Генитоуринарный менопаузальный синдром (ГУМС) – хроническое заболевание, возникающее в перименопаузальном возрасте, характеризующееся широким спектром субъективных симптомов и клинических признаков, таких как сухость влагалища, зуд, жжение, нарушения мочеиспускания, снижение любрикации и диспареуния, вызванных недостатком эстрогенов, и имеющее прогрессирующие течение [1–3]. Кроме того, ГУМС в значительной степени связан со стрессовым или смешанным недержанием мочи, гиперактивным мочевым пузырем и пролапсом стенок влагалища [4]. Симптомы ГУМС в той или иной степени присутствуют у 15% женщин в пременопаузе, тогда как у подавляющего большинства женщин (50-70%), они наблюдаются в постменопаузе [4]. При этом наиболее распространенным его проявлением является вульвовагинальная атрофия (ВВА) (снижение влажности – 93,7%, истончение стенок и исчезновение складок влагалища – 78,4%). Пациентки с ВВА по сравнению с женщинами без него в исследовании EVES имели более старший возраст (53 против 59 лет; р<0,001), тяжелые симптомы (индекс вагинального созревания <15 – 78 против 6%; р<0,05) и снижение сексуальной функции (7 против 12 баллов; р<0,001) [5]. ГУМС оказывает влияние на качество жизни женщин в постменопаузе, особенно на половую функцию, самовосприятие и образ тела [6].
В отличие от вазомоторных симптомов, которые со временем становятся менее выраженными, симптомы ГУМС прогрессируют и имеют тенденцию ухудшаться, если их не лечить, и редко разрешаются спонтанно [7], что определяет необходимость их раннего выявления и своевременного начала терапии. Основной целью лечения является облегчение симптомов. Согласно консенсусу Северо-Американского общества по менопаузе (NAMS) 2020 г., лечение первой линии при легких проявлениях состоит из негормональной терапии, такой как любриканты и увлажняющие средства, особенно для сексуально активных женщин; в то время как местная гормональная терапия считается «золотым стандартом» при среднетяжелом и тяжелом течении, или когда результаты лечения другими средствами неудовлетворительны [8]. Данное положение поддерживается многими авторами [1, 9–11]. Однако систематический обзор и метаанализ РКИ, выполненный в 2021 г. показал, что увлажняющие средства уступают эстрогенам по всем параметрам половой функции [12]. Новые терапевтические подходы с селективными модуляторами рецепторов эстрогена или лазерными технологиями рассматриваются как альтернативные варианты, но необходимы дальнейшие исследования для изучения эффективности и возможности внедрения в клиническую практику [1]. Долгосрочная безопасность вагинального дегидроэпиандростерона и агонистов/антагонистов эстрогенов отсутствуют [8, 10].
Слизистая оболочка нижних отделов полового тракта и мочевыделительной системы очень чувствительна к воздействию эстрогенов [1], что определяет патогенетическую обоснованность применения менопаузальной гормональной терапии (МГТ) эстрогенами. Мета-анализ 30 исследований продемонстрировал улучшение симптомов ВВА при использовании эстрогенов локально по сравнению с плацебо (ОШ=4,1; 95% ДИ: 1,9–8,9) [13]. Кроме того, ранее было показано, что местное применение эстриола лучше других средств устраняет атрофические изменения и эффективно у 80–90% пациенток, тогда как системные препараты МГТ эффективны в 50–75% случаев [14].
Эстриол применяется местно в низких дозах, как правило 0,05 мг на протяжении длительного времени (от 2–3 лет до пожизненного применения) [14–16]. В ряде исследований было продемонстрировано отсутствие системного действия и влияния на эндометрий, однако долгосрочные последствия и безопасность этого режима не достаточно изучены [1]. По данным Shifren J.L., вагинальные кольца с эстрогеном в очень низких дозах (7,5 мкг/день) или таблетки (10 мкг) не изменяли уровни эстрадиола в сыворотке, по сравнению с исходным уровнем и оставались в диапазоне от 3 до 11 пг/мл; в случае вагинальных эстрогеновых кремов (эстрадиол или конъюгированные эстрогены) концентрация эстрадиола в сыворотке варьировала в зависимости от частоты введения и дозы [10]. Имеется сообщение, что использование крема, содержащего эстрадиола валерат, может вызывать побочные эффекты, такие как болезненность молочных желез, гиперплазия эндометрия и вагинальное кровотечение у некоторых женщин [17]. Кроме того, эффект вагинальной терапии эстрогенами у женщин с тромбозом в анамнезе еще не изучен.
