Doppler evaluation of cerebral blood flow in the differential diagnosis of late-onset fetal growth restriction
Stoliarova E.V., Kholin A.M., Khodzhaeva Z.S., Gus A.I.
Background: Late-onset fetal growth restriction (FGR) is associated with an increased risk of stillbirth and neonatal morbidity. However, the differentiation between FGR and small-for-gestational-age (SGA) fetuses in the third trimester remains challenging. Fetal Doppler assessment traditionally plays a key role and is considered to be a potential tool for risk stratification and optimization of obstetric management in cases of FGR. In recent years, there has been an active discussion about the role of cerebral blood flow in the targeted monitoring of fetal health and the improvement of perinatal outcomes.
Objective: To study the diagnostic value of Doppler ultrasound parameters characteristic of centralization of fetal cerebral blood flow after 32 weeks gestation in the differential diagnosis of late-onset FGR and SGA, as well as their association with adverse perinatal outcomes.
Materials and methods: This was a prospective study that included 140 pregnant women after 32 weeks gestation, namely 72 patients with SGA fetuses and 68 patients with late-onset FGR, selected according to the guidelines of the Russian Society of Obstetricians and Gynecologists (RSOG). All participants underwent Doppler ultrasound assessment; the cerebroplacental ratio (CPR) and umbilicocerebral ratio (UCR) were calculated. The findings of the Doppler study, performed as close to delivery as possible, were used for analysis. Changes in Doppler parameters in both groups were studied, and their correlation with perinatal outcomes was assessed using logistic regression analysis.
Results: The comparison of perinatal outcomes showed that pregnant women with late-onset FGR compared to SGA fetuses had a higher rate of preterm birth, emergency delivery by cesarean section, birth of infants with a body weight below the 3rd percentile, and need for hospitalization of newborns in the neonatal intensive care unit (NICU). The parameters of Doppler ultrasound study (umbilical artery pulsatility index (UA PI), middle cerebral artery (MCA) PI, CPR, and UCR) in the late-onset FGR group more often exceeded the standard values. The median CPR was lower (1.19 vs. 1.70; p<0.001), and the median UCR was higher (0.84 vs. 0.59; p<0.001) in the late-onset FGR group compared to SGA. A significant association was found between UA PI, CPR, and UCR in pregnancies with FGR and adverse perinatal outcomes, such as preterm birth (OR: 76.3, p<0.001; 0.1, p<0.001; 26.5, p<0.001, respectively), emergency cesarean section due to fetal distress (OR: 4.1, p<0.05; 0.4, p=0.04; 4.5, p<0.05, respectively), neonatal hospitalization in the NICU (OR: 31.9, p<0.05; 0.1, p=0.04; 32.8, p<0.05, respectively), combined adverse perinatal outcome (OR: 13.3, p=0.002; 0.2, p=0.002; 11.2, p=0.05, respectively); however, ROC analysis (AUC, 95% CI) of the models for predicting combined adverse perinatal outcome showed low prognostic value of these parameters. The best diagnostic accuracy was demonstrated by the gestational age-adjusted centile values of CPR and UCR [0.695 (0.604–0.777) and 0.675 (0.582-0.758)], as well as the dichotomized by a predetermined centile values of UCR <5th centile and PCR>95th centile [0.641 (0.547–0.727) and 0.631 (0.538–0.718)]. No data were obtained to support the greater effectiveness of CPR or UCR. At the same time, extreme values characterizing the centralization of cerebral blood flow became more obvious and clearer for interpretation in case of using UCR.
Conclusion: Doppler parameters that characterize cerebral blood flow centralization, such as UA PI, MCA PI, CPR and UCR, are not effective enough to predict combined adverse perinatal outcome and its individual components in pregnancies with SGA fetuses and FGR. Gestational age-adjusted centile (continuous) values of CPR and UCR, as well as dichotomized by a pre-established cutoff centile value of CPR (<5th centile) and UCR (>95th centile) are more effective in predicting adverse perinatal outcomes. Further prospective studies are required to determine whether it is possible to include these parameters in clinical practice, as well as to find new markers that can predict adverse outcomes for children with late-onset FGR.
Authors’ contributions: Stoliarova E.V. – conducting the study, writing the text; Kholin A.M. – developing the concept of the study, conducting the study; Khodzhaeva Z.S. – supervision of the study, editing the text; Gus A.I. – developing the concept of the study, conducting the study.
Conflicts of interest: Authors declare lack of the possible conflicts of interests.
Funding: The study was carried out without sponsorship.
Ethical Approval: The study was approved by the Ethical Review Board of the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia.
