Phenotypic profile of peripheral blood mononuclear cells in preeclampsia
Objective: To investigate the phenotypic profile of mononuclear cells in the peripheral blood of pregnant women with preeclampsia.Krasnyi A.M., Kan N.E., Mirzabekova D.D., Tyutyunnik V.L., Panasenko E.A., Sadekova A.A.
Materials and methods: This study included 38 pregnant women. The study group included 20 women (10 with mild preeclampsia and 10 with severe preeclampsia). The control group comprised 18 women with healthy pregnancies. Flow cytometry was used to determine the expression by monocytes and lymphocytes of costimulatory inflammatory factors CD40, CD80, CD86, surface signaling molecules of the "don't eat me" pathway CD24 and CD47, surface costimulatory receptors CD28 and CD152, and Fc receptor CD16.
Results: Lymphocytes in the blood of pregnant women in the study group had increased expression of CD28 and CD16, monocytes had increased expression of CD152 and CD86, and there was a higher content of monocytes expressing CD16 in this group. Correlation analysis showed a relationship between the level of expression of CD152 and CD86 in monocytes and the content of monocytes expressing CD16 in women in both groups.
Conclusion: The relationship between the expression levels of CD16, CD152, and CD86 indicates the possibility of their involvement in the same CD86-mediated signaling pathway leading to the activation of the CD152 receptor, followed by the expression of the CD16 Fc receptor. These findings suggest that these factors may be potential markers for PE.
Authors’ contributions: Krasnyi A.M., Kan N.E., Mirzabekova D.D., Tyutyunnik V.L., Panasenko E.A., Sadekova A.A. – conception and design of the study, data collection and analysis, review of the relevant literature, statistical analysis, manuscript drafting, and editing.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Krasnyi A.M., Kan N.E., Mirzabekova D.D., Tyutyunnik V.L., Panasenko E.A., Sadekova A.A. Phenotypic profile of peripheral blood mononuclear cells in preeclampsia.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2023; (5): 68-74 (in Russian)
https://dx.doi.org/10.18565/aig.2023.27
Keywords
Preeclampsia (PE) remains one of the most common pregnancy complications of unclear etiology and is associated with adverse maternal and fetal outcomes [1–3]. Preeclampsia is thought to result from placental abnormalities, leading to placental hypoxia and the subsequent release of factors into the peripheral bloodstream, causing an abnormal immune system response. Immune maladaptation can lead to various maternal and fetal pathological conditions; therefore, the immunological theory of preeclampsia contributes significantly to this complication. [2, 4–6].
The effects of inflammation in preeclampsia are manifested by alterations in mononuclear cell polarization in a pro- or anti-inflammatory pathway [7–9]. The expression of CD16, an Fc receptor mediating cell-mediated antibody-dependent cytotoxicity in peripheral blood, by monocytes is known to correlate with the severity of pre-eclampsia [10–12]. According to the literature, in addition to CD16 expression, the pro-inflammatory activity of monocytes may be related to the membrane expression of pro-inflammatory cofactors such as CD86 and CD40 [13, 14].
Besides, fetal and maternal cells may be protected by the expression of so-called antiphagocytic proteins belonging to the 'don't eat me' signaling pathway, such as CD47 and CD24, which transmit signals through interaction with signal-regulating protein alpha (SIRPα) and the inhibitory receptor Ig-like lectin 10 (Siglec-10), respectively [15–17]. This mechanism may have an anti-inflammatory effect on monocytes. However, studies examining this mechanism in preeclampsia are lacking.
It has been suggested that T-lymphocyte activation may be enhanced by the costimulatory molecules CD28 and CD152, which are homologous to each other and bind competitively to CD80 and CD86 ligands, with CD28 mediating a proinflammatory and CD152 anti-inflammatory phenotype in preeclampsia [18, 19].
Given the above, it is of great interest to study the phenotypic profile of mononuclear cells in preeclampsia, namely the content of monocytes and lymphocytes expressing inflammatory factors CD40, CD80, CD86, surface signaling molecules of the 'don't eat me' pathway CD24 and CD47, surface costimulatory receptors CD28 and CD152, Fc-receptor CD16 and their expression levels.
The study of the above immune factors in preeclampsia may reveal new pathogenetic mechanisms and provide a basis for the identification of new markers for the early diagnosis of this complication.
This study aimed to investigate the phenotypic profile of mononuclear cells in the peripheral blood of pregnant women with preeclampsia.