Ученые пришли к выводу, что препарат, содержащий эстроген, для лечения ГУМС должен отвечать таким требованиям, как уменьшение симптомов при отсутствии системного действия и стимуляции чувствительных к эстрогену тканей, кроме влагалища, с минимальными побочными эффектами. Одним из направлений поиска лекарственных средств, отвечающих этим требованиям, является снижение дозы эстрогена [16].
В предварительном исследовании эффективности и безопасности вагинального геля с 0,005% эстриола, содержащего ультранизкую дозу эстриола на одно применение (50 мкг), для местного лечения ВВА, показано его превосходство по сравнению с плацебо в отношении индекса созревания эпителия и рН влагалища, устранения сухости и улучшении общей оценки симптомов при отсутствии изменений толщины эндометрия по сравнению с исходным [18].
Таким образом, актуальным является изучение средств, содержащих эстриол в ультранизких дозах, для лечения ГУМС.
Цель исследования: сравнить эффективность и безопасность лекарственных препаратов для вагинального введения, содержащих эстриол 50 мкг/г (гель) и 500 мкг/0,5 г (крем), в терапии постменопаузального атрофического вагинита.
Материалы и методы
Организация исследования. Работа выполнена на базе 8 исследовательских центров России с 2016 по 2018 гг. При создании протокола исследования учитывались положения Хельсинкской декларации Всемирной Медицинской Ассоциации (пересмотр 64-й Генеральной ассамблеи WMA (Форталеза, Бразилия, 2013 г.), документа «Международные этические рекомендации по проведению биомедицинских исследований с участием людей» (International Ethical Guidelines for Biomedical Research Involving Human Subjects), Национальным стандартом РФ ГОСТ Р52379-2005 «Надлежащая клиническая практика», другими законодательными и нормативными документами РФ. Протокол одобрен Советом по Этике МЗ РФ (выписка из протокола № 133 от 20.09.2016 г.). Клиническое исследование проводилось в соответствии с одобренной версией 2.0 от 27.10.2016 г. протокола RCT-BLS-01/12. Все изменения в исходной версии протокола были одобрены МЗ РФ до начала набора пациентов. Все пациентки дали письменное информированное добровольное согласие на участие в исследовании.
Дизайн. Многоцентровое открытое проспективное рандомизированное сравнительное клиническое испытание в параллельных группах с активным контролем III фазы.
Контингент. В скрининге приняли участие 123 пациентки, из них в исследование были включены 120 пациенток в постменопаузе в течение не менее 1 года после естественной или вызванной хирургическим вмешательством менопаузы с симптомами и признаками атрофического вагинита (коды МКБ-10: N95.2 Постменопаузный атрофический вагинит. N95.3 Состояния, связанные с искусственно вызванной менопаузой).
Расчет выборки. Основан на данных, представленных в клиническом испытании III фазы Cano A. et al. [18] и допущении о том, что Блиссель гель вагинальный, содержащий эстриол 50 мкг/г, обладает не меньшей эффективностью, что и вагинальный гель с эстриолом 50 мкг/г в вышеупомянутом исследовании. Размер выборки рассчитывался по формуле для двух параллельных групп исследования не меньшей эффективности (Chow S.C., 2003). При расчете использовали показатель значения зрелости вагинального эпителия (ЗЗВЭ), изменение которого по данным литературы на 15 баллов считалось значимым с клинической точки зрения со стандартным отклонением (SD) от 12 до 23 баллов. Для расчета размера выборки было принято стандартное отклонение, равное 20. Учитывая эффект плацебо в предыдущем исследовании равный 5 баллам, считали, что выявление разницы ЗЗВЭ в 10 баллов или более между лечением с помощью изучаемого препарата и препарата сравнения может свидетельствовать о меньшей эффективности, учитывая α – значение допустимой ошибки I рода, 0,05; β – значение допустимой ошибки II рода, 0,2. Вывод о не меньшей эффективности двух препаратов может быть сделан при попадании 95% доверительного интервала (ДИ) разницы средних значений ЗЗВЭ после терапии на 12 неделе в интервал не меньшей эффективности (10 баллов). Расчет необходимого объема выборки проводился в статистическом пакете R 3.2.2. Количество пациенток для обеспечения рекомендованной (не менее 80%) мощности статистического теста при уровне значимости α=0,05 для проведения данного исследования должно составлять не менее 50 в каждой из групп (100 во всем исследовании). Учитывая 20% пациентов, которые могут досрочно выбыть из исследования или данных, не пригодных для анализа, планировалось изначально рандомизировать 120 пациенток (по 60 в группе). Так как за период исследования выбыло всего 2 пациентки и 1 исключена из анализа эффективности, выбывание составило 2,5% от общей популяции, из чего можно сделать вывод, что статистическая мощность исследования соблюдена.