Patient Consent for Publication: The patients signed informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Stoliarova E.V., Kholin A.M., Khodzhaeva Z.S., Gus A.I. Doppler evaluation of cerebral blood flow in
the differential diagnosis of late-onset fetal growth restriction.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2025; (8): 88-98 (in Russian)
https://dx.doi.org/10.18565/aig.2025.184
Keywords
Late-onset fetal growth restriction (FGR) is associated with stillbirth, neonatal morbidity, and an increased risk of the brain damage in the fetus and newborn [1-3]. Differential diagnosis of FGR fetuses and small-for-gestational-age (SGA) fetuses is necessary to optimize pregnancy management tactics, including targeted monitoring of fetal condition and rational determination of delivery time [4]. There is a limited number of studies that have been conducted to determine the delivery time in case of this pathology. In addition, there is no agreement on which Doppler parameters best determine the risk of fetal distress [5]. Preterm delivery of these fetuses can lead to complications associated with prematurity and postterm delivery can result in further deterioration of the fetus, brain damage or stillbirth [6].
Despite the fact that FGR and SGA are clearly distinct conditions in terms of perinatal outcomes, their clinical differentiation is challenging [7]. In the third trimester of pregnancy, ultrasound fetometry may not be able to reliably identify fetuses with delayed growth, as their anthropometric measurements may be within normal ranges [8]. In addition, FGR may occur even when the fetal size exceeds the 10th percentile, suggesting that isolated fetometry may not be sensitive enough [2, 7].
Ultrasound Doppler assessment of fetoplacental and uteroplacental blood flow, along with assessment of fetal growth rate, plays a key role in the management of pregnant women with suspected FGR [9, 10]. Some low-weight fetuses in the third trimester of pregnancy do not reach their inherent growth potential, mainly due to placental insufficiency. This condition is often not detected by ultrasound Doppler assessment in the umbilical artery (UA), but it increases the risk of adverse perinatal outcomes and long-term complications [11].
The redistribution of fetal blood flow in favor of the brain or centralization of blood flow with decreased resistance in the cerebral arteries is a reaction to an unfavorable intrauterine environment characterized by hypoxemia [12]. The value of an isolated ultrasound Doppler assessment of the medial cerebral artery (MCA) for predicting an unfavorable outcome in cases of late-onset FGR is limited, but it significantly improves when it is combined with the data on blood flow in UA [13]. The degree of centralization of blood flow is quantified by calculating the cerebroplacental ratio (CPR) that refers to the ratio of the pulsatility index (PI) in MCA to the PI in UA. Its assessment demonstrates greater sensitivity than the assessment of these parameters individually [11]. Recent publications suggest that the umbilicocerebral ratio (UCR), which is an inversion of CPR, may be a more sensitive predictor of adverse perinatal outcomes [14–16]. Meanwhile, the role of UCR is still under debate, and it is currently unclear which of the indicators mentioned should be prioritized [17].
The aim of this study is to identify the diagnostic value of Doppler ultrasound parameters characteristic of centralization of fetal cerebral blood flow after 32 weeks gestation in the differential diagnosis of late-onset FGR and SGA, as well as their association with adverse perinatal outcomes.
Materials and methods
This was a prospective study conducted in the obstetric clinic of the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology in Moscow from 2023 to 2025.The study included 140 women with singleton pregnancy and gestational age at the time of inclusion in the study 32+0 to 36+6 weeks; the estimated fetal weight (EFW) or abdominal circumference (AC) should be less than the 10th percentile according to ultrasound fetometry. The diagnosis of late-onset FGR and SGA was made in accordance with the current clinical guidelines of the Russian Society of Obstetricians and Gynecologists, based on the criteria of the Delphi Consensus. The criteria for inclusion in the SGA group were EFW or AC according to ultrasound examination <the 10th, but more than the 3rd percentile with normal ultrasound Doppler examination parameters. The criteria for inclusion in the group of late-onset FGR were EFW/AC according to ultrasound <3rd percentile or less <10th percentile in combination with impaired blood flow according to the Doppler assessment (UA PI >95th percentile; MCA PI <5th percentile; CPR <5th percentile). The women were excluded from the study if they had multiple pregnancies, identified fetal structural or chromosomal abnormalities, intrauterine infection, or severe somatic pathology.