Materials and methods
The study was conducted at the V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia and was reviewed and approved by its Research Ethics Committee. All patients signed an informed consent form to participate in this study. The study included 38 pregnant women. The study group included 20 pregnant women with moderate-to-severe preeclampsia, and the control group consisted of 18 women with healthy pregnancies. The diagnosis of preeclampsia was established in accordance with the Clinical Practice Guidelines “Preeclampsia. Eclampsia. Edema, proteinuria, and hypertensive disorders during pregnancy, childbirth, and the postpartum period" (2021), developed by the Russian Society of Obstetricians and Gynecologists and approved by the Ministry of Health of the Russian Federation. Equipollent sampling was performed in both groups, with a median of 36 (33.6;38.6) weeks in the main group and 36.6 (36;37.4) weeks in the comparison group (p>0.05).
Inclusion criteria:
- For the study group: singleton pregnancies complicated by preeclampsia.
- For the control group: uncomplicated singleton pregnancy.
Exclusion criteria for both the groups:
- multiple pregnancies;
- autoimmune diseases and cancer;
- acute inflammatory disease;
- severe non-obstetric comorbidities.
The levels of CD28, CD80, CD86, CD40, CD152, CD24, CD47, and CD16 monocytes and lymphocytes expressed in the peripheral blood of pregnant women were determined by flow cytometry.
For phenotypic evaluation of mononuclear cells, whole blood samples were collected in sodium heparin tubes that were taken to the laboratory within 30 min. For analysis, 5 µL of blood was collected into three tubes, and 45 µL of PBS solution was added to each blood tube. In the next step, fluorescent-tagged antibody treatment was performed according to the manufacturer's protocol.
The analysis was performed by flow cytometry using a BD FACSCalibur flow cytometer (USA). The relative contents (%) of monocytes and lymphocytes were calculated as the number of each cell population relative to the total number of monocytes and lymphocytes. Expression of the factors studied by immune cells was assessed by the level of fluorescence of fluorescent-tagged antibodies to the antigens studied and is presented as fluorescence intensity relative units (RU).
Statistical analysis
Statistical analysis was performed using SPSS Statistics 17.0 and Microsoft Excel. Continuous variables were summarized as the median (Me) and interquartile range (Q1; Q3) and were compared with a nonparametric Mann–Whitney test; scaling (5%; Q1; Me; Q3; 95%) was plotted. Categorical variables were described as counts with percentages, and comparisons between groups were performed using the Fisher exact test. Spearman's coefficient was used for correlation analysis. Differences between the samples were considered statistically significant at p<0.05.
Results and discussion
The clinical and medical history characteristics of the patients in both the groups were comparable (Table 1).
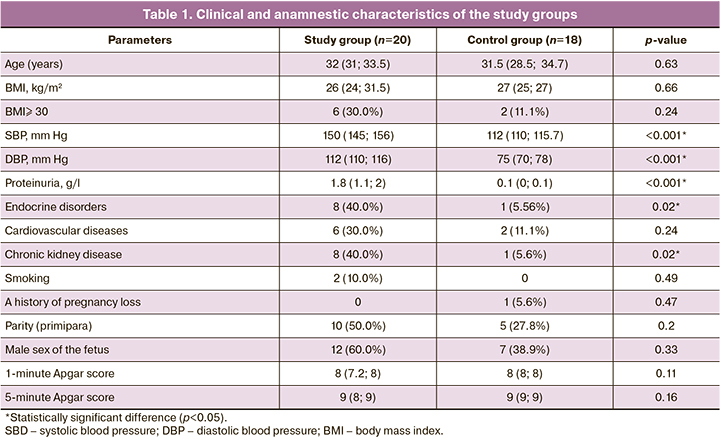
Pregnant women's age and BMI did not statistically differ between the study groups and were 32 (31; 33.5) years in the study group and 31.5 (28.5; 34.7) years in the control group (p=0.63); BMI was 26 (24; 31.5) and 27 (25; 27) kg/m2 by group, respectively (p=0.66).
Analysis of non-obstetric comorbidities revealed statistically significant differences (p=0.02) in the incidence of endocrine and chronic kidney disease; they occurred in eight women in the study group (40.0 %) and one case each in the control group. According to a number of researchers [20–22], cardiovascular diseases are considered risk factors for PE; no statistically significant difference (p=0.24) was found in our study. Potential risk factors such as smoking (p=0.49), parity (primiparous) (p=0.2), history of pregnancy loss (p=0.47), and male sex of the fetus (p=0.33) were also not statistically significantly different. The structure and frequency of gynecological diseases did not differ between the groups.