Критерии включения: возраст от 40 до 65 лет; некурящие, индекс массы тела: ≤37 кг/м2 и ≥19 кг/м2, постменопауза не менее 1 года, наличие не менее 1 субъективного симптома ВВА с исходными результатами цитологического исследования мазка ≤5% поверхностных клеток, рН влагалища >5,0, отсутствие признаков гиперплазии эндометрия у женщин с интактной маткой, отрицательные результаты анализов на ВИЧ, гепатиты В и С, сифилис, выполненных в течение последних 6 месяцев или во время скрининга, согласие пациентки следовать процедурам протокола.
Критерии невключения: гиперчувствительность к эстриолу и вспомогательным компонентам препаратов исследования, наличие противопоказания к терапии эстрогенами, лечение вульвовагинита за 15 дней до начала исследования, применение гормональной терапии, фитоэстрагенов или растительного лекарственного средства для лечения симптомов постменопаузы в течение 3 месяцев до включения в исследование (в т.ч. интравагинальное применение), исходная концентрация эстрадиола в плазме крови на скрининге >30 пг/мл, сопутствующая патология со стороны малого таза (пролапс тазовых органов II и более степени, маточное кровотечение, гиперплазия эндометрия, лейомиома матки или эндометриоз, фиброзно-кистозная мастопатия или узлы молочной железы, менопауза, вызванная хирургическим вмешательством по поводу эндометриоза или эстрогензависимой злокачественной опухоли, идиопатическая венозная тромбоэмболия (тромбоз глубоких вен, тромбоэмболия легочной артерии) в анамнезе или в настоящее время, текущая или недавняя (в течение полугода) артериальная тромбоэмболическая болезнь (стенокардия, инфаркт миокарда, инсульт, заболевание периферических артерий, др.), тяжелая патология сердечно-сосудистой системы, наличие коагулопатии, сахарный диабет, гипотиреоз или другое эндокринное заболевание, заболевания печени в острой стадии или в анамнезе, порфирия, почечная недостаточность.
Критерии исключения: отказ пациентки от участия в исследовании, появление психотических симптомов, развитие одного из критериев невключения во время участия в исследовании, нарушение процедур протокола, развитие нежелательного явления, вызывающего неприемлемый риск для здоровья пациентки в случае продолжения его участия в исследовании, возникновение или обострение сопутствующих соматических заболеваний, не связанных с приемом лекарственных препаратов, вызывающих необходимость в изменении процедур терапии и наблюдения, несовместимых с протоколом клинического исследования, беременность.
Рандомизация. 120 пациенток с ВВА, отобранных по итогам скрининга, были разделены на две группы из общего списка методом генерации случайных чисел с использованием программы WinPepi 11.65 (модуль ETCETERA 3.26), исходя из предположения о равной вероятности распределения пациентов в каждую из двух групп (р=1/2). Последовательность случайного распределения формировалась IT-специалистом, зачисление участников и назначение вмешательств выполнял врач амбулаторного звена, к которому обращались пациентки. Ослепление не проводилось. Анализ полученных данных проводился сотрудниками, не связанными с ведением больных, участвующих в исследовании, для создания условий независимой оценки результатов.
I группа (n=60) – получали гель вагинальный, содержащий эстриол 50 мкг/г (Блиссель, производитель Италфармако С.А., Испания), 1 доза = 50 мкг (1 г геля).
II группа (n=60) – получали крем вагинальный, содержащий эстриол 1 мг/г, 1 доза = 500 мкг (0,5 г крема).
Исследуемый и сравниваемый препараты применялись пациентками самостоятельно в течение 3 недель (21 день) ежедневно 1 раз в день на ночь. Далее проводилась поддерживающая терапия со снижением дозы до 2 раз в неделю (9 недель). Общая продолжительность терапии – 12 недель.
Дизайн и рандомизация с учетом выбывших представлены на рисунке 1.
