Женщины со сниженным овариальным резервом и «бедным» ответом на овариальную стимуляцию (ОС) – распространенная (9–24%) сложная категория пациенток в программах экстракорпорального оплодотворения (ЭКО), характеризующаяся высокой частотой отмены циклов и низкой частотой наступления беременности [1]. Согласно классификации POSEIDON (Patient-Oriented Strategies Encompassing Individualized Oocyte Number), принятой в 2016 г., неоднородная когорта пациенток с «бедным» ответом на ОС стратифицируется на 4 группы в соответствии с возрастом, уровнем антимюллерова гормона (АМГ), количеством антральных фолликулов (КАФ) и исходом предыдущей ОС (рисунок) [2].
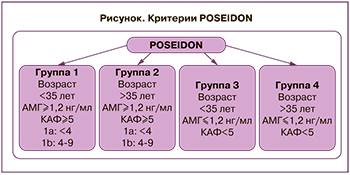
В настоящее время особый интерес вызывают пациентки в возрасте до 35 лет с нормальными параметрами овариального резерва (АМГ≥1,2 нг/мл, КАФ≥5) и непрогнозируемым «бедным» ответом на ОС (1-я группа POSEIDON), распространенность которого составляет 10–15% общего числа пациенток с нормальным овариальным резервом. Данная группа женщин демонстрирует лучший прогноз частоты наступления беременности по сравнению с пациентками старшего репродуктивного возраста и прогнозируемым «бедным» ответом на ОС [3, 4]. Необходимо детальное изучение патогенеза развития данного состояния для дальнейшей разработки алгоритма диагностики непрогнозируемого «бедного» ответа на ОС, а также персонифицированного подхода к ведению таких женщин, целью которого является увеличение количества полученных ооцитов в программе ЭКО [5].
Прогнозирование овариального ответа на гонадотропную стимуляцию является первостепенным этапом, определяющим успешность проведения программы ЭКО [6]. Несомненно, старший репродуктивный возраст женщины ассоциирован со снижением КАФ, меньшим количеством и худшим качеством ооцитов и эмбрионов, а также с низкой частотой наступления беременности [5]. По данным Li Y. et al. (2019), показатель кумулятивной частоты живорождения снижается в зависимости от возраста пациенток и их принадлежности к группам POSEIDON: 56,0, 30,1, 14,7 и 6,6% (POSEIDON 1, 2, 3 и 4 соответственно) [7]. В ретроспективном когортном исследовании Paffoni A. et al. (2022) также показано снижение частоты наступления беременности у женщин с неожиданным «бедным» ответом на ОС в зависимости от их принадлежности к возрастной группе: частота наступления беременности у женщин в возрасте <35 лет – 54% (p=0,036), 36–39 лет – 43% (p=0,048), 40–45 лет – 13% (p=0,72) [8].
К достоверным предикторам овариального ответа на стимуляцию, кроме возраста, также относят уровень АМГ, КАФ, уровень фолликулостимулирующего гормона (ФСГ) и индекс массы тела (ИМТ) женщины [9]. Однако эти маркеры не являются прогностически значимыми для пациенток с неожиданным «бедным» ответом на ОС при проведении программы ЭКО, что требует продолжения поиска значимых маркеров «бедного» ответа на ОС для 1-й группы POSEIDON.