The ultrasound assessment was performed using Voluson E8/E10 Expert (GE Healthcare, USA) with a 4D multifrequency curvilinear transducer (2-8 MHz). The gestational age was determined in accordance with the data of the crown rump length that was identified during the ultrasound screening in the first trimester. According to the guidelines of the International Society of Ultrasound in Obstetrics and Gynecology (ISUOG), measurements of the biparietal size, head circumference, AC, and femur length were performed to calculate EFW. EFW was determuned using the Hadlock formula [18]. Absolute values of EFW in grams were converted to percentile values based on the gestational age, using the INTERGROWTH-21st and FMF percentile tables [19, 20]. The parameters of impulse dopplerometry were automatically calculated on the ultrasound device after the analysis of 3 or more identical and consecutive curves at the time of maximum fetal rest, in the absence of tachycardia, and with an angle of radiation close to or equal to 0 degrees. MCA was assessed at the point of passage through the wing of the sphenoid bone, near the circle of Willis; UA was estimated in the area of the free loop of the umbilical cord. The latest available Doppler measurements were taken for analysis, and these measurements had to be within a range of no more than one week before delivery. Percentile values of ultrasound Doppler parameters (UA PI, MCA PI, uterine arteries (UtA) PI) were calculated based on graphs developed by the Fetal Medicine Foundation experts [19]. CPR was calculated as the ratio of UA PI to MCA PI, and the UCR was calculated as the ratio of MCA PI to UA PI, with the conversion of absolute values to relative values taking into account gestational age and using available percentile nomograms [15]. The medical information system Astraia software for women’s health v.26.1 (NEXUS/Astraia GmgH, Germany) was used to manage the patient database, calculate and interpret measurement results.
The comparison of the diagnostic accuracy of the Doppler parameters was carried out in relation to unfavorable outcomes for the fetus, newborn, and mother. Combined adverse perinatal outcome included at least one of the following events: instrumental delivery or emergency cesarean section for fetal distress, intrauterine fetal death, Apgar score at the 5th minute <7 points, impairment of the acid-base state (arterial blood pH <7.1 or excess of bases), hospitalization in the neonatal intensive care unit (NICU).
The prognostic value of Doppler parameters in predicting unfavorable perinatal outcomes was calculated using binary logistic regression; the diagnostic accuracy of the test was evaluated using ROC analysis with determination of the area under the characteristic curve (AUC). There was a comparison of the Doppler characteristics of UA PI, MCA PI, CPR, and UCR, namely, their absolute values, dichotomized absolute values (CPR<1; UCR>1), gestational age-adjusted centile values, dichotomized centile values with a threshold for CPR<5th centile, UCR>95th centile, UA PI>95th centile, MCA PI<5th centile.
Statistical analysis
The statistical significance of the model was checked using the criterion 2. With a p-value of less than 0.05, the null hypothesis that the model is insignificant was rejected. The quality of model compliance was checked using the Nagelkerke R-square, the Hosmer–Lemeshow test (with a p-value >0.05, the hypothesis of model consistency was accepted). The prognostic values of each dichotomized parameter were also assessed in terms of sensitivity, specificity, test accuracy, and positive and negative likelihood ratios.
To assess the normality of the distribution, the Shapiro–Wilk test (W test) was used. For quantitative data following a normal distribution, the mean (M) and standard deviation (SD) were used. In cases of non-normal data distribution, the median (Me) and quartiles (Q1–Q3) were applied. The comparison of quantitative variables was performed using an independent t-test and the Mann–Whitney test (U test). Categorical variables were compared using the χ2 test. Statistical significance was determined at p<0.05. Statistical calculations and drawing diagrams were performed using the JASP (version 0.19.3) and MedCalc (version 20.009) software.
Results
There was an analysis of the clinical and anamnestic characteristics, ultrasound Doppler parameters, obstetric and perinatal outcomes of 140 pregnancies at risk of developing FGR. Of these, antenatal FGR was detected in 68 cases and SGA was revealed in 72 cases (Table 1). In the general cohort of pregnant women, the median gestational age and EFW at the time of inclusion in the study were 37.1 (36.5–38.0) weeks and 2550 (2070–2786) years, respectively. The average age of pregnant women in the SGA and FGR groups was 31 (28–35) and 33 (29–38) years, respectively, and it did not differ significantly between the groups (p=0.052). The median body mass index (BMI) was 25 kg/m2, there were no significant differences between the groups (p=0.493). The incidence of hypertensive disorders of pregnancy, including gestational hypertension (GH) and preeclampsia (PE), was significantly higher in the late-onset FGR group (36.8% versus 12.5%; p=0.001); however, there were no differences in the incidence of gestational diabetes mellitus (GDM).