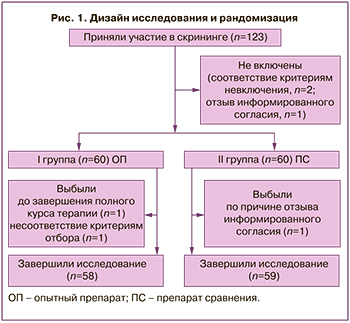
Методы исследования. Выполнялись: сбор демографических, антропометрических данных, анамнеза, регистрация жалоб, оценка субъективных симптомов заболевания, объективный осмотр, измерение жизненно важных параметров, гинекологический осмотр с оценкой клинических признаков ВВА и измерение рН влагалища, инструментальные обследования (трансвагинальное ультразвуковое исследование органов малого таза, измерение толщины М-эхо, ЭКГ, расширенная кольпоскопия), лабораторные исследования (клинический и биохимический анализ крови; общий анализ мочи; цитологическое исследование мазка с оценкой ЗЗВЭ; микроскопия мазка, окрашенного по Граму).
По результатам цитологического исследования мазка вычисляли величину ЗЗВЭ в баллах, которую рассчитывали по следующей формуле: ЗЗВЭ = 0,2 × (% парабазальных клеток) + 0,6× (% промежуточных клеток) + 1,0×(% поверхностных клеток).
Пациенткам был выдан дневник, который она заполняла самостоятельно, отмечая прием исследуемой и сопутствующей терапии, а также жалобы. Дневник просматривался исследователем на каждом визите пациентки для регистрации возможных нежелательных явлений (НЯ) в ходе исследования, а также контроля соблюдения пациенткой графика приема исследуемых лекарственных средств. Оценка всех признаков и симптомов НЯ, в том числе времени их возникновения и выраженности, проводилась ответственным исследователем на каждом визите и во время телефонных контактов с пациенткой со дня получения первой дозы препаратов исследования/сравнения до визита наблюдения.
Порядок обследования. Всего в исследовании планировалось проведение 4 визитов пациенток в исследовательский центр (скрининг, рандомизация, через 3 недели от начала терапии, через 12 недель в конце поддерживающей терапии) и 3 контакта по телефону (через 1 неделю после начала терапии, через 6 недель терапии и через 4 недели после окончания приема препаратов исследования/сравнения).
Критерии эффективности. Первичные: увеличение показателя ЗЗВЭ ≥15 баллов после 12 недель терапии в сравнении с исходным значением. Вторичные: увеличение показателя ЗЗВЭ после 3 недель терапии; частота облегчения или исчезновения симптомов ВВА, основанных на субъективной оценке (жалобах) пациентки, указанных на исходном визите, через 1, 3, 6 и 12 недель терапии; частота облегчения или исчезновения клинических признаков атрофии слизистой оболочки влагалища по данным гинекологического осмотра через 3 и 12 недель терапии, показатель рН влагалищного содержимого (изменение от исходного на 0,5 и более после 3 недель).
В настоящем сообщении представлен анализ эффективности в РР-популяции пациентов (Реr-Protocol population), отвечавших критериям включения/невключения, соблюдавших условия протокола и полностью завершивших курс лечения и запланированные периоды исследования.
Статистический анализ
Для статистической обработки результатов исследования использовали стандартный пакет статистических программ SPSS версии 22.0. Закон распределения признаков оценивали при помощи критерия Колмогорова–Смирнова. Количественные показатели представляли в виде М (SD), где М – среднее значение, а SD – среднее квадратичное отклонение, а также в виде Me (25%Q–75%Q), где Me – медианное значение показателя, а (25%Q–75%Q) – первый и третий квартили. Поскольку закон распределения большинства количественных показателей отличался от нормального, значимость различий оценивали при помощи непараметрических критериев. Анализ различий в независимых выборках проводили с помощью U-критерия Манна–Уитни. Качественные признаки отражали в виде абсолютных чисел (n) и относительных величин (%). Их различия оценивали при помощи критерия χ2. В данном исследовании принят уровень значимости р<0,05 при уровнях ошибки первого и второго рода α=5% и β=20% соответственно. Взаимосвязь лечебного фактора и исхода оценивали по величине ОШ с вычислением 95% ДИ по методу Woolf.
Результаты
Общая клиническая характеристика пациенток. Базовые демографические, анамнестические и клинические характеристики пациенток представлены в таблице 1.
Возраст пациенток в I и II группах варьировал от 40,0 до 65,0 и от 45,0 до 65,0 лет соответственно, составив в среднем 55,6 (5,2) и 56,0 (5,9) лет (р=0,86). Показатель индекса массы тела колебался от 20,1 до 35,3, в среднем – 25,8 (3,5) кг/м2 – в I группе и от 19,3 до 36,6, в среднем – 26,7 (3,8) кг/м2 – во II (р=0,22).