Причины развития непрогнозируемого «бедного» ответа на ОС до конца не ясны. Обсуждаются вероятные механизмы, задействованные в развитии непрогнозируемого субоптимального и «бедного» ответа на гонадотропную стимуляцию: влияние митохондриальной дисфункции и оксидативного стресса, действие вредных факторов окружающей среды, асинхронный рост фолликулов в цикле стимуляции яичников, назначение субоптимальных доз гонадотропинов, возникновение технических сложностей, ассоциированных с введением триггера овуляции и/или проведением трансвагинальной пункции яичников (ТВП) [5, 10]. Однако последние достижения в области репродуктивной медицины привели к созданию фармакогенетического подхода к ОС в программах ЭКО, основываясь на изучении генетических особенностей каждой пациентки. Предполагается, что генетическая вариабельность является ключевым фактором, прогнозирующим неожиданный «бедный» ответ на ОС [2, 5, 11]. Существуют данные о целесообразности проведения генетического скрининга у пациенток группы POSEIDON 1, поскольку он может предоставить персонифицированную информацию о состоянии репродуктивной системы женщины. Был выявлен ряд генетических полиморфизмов гонадотропинов, стероидных гормонов и их рецепторов, АМГ, ароматазы Р450 и дифференцированного фактора роста (GDF9), способствующих нарушению чувствительности яичников к гонадотропной стимуляции [12]. Обсуждается целесообразность дальнейших исследований для наилучшего понимания вклада генетических полиморфизмов в генез развития «бедного» и субоптимального ответа на ОС, а также разработки для фармакогенетического подхода к ОС у данной группы пациенток.
Целью фармакогенетического подхода является установление взаимосвязи между генетическими особенностями конкретной пациентки и индивидуальной реакцией на лекарственные препараты в зависимости от фармакокинетики и фармакодинамики [13]. Известно, что около 90% генетических вариаций генома человека представлено точечными мутациями, называемыми однонуклеотидными полиморфизмами (single nucleotide polymorphism, SNP). Благодаря развитию секвенирования и возможности сравнения различных генетических последовательностей были открыты более 10 миллионов SNP. Однонуклеотидные полиморфизмы встречаются в кодирующих (экзоны) и некодирующих (интроны) областях генома, а также в участках между генами [12]. Разнообразие последовательностей ДНК напрямую влияет на функцию гена, что является причиной индивидуальной реакции организма на воздействие специфических препаратов, вакцин и реакции в ответ на патогены [14]. Существуют данные, предполагающие роль SNP в развитии непрогнозируемого «бедного» ответа на ОС у пациенток с сохраненными параметрами овариального резерва (POSEIDON 1). Однако последовательные алгоритмы диагностики, генетические панели для скрининга, а также подходы к лечению в соответствии с индивидуальными характеристиками пациенток пока не разработаны [5, 11].
Обсуждается целесообразность исследования генетического профиля пациенток для выбора оптимальной схемы ОС, определения необходимой дозы препаратов, разработки наиболее экономически выгодного плана лечения, увеличения эффективности программы ЭКО и, наконец, сокращения времени до наступления беременности [5].
Фолликулостимулирующий гормон и его рецепторы
Особо важная роль в процессах фолликулогенеза и стероидогенеза отводится ФСГ и его рецептору. Ген рецептора ФСГ (follicle stimulating hormone receptor, FSHR) расположен на коротком плече 2 хромосомы в положении 16.3 и включает 10 экзонов и 9 интронов [15]. Активно обсуждаются значимость FSHR, экспрессирующегося гранулезными клетками, в физиологическом воздействии ФСГ на организм, а также его участие в патогенезе развития «бедного» ответа на ОС у пациенток группы POSEIDON 1.
В научной литературе все чаще встречаются исследования, направленные на изучение наиболее значимых однонуклеотидных полиморфизмов гена FSHR, локализующихся на 10 экзоне: FSHR 2039 A>G (Ash680Ser, rs6166), FSHR 919 A>G (Thr307Ala, rs6165), FSHR -29 G>A (rs1394205), FSHB -211 G>T (rs10835638) и их участие в развитии недостаточного ответа на ОС в программах ЭКО [5, 6, 11, 15]. Однако, несмотря на наличие некоторых противоречивых данных в научной литературе, достаточно доказательств, подтверждающих положительную корреляцию между генетической изменчивостью гена FSHR и результатами ОС [16].
В исследовании Kalinderi К. et al. (2018) установлено, что пациентки с генотипом Ser/Ser полиморфизма гена FSHR 2039 A>G (Asn680Ser) демонстрируют высокий уровень базального ФСГ, нуждаются в повышенных дозах рекомбинантного ФСГ (рФСГ), а также имеют меньшее количество аспирированных ооцитов в программе ЭКО по сравнению с пациентками с генотипом Asn/Asn (р=0,04) [12].