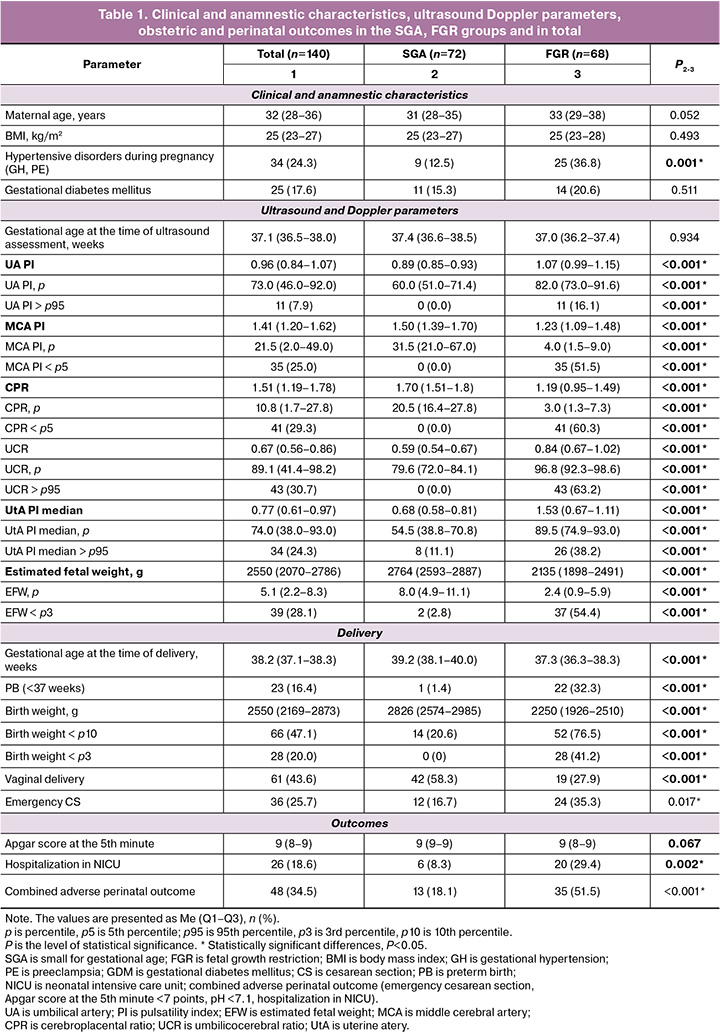
The median gestational age at the time of delivery was lower in the late-onset FGR group and it was 37.3 (36.3–38.3) weeks versus 39.2 (38.1–40.0) weeks, respectively, p<0.001. Only one (1.4%) case of preterm birth (PB) was recorded in the SGA group, while 22 (32.3%) cases were recorded in the late–stage FGR group, p<0.001. Pregnant women with FGR were more likely to have emergency cesarean section (24 (35.3%) versus 12 (16.7%), p=0.017) and more than two times less often vaginal delivery, compared to the SGA group (19 (27.9%) versus 42 (58.2%), p<0.001); there were no differences in the rate of operative vaginal deliveries. In most cases, emergency caesarean section was performed due to fetal indications, such as the detection of abnormal Doppler parameters, signs of fetal distress on the basis of CTG data, a lack of weight gain in the fetus, and acute fetal hypoxia in labor.
The median body weight at birth in the FGR group was significantly lower than that of the SGA group (2,250 g (1,926–2,510 g) versus 2,826 g (2,574–2,985 g); p<0.001). This is consistent with the lower median gestational age at birth. More than a third of newborns from the late-onset FGR group were born with a body weight of less than the 3rd percentile (28 (41.2%) versus 0; p<0.001). Newborns from the late-onset FGR group were more likely to require hospitalization in NICU (20 (28.6%) versus 6 (8.6%); p=0.002).
The differences in the parameters of ultrasound Doppler evaluation in the SGA and late-onset FGR groups are shown in Table 1. The gestational age at the time of ultrasound assessment did not significantly differ between the groups; however, pregnant women with late-onset FGR had Doppler assessment on average several days earlier (37.0 (36.2–37.4) versus 37.4 (36.6–38.5) weeks; p=0.934), which is explained by the earlier time of delivery in patients with altered ultrasound Doppler parameters or marked decrease in fetal weight. In the late-onset FGR group, the median UA was higher (1.07 (0.99–1.15) versus 0.89 (0.85–0.98); p<0.001), and an increase in UA PI >95th percentile was detected in 15.7% of cases. At the same time, ‘zero’ end-diastolic flow in UA was detected in three patients (4.3%); in the SGA group, the above changes were not detected. It should be noted that blood flow disorders in MCA were significantly more common than in UA (in 50% of cases in the late-onset FGR group), and MCA PI was lower than in the SGA group (4.0 (1.5–9.0) versus 31.5 (21.0–67.0); p<0.001). The median CPR in the late-onset FGR group was significantly lower than one in the SGA group (3.0 (1.3–7.3) versus 20.5 (16.4–27.8); p<0.001); the median UCR was higher (96.8 (92.3–98.6) versus 79.6 (72.0–84.1); p<0.001).