Индивидуальные показатели физикального осмотра по органам и системам у пациенток обеих групп не имели клинически значимых отклонений и были сопоставимы между группами.
Оценка эффективности лечения
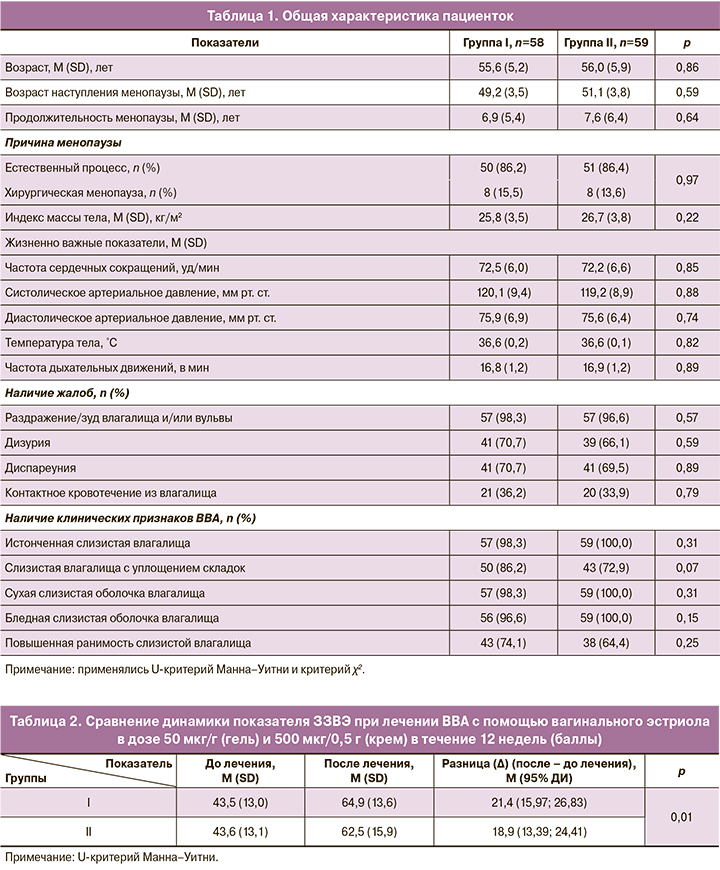
Первичный критерий. Сравнение результатов лечения ВВА с помощью лекарственных средств для вагинального введения, содержащих эстриол 50 мкг/г (гель) и 500 мкг/0,5 г (крем) показало следующие результаты. В обеих группах достигнуто сопоставимое увеличение показателя ЗЗВЭ более 15 баллов после 12 недель терапии – у 51,7% (30/58) пациенток в I группе и у 39,0% (23/59) – во II, р=0,23. Из рисунка 2 видно, что ЗЗВЭ увеличился в I группе с 43,5 (13,0) до 64,9 (13,6) баллов (р=0,01), во II – с 43,6 (13,1) до 62,5 (15,9) баллов (р=0,01).
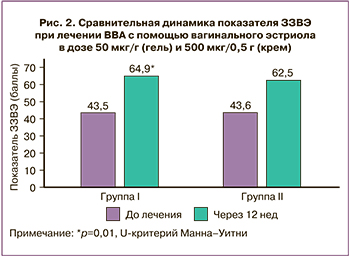
Поперечные сравнения величины изменений между группами от исходного уровня для ЗЗВЭ представлены в таблице 2.
Заключение о не меньшей эффективности лекарственного средства, содержащего эстриол 50 мкг/г (гель) по сравнению с 500 мкг/0,5 г (крем) было сделано на основании следующих расчетов.
Нулевая гипотеза Н0: μt-μr≤delta, альтернативная гипотеза: Н1: μt-μr>delta, где delta=10 – интервал не меньшей эффективности, μt – среднее изменение ЗЗВЭ к 12 неделе терапии в I группе, μr – во II.
В соответствии с протоколом за интервал не меньшей эффективности исследуемого препарата по отношению к препарату сравнения была принята величина 10 баллов (Δ) – разница между средними значениями изменения ЗЗВЭ от исходного уровня после 12 недель терапии.
