых в рамках данного исследования не изучался (рис. 2). По классам большую часть белков представляют ингибиторы протеаз, которые, возможно, оказывают влияние на развитие патологического процесса, ингибируя апоптотические ферменты.
Далее был проведен ANOVA-тест с отклонением p-value менее 0,01: из 128 характерных белков было выявлено 37 достоверно изменяющихся в различных группах. Среди них присутствуют белки, которые представляют интерес в качестве потенциальных маркеров опухолевых изменений. Белки KLK6 и FBLN1 выполняют защитную функцию при развитии злокачественного новообразования, в частности, KLK6 ингибирует эпителиально- мезенхимальную трансформацию [13], а FBLN1 подавляет инвазию опухолевых клеток [14]. Также у пациентов в группах с неоплазиями шейки матки снижается уровень белка SERPINB3 [15], участвующего в иммунном ответе против опухолевых клеток и индуцирующего эпителиально- мезенхимальные трансформации, и повышается уровень SERPINB4, являющегося ингибитором протеазы и участвующим в модуляции иммунного ответа организма против опухолевых клеток [16].
Среди белков, значимо изменяющиеся в группах ASCUS, LSIL, HSIL, стоит отметить увеличение уровня белков TGM1 и ANXA3, участвующих в клеточной пролиферации и миграции эндотелиальных клеток соответственно (табл. 2). Наблюдается повышение уровня белка GM2A, коррелирующее с активностью опухолевых клеток [17]. Увеличение уровня белка SERPINB13, одной из функций которого является снижение апотоза кератиноцитов, может быть связано с выработкой атипическими клетками кератина и дальнейшим его накоплением [18].
Проводился анализ достоверно изменяющихся белков для каждой из трех групп ASCUS, LSIL, HSIL, попарно с NILM (табл. 2). Были выявлены 9 белков, которые достоверно изменяются во всех трех группах, среди них белки FBLN1, KLK6. Среди данных потенциальных биомаркеров неопластической трансформации эпителия шейки матки следует особо отметить белок S100A9 [19].
В результате в данной работе были определены белковые панели, специфичные для различных форм ВПЧ-ассоциированных поражений шейки матки (ASCUS, LSIL и HSIL). Первая группа белков (P4HB, HSPA8, C4BPA и др.) характеризуют ранние изменения, ассоциированные с ВПЧ-инфекцией, и связана с поражением эпителия шейки матки, в том числе с проникновением вируса в клетку и его транскрипцией, нарушением функции системы комплемента. Вторая группа белков (PRDX5, YWHAE, LRG1 и др.) принимает непосредственное участие в развитие неоплазий шейки матки и их прогрессировании и характеризует поздние изменения, в частности, снижение процесса апоптоза, нарушение дифференцировки и созревания эпителия, трансформации атипических клеток. Таким образом, анализ протеома ЦВЖ позволяет изучать молекулярные механизмы патогенеза ВПЧ-ассоциированных заболеваний шейки матки и дифференцировать изменения эпителия на ранних этапах развития.