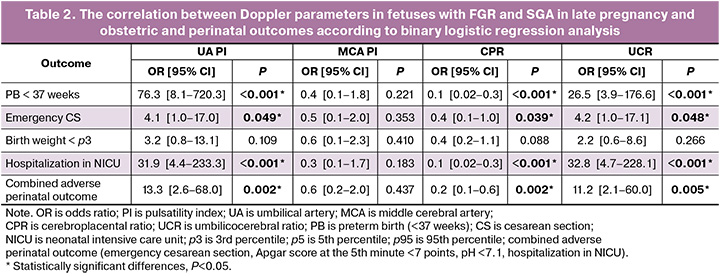
Table 2 shows the results of binary logistic regression analysis. Such Doppler parameters as UA PI, CPR, and UCR showed a significant association with preterm birth up to 37 weeks (OR: 76.3 [8.1–720.3], p<0.001; OR: 0.1 [0.02–0.3], p<0.001; OR: 26.5 [3.97–176.59], p=0.028, respectively], emergency cesarean section (OR: 4.1 [1.0–17.0], p<0.05; OR: 0.4 [0.1–1.0], p=0.039; OR: 4.2 [1.0–17.1], p=0.048, respectively), hospitalization in NICU (OR: 31.9 [4.4–233.3], p<0.001; OR: 0.1 [0.02–0.3], p<0.001; OR: 32.8 [4.7–228.1], p<0.001, respectively), combined adverse perinatal outcomes (OR: 13.3 [2.6–68.0], p=0.002; OR: 0.2 [0.1–0.6], p=0.002; OR: 11.2 [2.1–60.0], p=0.005, respectively), however, MCA PI showed no significant association with the above outcomes (OR: 0.4 [0.1–1.8], p=0.221; OR: 0.5 [0.1–2.0], p=0.353; OR: 0.3 [0.1–1.7], p=0.183; OR: 0.6 [0.2–2.0], p=0.437). The correlation of all four Doppler parameters (UA PI, MCA PI, CPR and UCR) with birth weight of less than the 3rd percentile was not revealed as well.
The comparison of the diagrams of the distributions of the CPR and UCR values shows that the UCR distribution has a symmetrical shape with approximately equal deviations from the median in both directions (Fig. 1A). In contrast, the CPR distribution is asymmetrical, with a shift to the left (Fig. 1B). The UCR values (>1), which are critical in assessing the cases of FGR, demonstrate ‘outliers’ more clearly than the CPR values (<1).
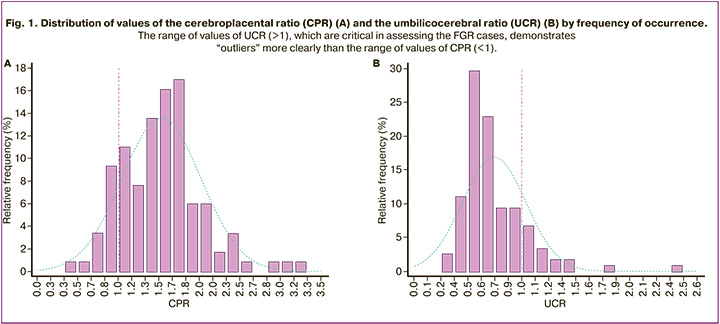
Table 3 presents the data on the assessment of the effectiveness of predicting a combined perinatal outcome in the study population. The areas under the ROC curves, AUC (95% CI), UA PI, MCA PI, CPR and UCR are similar and correspond to low diagnostic accuracy (AUC<0.7) for absolute, centile values and dichotomous absolute and the centile threshold values of the Doppler parameters. For the MCA PI, the area under the ROC curve for the absolute parameter values was 0.542 (0.450–0.636), p=0.414, that is, the model did not show diagnostic effectiveness. For the centile parameter values, the area was 0.606 (0.511–0.694), p=0.048, that is, the diagnostic effectiveness of the model is low. The Nagelkerke R-square and the Hosmer–Lemeshow test for compliance also showed poor compliance of the model in terms of MCA PI and outcome. As for CPR and UCR, the efficiency of absolute, centile values and dichotomous centile values (CPR<5th centile and UCR>95th centile) was statistically significantly higher than that of dichotomous absolute values (CPR<1 and UCR>1). The criteria and compliance tests showed poor or moderate model compliance for these variables and outcomes.

Sensitivity analysis was performed for dichotomized variables. It showed identical results for CPR <5th centile and UCR >95th centile, CPR <1 and UCR >1, as well as similar results for UA PI >95th centile and MCA PI <5th centile. When dichotomized initial values of CPR and UCR were used, the results were generally low. In case of dichotomized centile values, specificity (78.1% [66.0–87.5]) and a positive likelihood ratio (2.29) demonstrated moderate effectiveness in detecting a combined adverse perinatal outcome. Similar results were demonstrated by dichotomized centile values of UA PI, MCA PI: specificity is 89.1% [78.8–95.5] and 79.9% [67.8–88.7], with a positive likelihood ratio of 3.05 and 2.37, respectively.