Изменение ЗЗВЭ от исходного составило µt=21,4 (21,1) баллов и µr=18,9 (21,6) баллов соответственно, Δ=2,47 (95% ДИ: -4,07;9,01, р=0,27). Верхняя граница ДИ не превышала 10 баллов разницы изменения ЗЗВЭ за указанный период. Сделан вывод о не меньшей эффективности вагинального эстриола в дозе 50 мкг/г (гель) по сравнению с 500 мкг/0,5 г (крем) при заданном интервале не меньшей эффективности μt-μr≤Δ(10).
Вторичные критерии. Сравнение динамики увеличения показателя ЗЗВЭ более 15 баллов от исходного после 3 недель терапии в группах с использованием лекарственных средств, содержащих эстриол 50 мкг/г (гель) и 500 мкг/0,5 г (крем) представлено на рисунке 3, где показано, что в I группе увеличение показателя произошло с 43,5 (13,0) до 59,5 (16,3) (+16,0, р=0,01) баллов, во II – с 43,6 (13,1) до 59,4 (17,0) (+15,8, р=0,01) баллов. Значимое увеличение ≥15 баллов по сравнению с исходным уровнем через 3 недели наблюдалось у 37,9% (22/58) и у 32,2% (19/59) пациенток в соответствующих группах (р=0,65).
Динамика облегчения или исчезновения симптомов ВВА, основанная на субъективной оценке (жалобах) пациентки, представлена в таблице 3.
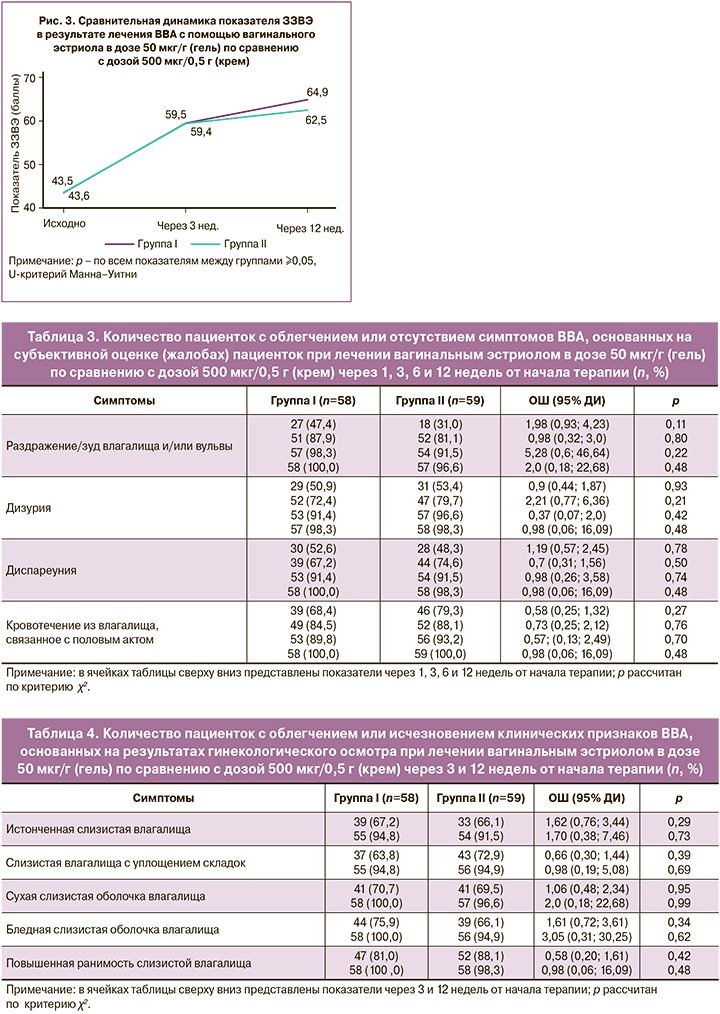
Анализ данных таблицы 3 демонстрирует положительную динамику в снижении частоты симптомов ВВА и других симптомов ГУМС в обеих группах без статистически значимой разницы между ними.
Доля пациенток с облегчением или исчезновением клинических признаков ВВА по результатам гинекологического осмотра на исходном визите, после 3 и 12 недель лечения представлена в таблице 4.
Результаты гинекологического осмотра показали также положительную динамику симптомов, которые были нивелированы почти у всех пациенток в обеих группах, и разница между ними не была статистически значимой.
Динамика средних показателей рН в процессе лечения представлена на рисунке 4. После 3 недель терапии значение рН влагалища в обеих группах снизилось в среднем на 0,7 (0,56) и 0,65 (0,73) ед. соответственно. Показатель рН на этом этапе достиг значения <5,0 у 25,9% (15/58) и 22,0% (13/59) соответственно (р=0,19), снижение его не менее 0,5 ед. наблюдалось у 74,1% (43/58) и 64,4% (38/59) соответственно (р=0,35).