Bayraktar B. et al. (2022) подтвердили данные о влиянии полиморфизма гена FSHR 2039 A>G на развитие непрогнозируемого «бедного» ответа на ОС. Так, у гомозиготных носителей генотипа Ser/Ser данного полиморфизма отмечался более низкий уровень эстрадиола в день ТВП, и пик прогестерона в лютеиновую фазу стимулированного цикла был ниже (р=0,018 и р=0,016 соответственно) по сравнению с носителями генотипа Asn/Ser. Статистически значимое снижение количества аспирированных ооцитов наблюдалось у пациенток с генотипом Ser/Ser в сравнении с генотипом Asn/Ser (р=0,009). Соответственно, число пациентов с «бедным» ответом на ОС было выше в группе носителей генотипа Ser/Ser полиморфизма гена FSHR 2039 A>G (р=0,011) [17]. Эти данные также отмечены в метаанализе, проведенном Pabalan N. et al. в 2014 г., что подтверждает целесообразность применения повышенных доз рФСГ у носителей данных полиморфизмов для преодоления низкой чувствительности яичников на ОС [18].
В работе Mayorga P. et al. (2000) была продемонстрирована необходимость назначения меньших доз рФСГ во время ОС у женщин с генотипом Asn/Asn полиморфизма гена FSHR 2039 A>G по сравнению с носителями генотипа Ser/Ser для достижения сопоставимой концентрации эстрадиола в день введения триггера овуляции, что указывает на меньшую чувствительность к ФСГ и более слабый ответ на препараты гонадотропинов у носителей генотипа Ser/Ser данного полиморфизма [15].
Систематический обзор и метаанализ 2018 г., включающий 4425 пациенток с бесплодием, показали, что количество полученных ооцитов было значительно ниже среди женщин с генотипом Ser/Ser полиморфизма гена FSHR 2039 A>G по сравнению с генотипом Asn/Asn (p=0,01), что также указывает на корреляцию гомозиготного носительства аллеля 680Ser FSHR 2039 A>G и низкой чувствительности к рФСГ в протоколах ЭКО [2].
К аналогичным результатам пришли Alviggi С. et al. (2016), продемонстрировав, что полиморфизм гена FSHR 2039 A>G (Asn680Ser) имел прямую корреляцию с неожиданным «бедным» ответом на ОС у женщин с генотипом Ser/Ser (р=0,02) [19]. В метаанализе, проведенном Tang H. et al. (2015), сообщается, что количество ооцитов, полученных после ТВП, было значительно ниже у женщин с генотипом Ser/Ser полиморфизма гена FSHR 2039 A>G по сравнению с женщинами с генотипами Asn/Asn и Asn/Ser (P<0,001) при получении одинаковых доз рФСГ. Однако не было отмечено разницы показателя частоты наступления беременности в разных группах (P=0,454) [20]. Эти данные также подтверждаются в исследовании Huang X. et al. (2015) [21].
При исследовании полиморфизма гена FSHR 919 A>G в работе Yan Y. et al. (2013) продемонстрировано, что у пациенток с гомозиготным генотипом Ala/Ala полиморфизма гена FSHR 919 A>G отмечался более высокий уровень базального ФСГ (p<0,05), и им требовалось проведение более продолжительной ОС по сравнению с другими женщинами. Также у данных пациенток отмечался высокий риск неожиданного «бедного» ответа на ОС (р=0,0001) [22].
Однако в метаанализе, проведенном Achrekar S. et al. (2009), не было установлено существенной разницы в среднем количестве полученных ооцитов среди носителей полиморфизма гена FSHR 919 A>G (Thr307Ala) с разными вариантами генотипов у пациенток с сохраненными параметрами овариального резерва [23].