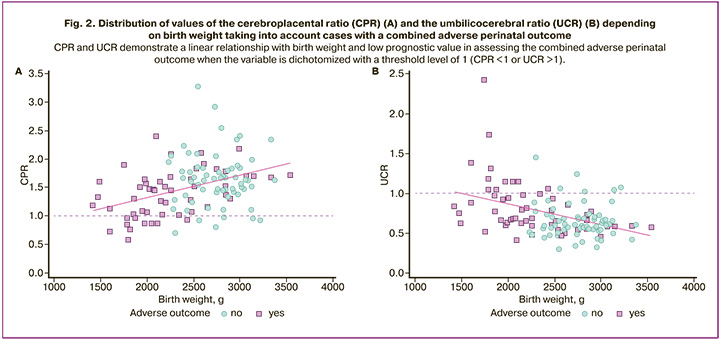
The scattering diagram of the values of CPR and UCR (Fig. 2) shows a linear relationship between the studied variables and birth weight and the low prognostic value of a dichotomous variable with a threshold level of 1 (CPR<1 or UCR>1) when assessing combined unfavorable perinatal outcomes.
Discussion
The results of our study revealed significant differences in perinatal outcomes between the groups of pregnant women with late-onset FGR and SGA. Such Doppler parameters as UA PI, CPR, and UCR have been shown to be significantly associated with adverse perinatal outcomes in pregnancies with FGR, in particular with preterm birth, emergency cesarean section due to fetal distress, hospitalization in NICU, and combined adverse perinatal outcomes. However, they have low diagnostic accuracy when these indicators are used as predictive models. Thus, in the study the most precise diagnostic results were achieved by the gestational age-adjusted centile (continuous) values of CPR and UCR, as well as the dichotomized values of CPR (<5th centile) and UCR (>95th centile) based on a preset centile. No data has been obtained to support a higher efficiency of CPR or UCR. At the same time, the extreme values characterizing the centralization of cerebral blood flow became more obvious and evident when using UCR.
The differential diagnosis of late-onset FGR and SGA is of great clinical importance for choosing the optimal pregnancy management tactics that includes targeted monitoring and determining the delivery time. Dopplerometry is the most important tool in the evaluation of FGR. Ultrasound Doppler assessment of UA is not a sufficiently informative parameter, since UA PI in late pregnancy most often remains within the normative range. The longitudinal cohort study conducted by Oros D. et al. (2011) showed that less than 3% of fetuses with EFW <10th percentile had an increase in PI in UA [21]. The observational study conducted by Meler E. et al. (2021), which included more than a thousand small fetuses, demonstrated that a change in blood flow in UA was present in less than 6% of diagnosed cases and in 10% immediately before delivery [22]. Thus, a dynamic increase in UA should not be expected in cases of late-onset FGR. In our study, abnormalities in UA PI were detected in 16.1% of cases in the FGR group and in 7.9% of cases among all fetuses with an estimated body weight < 10th centile.
For the first time, Arabeille P. et al. (1994) proposed to evaluate changes in blood flow occurring in the cerebrovascular and placental areas by calculating the ratio of the values of MCA PI and UA PI, since it better detects early signs of fetal distress [23]. Later, Flood K. et al. (2014) in a multicentered PORTO study showed that CPR values of less than 1 were associated with an 11-fold increased risk of adverse outcomes [24].
A growing number of studies have confirmed the association between low CPR and adverse outcomes for newborns, particularly with regard to perinatal mortality and morbidity. The meta-analysis conducted by Villalain C. et al. (2018) included 4,300 pregnant women and showed that the accuracy of CPR for predicting perinatal mortality ranged from moderate to high (with a sensitivity of 93% and a specificity of 76%) and it was low for predicting combined adverse perinatal outcomes. However, CPR showed the strongest association with the rate of emergency cesarean section in comparison with other ultrasound Doppler parameters [25].
Moreover, blood flow disorders in MCA and decreased CPR are more likely to occur immediately before delivery that can be indicative of the progression of placental insufficiency. In a study by Meler E. et al. (2021), low CPR was only detected in 22.3% of pregnant women with insufficient fetal growth, but impaired blood flow in MCA was recorded only in 8.3% of cases [22]. In our study, a decrease in CPR was observed more often than abnormal blood flow in MCA, which indicates a high sensitivity of this indicator.
The differences in the indicators of CPR and UCR were first described by Gramellini D. et al. in 1992 in case of fetal distress, after which the clinical value of the above ratio was discussed in a number of other studies [26]. According to Wolf H. et al. (2020), the greater efficiency of UCR is associated with an asymmetric distribution of values and it facilitates the detection of pathological changes [27]. In our study, the distribution of UCR was also asymmetric, which could potentially enhance its usefulness in diagnosing late-onset FGR. The presented results correlate with previously published studies by Di Mascio D. et al. (2020) and Stumpfe F.M. et al. (2023), where it was noted that both ratios are equally associated with various adverse outcomes, including preterm birth [17, 28].
The authors of the TRUFFLE study argue that since fetal Doppler disorders are more severe in patients with low resistance to blood flow in MCA and high resistance in UA, CPR tends to an asymptote approaching zero, while the UCR tends towards infinity. This emphasizes the differences between abnormal values and leads to a more informative clinical picture [29]. Other experts, on the contrary, expressed some concerns, as the inversion of CPR can have a significant impact on its distribution and interpretation of the resulting UCR. This raises some statistical issues that should be considered when analyzing UCR and comparing it to CPR [27]. Our research, along with the work of Di Mascio D. et al. (2021), contributes to a deeper understanding of the benefits and characteristics of each studied relationship [30].