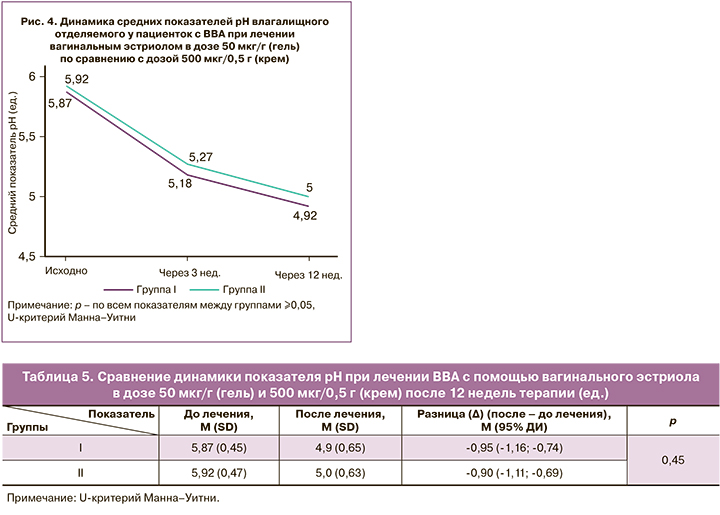
После 12 недель терапии значение рН влагалища в обеих группах снизилось в среднем на 0,95 (0,8) ед. и достигло среднего значения рН 4,9 (0,65) ед. в I группе и на 0,9 (0,83) ед. со средним значением рН 5,0 (0,63) ед. – во II (табл. 5). Показатель рН достиг значения <5,0 у 53,5% (31/58) и 45,8% (27/59) пациенток соответственно (р=0,52), снижение его не менее 0,5 ед. наблюдалось у 82,8% (48/58) и 83,1% (49/59) соответственно (р=0,84).
В целом, в результате проведенного сравнительного анализа эффективности препаратов можно утверждать, что лекарственное средство «Блиссель», гель вагинальный, содержащий эстриол в суточной дозировке 50 мкг/г, в 10 раз меньшей, чем у препарата крем вагинальный в суточной дозировке 500 мкг/0,5 г, обладает не меньшей эффективностью при применении по указанной схеме.
Оценка безопасности
В ходе клинического исследования было зарегистрировано всего 15 НЯ: 5 (33,3%) – в группе препарата «Блиссель» и 10 (66,7%) в группе препарата сравнения. Серьезные нежелательные явления не зарегистрированы. Из 15 зарегистрированных НЯ 13 (86,7%) были расценены, как легкие, 1 (6,7%) – средней степени тяжести (местная реакция), 1 (6,7%) – тяжелое (не связанное с приемом препарата – перелом правой лодыжки). Все НЯ разрешились. В случае 14 НЯ изменения в исследуемой терапии (схема, дозировка) не потребовались. В случаях 8 (53,3%) НЯ потребовалась медицинская коррекция (симптоматическая терапия). Одна пациентка из группы исследуемого препарата отказалась от дальнейшего участия из-за НЯ (местная реакция на применение препарата средней степени тяжести – чувство распирания во влагалище, тяжесть внизу живота, дискомфорт при мочеиспускании, которая возникала после интравагинального введения препарата и проходила сама примерно через 12 ч без применения терапии). Связь с приемом препарата исследования/препарата сравнения по мнению исследователей, представлена следующим образом: 9 – не связано (60%), 4 – возможно связано (общие симптомы – 27%, зарегистрированы в группе II), 2 – вероятно связано (тянущие боли внизу живота, напряженность в молочных железах, болезненность сосков – 13%, в группе I).
В результатах лабораторных, инструментальных исследований не было зарегистрировано клинически значимых изменений во время исследования в сравниваемых группах. При физикальном осмотре было зарегистрировано одно клинически значимое отклонение, связанное с зарегистрированным НЯ. В результатах оценки жизненно важных показателей на визитах не было зарегистрировано клинически значимых изменений. Непредвиденные нежелательные реакции и серьезные НЯ не были зарегистрированы во время проведения исследования.
Изучение показателей толщины эндометрия перед началом и через 12 недель лечения показали отсутствие динамики – в I группе они были соответственно – 2,29 (1,17) и 2,4 (1,17) мм, во II – 2,26 (1,05) и 2,31 (1,07) мм соответственно.