The SBP, DBP, and proteinuria levels were higher in the study group due to the inclusion criteria. Blood pressure amounted to SBP of 150 (145; 156) mm Hg and DBP of 112 (110; 116) mm Hg in the study group and SBP 112 (110; 115.7) mm Hg and DBP 75 (70; 78) mm Hg in the control group (p<0.001); the level of proteinuria was 1.8 g/l and 0.1 g/l, respectively (p<0.001).
It should be noted that there were no statistically significant differences in the babies' Apgar scores at birth between the study groups.
The CD28, CD80, CD86, CD40, CD152, CD24, CD47, and CD16 levels expressed by monocytes and peripheral blood lymphocytes of pregnant women were analyzed using flow cytometry to examine the phenotypic characteristics of lymphocytes and monocytes in the peripheral blood stream. The data obtained are listed in Table 2.
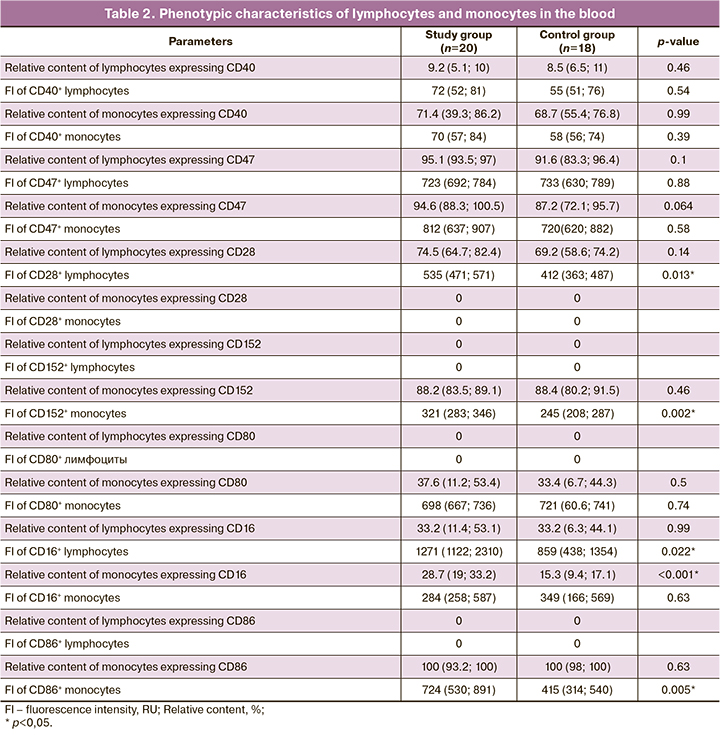
A higher CD16+ monocyte count was observed in preeclampsia (28.7% and 15.3 %, respectively) than in the control group, confirming previous findings [10, 23]. The relative proportion of lymphocytes expressing CD16 did not differ between groups (33.2%). The monocyte expression of CD16 did not differ between the groups and was 284 and 349 RU, respectively (p=0.63); however, the evaluation of lymphocyte expression revealed statistically significant differences (1271 and 859 RU) between the groups (p=0.022).
The hypothesis that the "don't eat me" mechanism might activate monocytes through the anti-inflammatory pathway in preeclampsia was not confirmed in this study as we found no statistically significant difference between the study groups. The relative CD47+ lymphocyte and CD47+ monocyte counts and CD47 expression levels in lymphocytes and monocytes did not differ.
The relative content of monocytes and lymphocytes expressing the proinflammatory factor CD40 and its expression level in PE also showed no statistically significant increase relative to the control group. The relative CD40+ lymphocyte content was 9.2% in the study group and 8.5% in the control group (p=0.46), while the relative CD40+ monocyte content was 71.4% and 68.7%, respectively (p=0.99). The expression levels of CD40 by lymphocytes were 72 and 55 (p=0.54) in the study and control groups, respectively; in monocytes, they were 70 and 58 units per group, respectively (p=0.39).