При изучении полиморфизма гена FSHR -29 G>A (rs1394205), согласно данным исследования Maruška Č. et al. (2019), было показано, что у пациенток с генотипом G/G полиморфизма гена FSHR -29 G>A отмечался более низкий уровень АМГ (р=0,016), что требовало применения более высоких доз рФСГ в протоколах ОС (р=0,036) по сравнению с носителями A/A или A/G генотипов данного полиморфизма. Авторы также отмечают, что пациентки с генотипом А/А полиморфизма гена FSHR 2039 A>G имели более высокий уровень базального ФСГ, чем пациентки с генотипами A/G или G/G (р=0,043) [24].
В другом исследовании Desai S.S. et al. (2011) генотип А/А полиморфизма гена FSHR -29 G>A (rs1394205) также ассоциировался с получением меньшего количества ооцитов в результате программ ЭКО по сравнению с носителями генотипа G/G данного полиморфизма (р=0,001). Результаты исследования также подтверждают предыдущие выводы о целесообразности применения более высоких доз препаратов ФСГ в связи с низкой чувствительностью у гомозиготных носителей аллеля А полиморфизма гена FSHR -29 G>A [25].
Polyzos N. et al. (2021) обнаружили, что наличие носительства аллеля G полиморфизма гена FSHR 2039 A>G напрямую коррелирует с получением меньшего количества ооцитов по сравнению с генотипом А/А (95% CI -2,82–-0,11). В свою очередь, аллель А полиморфизма гена FSHR -29 G>A (rs1394205) ассоциируется со значительным снижением количества полученных ооцитов по сравнению с генотипом G/G (95% CI -3,06–-0,31). Также отмечалось наибольшее количество женщин с «бедным» ответом на ОС с генотипом A/G полиморфизма гена FSHR 2039 A>G (55,9%, n=57) по сравнению с генотипом А/А данного полиморфизма (95% CI 1,08–3,24) [26].
Как известно, α-субъединица является общей для ряда гормонов: ФСГ, лютеинизирующего гормона (ЛГ), тиреотропного гормона и хорионического гонадотропина человека (ХГЧ); тогда как β-субъединица (FSHB) содержит в себе специфическую для гормона информацию и отвечает за связывание с FSHR. Ген FSHB содержит промоторный полиморфизм -211 G>T (rs10835638), который локализуется выше участка начала транскрипции [27].
В исследовании Trevisan C. et al. (2019) отмечено, что носители генотипа G/T полиморфизма гена FSHB -211 G>T чаще демонстрировали «бедный» ответ на ОС по сравнению с генотипом G/G (47,4% против 26,5%, p=0,010), имели меньшее КАФ (8,0 против 10,0, p=0,03) на момент вступления в программы ЭКО, количество полученных ооцитов (3,0 против 5,0, p=0,03), количество ооцитов MII (3,0 против 4,0, p=0,02) и число эмбрионов (2,0 против 3,0, p=0,02) [28]. В нескольких исследованиях также отмечается повышение базального уровня ФСГ у носителей гомозиготного генотипа T полиморфизма гена FSHB -211 G>T [27, 29].
В связи с ограниченностью данных необходимо проведение дальнейших исследований о вкладе различных SNP гена FSHR в формирование непрогнозируемого «бедного» ответа на ОС в программе ЭКО.
Лютеинизирующий гормон и β-субъединица ЛГ
ЛГ является гетеродимерным гликопротеином. Он, как и другие гипофизарные гормоны, необходим для нормальной работы репродуктивной системы. ЛГ активно принимает участие в процессах фолликулогенеза, овуляции и непосредственно в лютеинизации доминантного фолликула, а также способствует экспрессии андрогенов, являющихся субстратом для синтеза эстрадиола в яичниках, воздействуя через рецепторы ЛГ (LHR/LHСGR) на поверхности тека клеток. LHR также известен как LHСGR, поскольку ЛГ и ХГЧ являются эндогенными лигандами для рецептора ЛГ [30]. Ген LHСGR состоит из 11 экзонов, расположен на коротком плече 2 хромосомы в положении 16.3. Известно, что ген LHСGR имеет более 520 SNPs и, по крайней мере, 300 полиморфизмов гена LHCGR, вероятно, оказывающих влияние на состояние репродуктивной системы [31].