В целом, оба препарата (содержащий эстриол в дозе 50 мкг/г (гель) по сравнению с дозой 500 мкг/0,5 г (крем)) переносились хорошо; установленные НЯ согласуются с известной информацией о безопасности препаратов; количество зарегистрированных случаев сопоставимо в обеих сравниваемых группах.
Обсуждение
Женщины проводят около одной трети своей жизни в состоянии дефицита эстрогенов. Поэтому эксперты подчеркивают, что необходимо соответствующее лечение острых симптомов и профилактика последствий хронического дефицита эстрогенов. Международные руководства призывают к использованию самых низких эффективных доз гормонов для облегчения вазомоторных симптомов, которые являются основным показанием к МГТ [19]. В начале XXI в. стали доступны лекарственные средства с ультранизкими дозами (25% от предыдущей стандартной дозы) эстрогена, одобренные FDA. Эти препараты эффективно облегчают симптомы менопаузы, такие как вазомоторные симптомы и атрофия влагалища, и потенциально защищают от потери костной массы [20].
Симптомы ВВА можно безопасно и эффективно контролировать с помощью местной терапии эстрогенами, что снижает риски, связанные с длительной системной МГТ. Вагинальная таблетка с ультранизкой дозой 10 мкг эстрадиола является самой низкой из доступных доз, и годовая экспозиция эстрадиола составляет всего 1,14 мг [21]. Изучена эффективность крема, содержащего эстрадиол 0,003% (15 мкг), который уменьшал выраженность сухости влагалища, снижал рН, увеличивал процент поверхностных клеток и уменьшал – парабазальных, снижал диспареунию с хорошими показателями безопасности [22]. Однако ранее было показано, что эстрадиол, даже при влагалищном введении, не исключает риска системного действия, что особенно актуально для женщин с высоким риском тромбоэмболических осложнений и перенесенным раком молочной железы, а их долгосрочные последствия недостаточно изучены [16].
В рандомизированном, двойном слепом, плацебо-контролируемом исследовании фазы II, опубликованном в 2020 г., изучалась эффективность и безопасность вагинального геля с ультранизкой дозой 0,005% эстриола для лечения ВВА у женщин в постменопаузе с ранним раком молочной железы, получавших нестероидные ингибиторы ароматазы. При положительном клиническом эффекте наблюдались небольшие колебания ФСГ и ЛГ, которые оставались в пределах постменопаузального диапазона, уровни эстриола первоначально повышались и нормализовались к 12 неделям, а эстрадиол и эстрон оставались на неопределяемом уровне на протяжении всего исследования [23].
Клинический эффект был оценен в другом недавнем исследовании (2022 г.) при использовании сверхмалой дозы 0,005% вагинального геля эстриола, которая приводила к быстрому улучшению большинства симптомов и признаков ГУМС. Этот клинически значимый ответ наблюдался с первых дней лечения, подтверждая быстрое начало и прогрессирующее действие [24], что совпадает с результатами настоящего исследования. Другими исследователями показано также положительное действие в отношении сексуального функционирования и повышения качества жизни со значительным улучшением общего показателя соматических аспектов [25].
Систематический обзор 53 исследований эффективности и безопасности вагинальных препаратов эстрогена для лечения ГУМС показал, что по сравнению с плацебо все вагинальные эстрогены продемонстрировали превосходство в объективных и субъективных конечных точках ГУМС, тогда как в некоторых исследованиях было продемонстрировано превосходство над плацебо в урогенитальных симптомах. Не наблюдалось существенной разницы между различными дозировками и лекарственными формами вагинальных эстрогеновых препаратов [16].
Настоящее исследование продемонстрировало высокую эффективность и безопасность вагинального геля в ультранизкой дозе (50 мкг/г) в лечении ВВА, которое было сопоставимо с вагинальным кремом в стандартной дозировке (500 мкг/0,5 г), что открывает новые возможности для лечения ВВА.
Ограничения исследования. Настоящий анализ был ограничен 12-недельным наблюдением, что исключало долгосрочную оценку эффектов эстриола. Необходимы дальнейшие исследования с использованием большего размера выборки (со сбалансированной рандомизацией) для оценки долгосрочных эффектов.
Заключение
Эффективность ультранизкой дозы 50 мкг/г вагинального геля эстриола в улучшении симптомов и клинических признаков ВВА аналогична препарату вагинального крема с эстриолом в стандартной дозе (500 мкг/0,5 г). Безопасность обоих лекарственных средств сопоставима.