The prospective design and high quality of targeted monitoring are the strengths of our study. Unlike most similar studies, pregnant women with SGA were included in the control group, which made it possible to characterize in detail the differences between pregnant women with low EFW with and without the risk of its decompensation.
The relatively small sample size is the main limitation of our study. In addition, it was not possible to assess the role of ultrasound Doppler parameters in detecting antenatal fetal death which is the most significant, but rather rare outcome of pregnancy; it was due to the fact that this complication was not recorded in the presented cohort. It should be noted, that due to the small number of cases (6 newborns), it was not possible to assess the relationship between Doppler parameters and indicators of the acid-base balance of blood. Neonatal and infant morbidity, as well as neurological outcomes in children during the first two years of life in the FGR and SGA groups also require further investigation.
The prerequisite for conducting this work was the idea of the possibility of optimizing management strategies, targeted monitoring, and a more informed choice of the time and mode of delivery for pregnant women with late-onset FGR/SGA and the highest risk of adverse perinatal outcomes. Intrauterine decompensation of the fetus in late pregnancy occurs due to the mechanism of centralization of blood flow. Therefore, an isolated ultrasound Doppler assessment of UA can give a false impression of the satisfactory condition of the fetus and may affect management tactics. Low CPR values and high UCR values are equally associated with adverse perinatal outcomes in pregnant women with late-onset FGR. The results of this study are consistent with other research findings on the use of CPR in FGR, which have demonstrated a correlation with adverse perinatal outcomes, but are not strong predictors on their own. In addition to the already known information, we have added a comparison of CPR and UCR in their most commonly used values, including initial values and centiles, as well as both continuous and dichotomous measurements.
Conclusion
Doppler parameters that characterize cerebral blood flow centralization, such as UA PI, MCA PI, CPR and UCR, are not effective enough to predict combined adverse perinatal outcome and its individual components in pregnancies with SGA fetuses and FGR. Gestational age-adjusted centile (continuous) values of CPR and UCR, as well as dichotomized by a pre-established cutoff centile value of CPR (<5th centile) and UCR (>95th centile) are more effective in predicting adverse perinatal outcomes. Further prospective studies are required to determine whether it is possible to include these parameters in clinical practice, as well as to find new markers that can predict adverse outcomes for children with late-onset FGR.
References
- Baschat A.A. Planning management and delivery of the growth-restricted fetus. Best Pract. Res. Clin. Obstet. Gynaecol. 2018; 49: 53-65. https://dx.doi.org/10.1016/j.bpobgyn.2018.02.009
- Lees C.C., Stampalija T., Baschat A., da Silva Costa F., Ferrazzi E., Figueras F. et al. ISUOG Practice Guidelines: diagnosis and management of small-for-gestational-age fetus and fetal growth restriction. Ultrasound Obstet. Gynecol. 2020; 56(2): 298-312. https://dx.doi.org/10.1002/uog.22134
- Malhotra A., Ditchfield M., Fahey M.C., Castillo-Melendez M., Allison B.J., Polglase G.R. et al. Detection and assessment of brain injury in the growth-restricted fetus and neonate. Pediatr. Res. 2017; 82(2): 184-93. https://dx.doi.org/10.1038/pr.2017.37
- Figueras F., Savchev S., Triunfo S., Crovetto F., Gratacos E. An integrated model with classification criteria to predict small-for-gestational-age fetuses at risk of adverse perinatal outcome. Ultrasound Obstet. Gynecol. 2015; 45(3): 279-85. https://dx.doi.org/10.1002/uog.14714
- Ghi T., Frusca T., Lees C.C. Cerebroplacental ratio in fetal surveillance: an alert bell or a crash sound? Am. J. Obstet. Gynecol. 2016; 214(2): 297-98. https://dx.doi.org/10.1016/j.ajog.2015.09.097
- Mylrea-Foley B., Thornton J.G., Mullins E., Marlow N., Hecher K., Ammari C. et al. Perinatal and 2-year neurodevelopmental outcome in late preterm fetal compromise: the TRUFFLE 2 randomised trial protocol. BMJ Open. 2022; 12(4): e055543. https://dx.doi.org/10.1136/bmjopen-2021-055543