По данным ретроспективного исследования Ramaraju G. et al. (2021), наблюдалось получение большего числа аспирированных ооцитов, МII ооцитов, эмбрионов и повышение частоты наступления беременности (р=0,049) при добавлении препаратов ЛГ пациенткам с генотипами А/А и А/G полиморфизма rs2293275 (Ser312Asn) гена LHCGR [32].
ЛГ также состоит из α- и β-субъединиц. Известно, что гормоны ФСГ и ЛГ содержат общую α-субъединицу, в то время как β-субъединица является гормонспецифичной и содержит рецепторсвязывающий домен. Она локализуется на хромосоме 11p13 и имеет 3 экзона, для гена β-субъединицы ЛГ также характерно наличие полиморфизма. Наиболее часто встречающимся вариантом является полиморфизм гена v-β-ЛГ, обладающий меньшей биологической активностью [33].
В 2013 г. Alviggi C. et al. было проведено ретроспективное исследование, включающее 220 пациенток с нормальными параметрами овариального резерва. Частота встречаемости полиморфизма гена v-β-ЛГ составила 10,9%. Также представлены данные о необходимости применения более высоких доз рФСГ у пациенток с полиморфизмом гена v-β-ЛГ по сравнению с пациентками с распространенным генотипом для получения оптимального овариального ответа на стимуляцию яичников (2435,86±932,8 ЕД против 1959,8±736,45 ЕД; p=0,048) [33]. По результатам обсервационного исследования Altmae S. et al. (2011), проведенного с участием 60 женщин с нормальными параметрами овариального резерва, отмечено, что пациентки с полиморфизмом гена v-β-ЛГ были менее восприимчивы к препаратам рФСГ, и этим пациенткам целесообразно назначение более высоких доз рФСГ в связи со сниженной чувствительностью к данным препаратам [34]. Обсуждается целесообразность использования препаратов рЛГ вместо увеличения дозы рФСГ у пациенток с полиморфизмом гена v-β-ЛГ [35].
Эстрогены и эстрогеновые рецепторы
Хорошо изучено активное участие эстрогенов в процессах фолликулогенеза, овуляции и подготовке эндометрия к процессу имплантации эмбриона. Эстрогены оказывают действие через эстрогеновые рецепторы (ЕSRs). Гены рецепторов эстрогенов расположены на длинных плечах хромосом 6 (6q25.1) и 14 (14q23.2) соответственно [12]. Выявлено, что ген ESR1 достаточно полиморфен и включает около 2200 SNPs, в то время как ген ESR2 содержит около 720 SNPs [12, 36].
В литературе встречаются данные о связи полиморфизмов rs2234693 гена ESR1 и rs4986938 гена ESR2 c развитием непрогнозируемого «бедного» ответа на ОС у пациенток с сохраненными параметрами овариального резерва. Так, в исследовании DeMattos C. et al. (2014) была отмечена прямая корреляция генотипа Т/Т полиморфизма rs2234693 гена ESR1 и генотипа G/G полиморфизма rs4986938 гена ESR2 с более продолжительным проведением ОС в программе ЭКО и указана целесообразность применения повышенных доз препаратов гонадотропинов (p<0,03) [36]. Также в работе Čuš М. et al. (2019) выявлена ассоциация генотипа Т/Т полиморфизма rs2234693 гена ESR1 с более частым развитием «бедного» ответа на ОС [24].
По данным исследования Boudjenah R. et al. (2012), было получено большее количество зрелых ооцитов у пациенток, гомозиготных по G аллелю полиморфизма rs49866938 гена ESR2, по сравнению с гетерозиготными носителями по А аллелю (8,1±4,2 и 7,2±4,0 соответственно; p=0,0017). Авторами показано, что гомозиготным носителям по А аллелю rs49866938 полиморфизма гена ESR2 требовалась большая доза рФСГ (2706±879МЕ) во время ОС, чем женщинам, гомозиготным или гетерозиготным по G аллелю данного полиморфизма, для получения сопоставимого числа ооцитов (2375±752 МЕ и 2145±744 МЕ соответственно) [37]. Motawi T. et al. (2017) обнаружили взаимосвязь сочетания генотипа А/А полиморфизма rs49866938 гена ESR2 и генотипа Ala/Ala полиморфизма гена FSHR 919 A>G в генезе развития непрогнозируемого «бедного» ответа на ОС у пациенток в возрасте <35 лет (p<0,001) [38].