According to the literature, the costimulatory molecules CD28 and CD152, which are homologous to each other, are known to bind competitively to CD80 and CD86 ligands. CD28 and CD152 have opposite effects on T cell stimulation: CD28 provides a proinflammatory response and enhances cytotoxic activation of T lymphocytes, whereas CD152 implements an anti-inflammatory response [18, 19]. Despite data on CD152 expression on the surface of lymphocytes, Oyewole-Said D. et al. (2020) [24], Tiemann M. et al. (2021) [25] showed that this marker is also expressed by other immune cells, including monocytes. In this study, the expression of CD28 by lymphocytes, as well as CD152 and CD86 by monocytes, was found to be increased in preeclampsia, with monocytes expressing almost no CD28 and lymphocytes expressing CD152 and CD86. The relative proportion of lymphocytes expressing CD28 did not differ between the groups, and was 74.5% and 69.2%, respectively (p=0.14). The level of CD28 expression by lymphocytes in the PE group was significantly different from that in the control group at 535 RU (p=0.013). The relative content of monocytes expressing CD152 did not differ either, with an expression level of 321 RU in the study group and 245 RU in the control group (p=0.002).
The relative CD80+ monocyte count and CD80 expression levels in monocytes showed no significant differences. The relative content of CD86+ monocytes in both groups was 100%, but the expression level of CD86 by monocytes in the study group was significantly higher at 724 and 415 RU, respectively (p=0.005). The most significant results are shown in Figure 1.
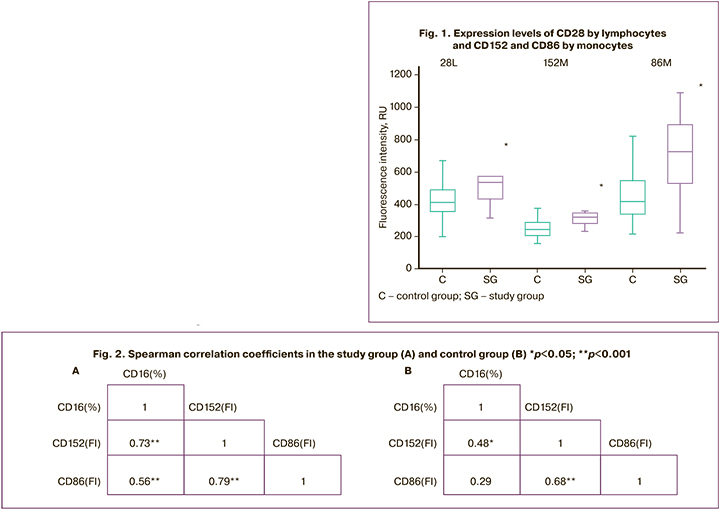
Correlation analysis revealed a strong correlation between the expression levels of CD152 and CD86 in monocytes and the relative amount of CD16+ monocytes (Fig. 2). This result may indicate a possible activation of the signaling pathway in which CD152 binds to the CD86 receptor, leading to monocyte expression of CD16. These findings also show that CD28 enhances the cytotoxic activity of T lymphocytes in preeclampsia.
Conclusion
Analysis of the phenotypic characteristics of immune cells potentially involved in the development of preeclampsia is not only of scientific importance, contributing to the understanding of the pathogenesis of preeclampsia, but is also of practical value. Thus, the increased expression of CD28 by lymphocytes and CD86 and CD152 by monocytes has diagnostic potential, and these factors can be considered novel markers of preeclampsia. The observed relationship between CD16, CD152, and CD86 indicates their possible involvement in the same signaling pathway. The study of this signaling pathway may reveal new mechanisms underlying this pregnancy complication, and establish diagnostic and prognostic criteria for preeclampsia.
References
- Министерство здравоохранения Российской Федерации. Преэклампсия. Эклампсия. Отеки, протеинурия и гипертензивные расстройства во время беременности, в родах и послеродовом периоде. Федеральные клинические рекомендации (протокол лечения). М.; 2021. 81с. [Ministry of Health of the Russian Federation. Preeclampsia. Eclampsia. Edema, proteinuria and hypertensive disorders during pregnancy, childbirth and the postpartum period. Federal clinical guidelines (treatment protocol). Moscow; 2021. 81p.(in Russian)].
- ACOG Practice Bulletin No. 202: Gestational hypertension and preeclampsia. Obstet. Gynecol. 2019; 133(1): 1. https://dx.doi.org/10.1097/AOG.0000000000003018.
- Melchiorre K., Giorgione V., Thilaganathan B. The placenta and preeclampsia: villain or victim? Am. J. Obstet. Gynecol. 2022; 226(Suppl. 2): S954-62.https://dx.doi.org/10.1016/j.ajog.2020.10.024.