Напротив, в исследовании Sindiani А. et al. (2021) не наблюдалось статистически значимой разницы в исходах ОС у пациенток – носителей полиморфизма rs4986938 гена ESR2 и полиморфизма гена FSHR 919 A>G (Thr307Ala) (p=0,433 и p=0,696 соответственно) [39]. Также противоречивые данные были получены Altmae S. et al. (2007), указывающие на то, что, вероятно, наличие полиморфизма rs4986938 гена ESR2 не влияет на результаты ОС в программах ЭКО [40]. В связи с противоречивыми данными необходимо проведение дальнейших исследований для определения влияния полиморфизмов эстрогеновых рецепторов на ОС в программах ЭКО.
Антимюллеров гормон и его рецептор
АМГ считается наиболее значимым прогностическим маркером эффективности программы ЭКО, отражающим репродуктивный потенциал женщины. АМГ представляет собой гликопротеин, относящийся к суперсемейству трансформирующего фактора роста β (TGF-β). Экспрессия гормона происходит гранулезными клетками преантральных и малых антральных фолликулов. АМГ кодируется геном AMH, локализованным на 19 хромосоме. АМГ действует через рецептор АМГ II типа (AMHR2), который кодируется геном, расположенным на длинном плече 12 хромосомы 12q13 [41, 42].
В работе Čuš M. et al. (2019) продемонстрировано, что носителям генотипа G/G полиморфизма rs3741664 гена AMHRII требовалось применение более высоких доз рФСГ во время ОС по сравнению с носителями генотипа A/G данного полиморфизма у женщин с непрогнозируемым «бедным» ответом на ОС [24].
В работе Yoshida Y. et al. (2014) показано, что количество полученных ооцитов (28,5%) в программе ЭКО и частота наступления беременности (0%) были значительно ниже у носителей генотипа G/G полиморфизма rs2002555 гена AMHR II [43]. Однако в исследовании Meireles A. et al. (2021) эти данные опровергаются [44]. В связи с единичными исследованиями и отсутствием однозначного мнения о роли полиморфизма гена AMHR II в развитии непрогнозируемого «бедного» ответа на ОС, необходимо продолжить исследования на более крупной выборке пациенток.
Ароматаза (CYP19)
Цитохром Р450 является основным ферментом, участвующим в синтезе эстрогенов из андрогенов, и включает в себя 2 вида цитохрома: Р450 редуктаза и Р450 ароматаза. Цитохром Р450 ароматаза является основным ферментом и кодируется геном CYP19A1, состоящим из 9 кодирующих экзонов (II–Х)
и 5’-нетранслируемой области. Он расположен в коротком плече 15-й хромосомы 15q21.1 и экспрессируется во множестве тканей, таких как гранулезные клетки яичников, желтое тело, молочные железы, печень, мышцы, а также головной мозг [45]. Генетические изменения по данному локусу могут изменить активность ароматазы и в результате влиять на продукцию стероидных гормонов яичниками. Наиболее изученным полиморфизмом гена CYP19 является короткий тетрануклеотидный тандемный повтор (ТТТА)n, локализующийся в 4-м интроне CYP19 и участвующий в регуляции экспрессии стероидных гормонов [12, 46].