- Yagel S., Cohen S.M., Goldman-Wohl D. An integrated model of preeclampsia: a multifaceted syndrome of the maternal cardiovascular-placental-fetal array. Am. J. Obstet. Gynecol. 2022; 226(Suppl. 2): S963-72. https://dx.doi.org/10.1016/j.ajog.2020.10.023.
- El-Sayed A.A.F. Preeclampsia: a review of the pathogenesis and possible management strategies based on its pathophysiological derangements. Taiwan. J. Obstet. Gynecol. 2017; 56(5): 593-8. https://dx.doi.org/10.1016/j.tjog.2017.08.004.
- Overton E., Tobes D., Lee A. Preeclampsia diagnosis and management. Best Pract. Res. Clin. Anaesthesiol. 2022; 36(1): 107-21. https://dx.doi.org/10.1016/j.bpa.2022.02.003.
- Callahan M.K., Postow M.A., Wolchok J.D. Targeting T cell co-receptors for cancer therapy. Immunity. 2016; 44(5): 1069-78. https://dx.doi.org/10.1016/j.immuni.2016.04.023.
- Attanasio J., Wherry E.J. Costimulatory and coinhibitory receptor pathways in infectious disease. Immunity. 2016; 44(5): 1052-68. https://dx.doi.org/10.1016/j.immuni.2016.04.022.
- Zhang Q., Vignali D.A. Co-stimulatory and co-inhibitory pathways in autoimmunity. Immunity. 2016; 44(5): 1034-51. https://dx.doi.org/10.1016/j.immuni.2016.04.017.
- Tang M.X., Zhang Y.H., Hu L., Kwak-Kim J., Liao A.H. CD14++ CD16+ HLA-DR+ monocytes in peripheral blood are quantitatively correlated with the severity of pre-eclampsia. Am. J. Reprod. Immunol. 2015; 74(2): 116-22. https://dx.doi.org/10.1111/aji.12389.
- Alahakoon T.I., Medbury H., Williams H., Fewings N., Wang X.M., Lee V.W. Characterization of fetal monocytes in preeclampsia and fetal growth restriction. J. Perinat. Med. 2019; 47(4): 434-8. https://dx.doi.org/10.1515/jpm-2018-0286.
- Yeap W.H., Wong K.L., Shimasaki N., Teo E.C., Quek J.K., Yong H.X. et al. CD16 is indispensable for antibody-dependent cellular cytotoxicity by human monocytes. Sci. Rep. 2016; 6: 34310. https://dx.doi.org/10.1038/srep34310.
- Peng Y., Luo G., Zhou J., Wang X., Hu J., Cui Y. et al. CD86 is an activation receptor for NK cell cytotoxicity against tumor cells. PLoS One. 2013; 8(12): e83913. https://dx.doi.org/10.1371/journal.pone.0083913.
- Horton H.M., Bernett M.J., Peipp M., Pong E., Karki S., Chu S.Y. et al. Fc-engineered anti-CD40 antibody enhances multiple effector functions and exhibits potent in vitro and in vivo antitumor activity against hematologic malignancies. Blood. 2010; 116(16): 3004-12. https://dx.doi.org/10.1182/blood-2010-01-265280.
- Bradley C.A. CD24 – a novel 'don't eat me' signal. Nat. Rev. Cancer. 2019; 19(10): 541. https://dx.doi.org/10.1038/s41568-019-0193-x.
- Hayat S.M.G., Bianconi V., Pirro M., Jaafari M.R., Hatamipour M., Sahebkar A. CD47: role in the immune system and application to cancer therapy. Cell. Oncol. (Dordr.). 2020; 43(1): 19-30. https://dx.doi.org/10.1007/s13402-019-00469-5.
- Barkal A.A., Brewer R.E., Markovic M., Kowarsky M., Barkal S.A., Zaro B.W. et al. CD24 signalling through macrophage Siglec-10 is a target for cancer immunotherapy. Nature. 2019; 572(7769): 392-6. https://dx.doi.org/10.1038/s41586-019-1456-0.
- Hattori H., Okano M., Yoshino T., Akagi T., Nakayama E., Saito C. et al. Expression of costimulatory CD80/CD86-CD28/CD152 molecules in nasal mucosa of patients with perennial allergic rhinitis. Clin. Exp. Allergy. 2001; 31(8): 1242-9. https://dx.doi.org/10.1046/j.1365-2222.2001.01021.x.