Altmäe S. et al. (2009) предположили связь малого числа повторов (ТТТА)n в гене CYP19, в частности del-(ТТТА)7, со сниженным КАФ (r=0,21; р=0,018), а также со снижением чувствительности яичников на гонадотропную стимуляцию (р=0,039), что потребовало увеличения дозы рФСГ для достижения лучшего результата [47]. В работе Lazaros A. et al. (2012) было показано, что у женщин, гомозиготных по коротким CYP19 (TTTA)n аллелям, включая носителей CYP19 (TTTA) 7 аллеля, отмечались более высокие уровни ФСГ на 3-й день менструального цикла (p<0,003), меньшее КАФ (p<0,02) и меньшее число фолликулов в день ТВП (p<0,01) по сравнению с женщинами с длинными аллелями. Однако разницы в частоте наступлении беременности отмечено не было. Авторы предполагают, что высокий сывороточный уровень ФСГ связан с отрицательным влиянием коротких CYP19 (TTTA)n аллелей на активность ароматазы. Данные результаты свидетельствуют о вероятной целесообразности определения количества повторов CYP19(TTTA)n в качестве предиктора неожиданного «бедного» ответа на ОС, что может помочь в разработке персонифицированного подхода к ОС у таких пациенток [48]. Однако необходимо проведение дальнейших исследований на большей выборке пациенток.
Дифференцированный фактор роста GDF9
Дифференцированный фактор роста 9 (Growth differentiation factor 9, GDF9) относится к суперсемейству TGF-β. Ген GDF9 локализуется на 5-й хромосоме 5q31.1 [12]. Он активно экспрессируется ооцитами и принимает участие в процессах раннего фолликулярного роста, регулируя экспрессию генов на преовуляторной стадии, стимулирует пролиферацию клеток гранулезы и рост клеток кумулюса, а также ингибирует апоптоз фолликулов [12, 49]. GDF9 способствует поддержанию метаболических каскадов, таких как гликолиз и биосинтез стероидов, и индуцирует сигнальный путь EP2, необходимый для синтеза прогестерона в клетках гранулезы [50, 51]. Существуют исследования, указывающие на повышенные уровни гормонов ФСГ и ЛГ, меньший объем яичников, а также отсутствие развития фолликулогенеза у мышей с нокаутом по данному гену [52].
В настоящее время изучается связь полиморфизма гена GDF9 с развитием «бедного» ответа на ОС. В исследовании SerdyńskaSzuster M. et al. (2016) продемонстрирована связь генотипов G/A и G/G полиморфизма rs10491279 гена GDF9 (P=0,0008) с развитием «бедного» ответа на ОС и снижением частоты оплодотворения [53].
Bilibio J. et al. (2020) обнаружили, что редкий аллель полиморфизма гена GDF9 C398G у пациенток с нормальными параметрами овариального резерва напрямую ассоциируется со снижением числа преовуляторных фолликулов >17 мм в день введения триггера овуляции (4,33 vs. 6,49, p=0,001), уменьшением количества зрелых ооцитов (5,38 vs. 8,84, p=0,017) по сравнению с группой контроля [54]. Аналогичные данные были получены в работе Meireles A. et al. [55].
Таким образом, единичные исследования свидетельствуют о вкладе полиморфизмов гена GDF9 в формирование неожиданного «бедного» ответа на ОС в программе ЭКО.
Заключение
Несомненно, применение фармакогенетического подхода к ведению пациенток с нормальными параметрами овариального резерва и непрогнозируемым «бедным» и субоптимальным ответом на ОС в программе ЭКО является многообещающей концепцией в развитии персонализированной медицины. Несмотря на исследования, проведенные с целью изучения патогенетических причин развития непрогнозируемого «бедного» ответа на ОС, роль однонуклеотидных полиморфизмов генов гонадотропинов, стероидных гормонов и их рецепторов, АМГ, ароматазы Р450 и дифференцированного фактора роста (GDF9) в генезе развития непрогнозируемого «бедного» и субоптимального ответа на ОС до конца не ясна, что требует продолжения дальнейших исследований. Априорное понимание индивидуальной генетической вариабельности в популяции пациенток с непрогнозируемым «бедным» овариальным ответом и сохраненными параметрами овариального резерва, наряду с известными клинико-лабораторными маркерами, поможет в разработке персонифицированных протоколов ОС в программе ЭКО.