- Kennedy A., Waters E., Rowshanravan B., Hinze C., Williams C., Janman D. et al. Differences in CD80 and CD86 transendocytosis reveal CD86 as a key target for CTLA-4 immune regulation. Nat. Immunol. 2022; 23(9): 1365-78.https://dx.doi.org/10.1038/s41590-022-01289-w.
- Крецу В.Н., Савичева А.М., Ордиянц И.М. Факторы перинатального риска развития преэклампсии у беременных. Акушерство и гинекология: новости, мнения, обучение. 2020; 8(3): 16-9. [Cretsu V.N., Savicheva A.M., Ordiyants I.M. Perinatal risk factors for preeclampsia in pregnant women. Obstetrics and Gynecology: News, Opinions, Training. 2020; 8(3): 16-9.(in Russian)]. https://dx.doi.org/10.24411/2303-9698-2020-13002.
- Chaemsaithong P., Sahota D.S., Poon L.C. First trimester preeclampsia screening and prediction. Am. J. Obstet. Gynecol. 2022; 226(Suppl. 2): S1071-97. e2. https://dx.doi.org/10.1016/j.ajog.2020.07.020.
- Shen M., Smith G.N., Rodger M., White R.R., Walker M.C., Wen S.W. Comparison of risk factors and outcomes of gestational hypertension and pre-eclampsia. PLoS One. 2017; 12(4): e0175914. https://dx.doi.org/10.1371/journal.pone.0175914.
- Борис Д.А., Волгина Н.Е., Красный А.М., Тютюнник В.Л., Кан Н.Е. Прогнозирование преэклампсии по содержанию CD16-негативных моноцитов. Акушерство и гинекология. 2019; 7: 49-55. [Boris D.A., Volgina N.E., Krasnyi A.M., Tyutyunnik V.L., Kan N.E. Prediction of preeclampsia on the couts of CD-16 negative monocytes. Obstetrics and Gynecology. 2019; (7): 49-55. (in Russian)]. https://dx.doi.org/10.18565/aig.2019.7.49-55.
- Oyewole-Said D., Konduri V., Vazquez-Perez J., Weldon S.A., Levitt J.M., Decker W.K. Beyond T-cells: functional characterization of CTLA-4 expression in immune and non-immune cell types. Front. Immunol. 2020; 11: 608024. https://dx.doi.org/10.3389/fimmu.2020.608024.
- Tiemann M., Atiakshin D., Samoilova V., Buchwalow I. Identification of CTLA-4-positive cells in the human tonsil. Cells. 2021; 10(5): 1027.https://dx.doi.org/10.3390/cells10051027.
Received 31.01.2023
Accepted 04.04.2023
About the Authors
Aleksey M. Krasnyi, PhD, Head of the Cytology Laboratory, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology,Ministry of Health of Russia, +7(495)438-22-72, alexred@list.ru, https://orcid.org/0000-0001-7883-2702, 117997, Russia, Moscow, Ac. Oparina str., 4.
Natalia E. Kan, Professor, MD, PhD, Deputy Director of Science, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(926)220-86-55, kan-med@mail.ru, Researcher ID: B-2370-2015, SPIN-код: 5378-8437, Authors ID: 624900, Scopus Author ID: 57008835600, https://orcid.org/0000-0001-5087-5946, 117997, Russia, Moscow, Ac. Oparina str., 4.
Dzhamilia D. Mirzabekova, PhD student, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(920)984-94-07, Jamilya1705@yandex.ru, https://orcid.org/0000-0002-2391-3334, 117997, Russia, Moscow, Ac. Oparina str., 4.
Victor L. Tyutyunnik, Professor, MD, PhD, Leading Researcher at the Research and Development Service, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(903)969-50-41, tioutiounnik@mail.ru. Researcher ID: B-2364-2015, SPIN-код: 1963-1359,
Authors ID: 213217, Scopus Author ID: 56190621500, https://orcid.org/0000-0002-5830-5099, 117997, Russia, Moscow, Ac. Oparina str., 4.
Ekaterina A. Panasenko, Researcher at the Cytology Laboratory, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(495)438-22-72, e_panasenko@oparina4.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.
Alsu A. Sadekova, PhD, Researcher at the Cytology Laboratory, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(495)438-22-72, a_sadekova@oparina4.ru, https://orcid.org/0000-0003-4726-7477,
117997, Russia, Moscow, Ac. Oparina str., 4.