- Lees C.C., Romero R., Stampalija T., Dall'Asta A., DeVore G.A., Prefumo F. et al. Clinical opinion: the diagnosis and management of suspected fetal growth restriction: an evidence-based approach. Am. J. Obstet. Gynecol. 2022; 226(3): 366-78. https://dx.doi.org/10.1016/j.ajog.2021.11.1357
- Sovio U., White I.R., Dacey A., Pasupathy D., Smith G.C.S. Screening for fetal growth restriction with universal third trimester ultrasonography in nulliparous women in the Pregnancy Outcome Prediction (POP) study: a prospective cohort study. Lancet. 2015; 386(10008): 2089-97. https://dx.doi.org/10.1016/S0140-6736(15)00131-2
- Stampalija T., Wolf H., Mylrea-Foley B., Marlow N., Stephens K.J., Shaw C.J. et al. Reduced fetal growth velocity and weight loss are associated with adverse perinatal outcome in fetuses at risk of growth restriction. Am. J. Obstet. Gynecol. 2023; 228(1): 71.e1-e10. https://dx.doi.org/10.1016/j.ajog.2022.06.023
- Mylrea-Foley B., Lees C. Clinical monitoring of late fetal growth restriction. Minerva Obstet. Gynecol. 2021; 73(4): 462-70. https://dx.doi.org/10.23736/S2724-606X.21.04845-4
- Martinez J., Boada D., Figueras F., Meler E. How to define late fetal growth restriction. Minerva Obstet. Gynecol. 2021; 73(4): 409-14. https://dx.doi.org/10.23736/S2724-606X.21.04775-4
- Liu W., Liu J., Lou X., Zheng D., Wu B., Wang D.J. et al. A longitudinal study of cerebral blood flow under hypoxia at high altitude using 3D pseudo-continuous arterial spin labeling. Sci. Rep. 2017; 7: 43246. https://dx.doi.org/10.1038/srep43246
- Conde-Agudelo A., Villar J., Kennedy S.H., Papageorghiou A.T. Predictive accuracy of cerebroplacental ratio for adverse perinatal and neurodevelopmental outcomes in suspected fetal growth restriction: systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2018; 52(4): 430-41. https://dx.doi.org/10.1002/uog.19117
- Stampalija T., Arabin B., Wolf H., Bilardo C.M., Lees C. Is middle cerebral artery Doppler related to neonatal and 2-year infant outcome in early fetal growth restriction? Am. J. Obstet. Gynecol. 2017; 216(5) : 521.e521-e513. https://dx.doi.org/10.1016/j.ajog.2017.01.001
- Acharya G., Ebbing C., Karlsen H.O., Kiserud T., Rasmussen S. Sex-specific reference ranges of cerebroplacental and umbilicocerebral ratios: longitudinal study. Ultrasound Obstet. Gynecol. 2020; 56(2): 187-95. https://dx.doi.org/10.1002/uog.21870
- Familiari A., Neri C., Vassallo C., Di Marco G., Garofalo S., Martino C. et al. Fetal Doppler parameters at term in pregnancies affected by gestational diabetes: role in the prediction of perinatal outcomes. Ultraschall Med. 2020; 41(6): 675-80. https://dx.doi.org/10.1055/a-0753-0120
- Di Mascio D., Rizzo G., Buca D., D'Amico A., Leombroni M., Tinari S. et al. Comparison between cerebroplacental ratio and umbilicocerebral ratio in predicting adverse perinatal outcome at term. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020; 252: 439-43. https://dx.doi.org/10.1016/j.ejogrb.2020.07.032
- Hadlock F.P., Harrist R.B., Martinez-Poyer J. In utero analysis of fetal growth: a sonographic weight standard. Radiology. 1991; 181(1): 129-33. https://dx.doi.org/10.1148/radiology.181.1.1887021
- Ciobanu A., Wright A., Syngelaki A., Wright D., Akolekar R., Nicolaides K.H. Fetal Medicine Foundation reference ranges for umbilical artery and middle cerebral artery pulsatility index and cerebroplacental ratio. Ultrasound Obstet. Gynecol. 2019; 53(4): 465-72. https://dx.doi.org/10.1002/uog.20157
- Nicolaides K.H., Wright D., Syngelaki A., Wright A., Akolekar R. Fetal Medicine Foundation fetal and neonatal population weight charts. Ultrasound Obstet. Gynecol. 2018; 52(1): 44-51. https://dx.doi.org/10.1002/uog.19073
- Oros D., Figueras F., Cruz-Martinez R., Meler E., Munmany M., Gratacos E. Longitudinal changes in uterine, umbilical and fetal cerebral Doppler indices in late-onset small-for-gestational age fetuses. Ultrasound Obstet. Gynecol. 2011; 37(2): 191-5. https://dx.doi.org/10.1002/uog.7738
- Meler E., Mazarico E., Eixarch E., Gonzalez A., Peguero A., Martinez J. et al. Ten-year experience of protocol-based management of small-for-gestational-age fetuses: perinatal outcome in late-pregnancy cases diagnosed after 32 weeks. Ultrasound Obstet. Gynecol. 2021; 57(1): 62-9. https://dx.doi.org/10.1002/uog.23537
- Arbeille P., Maulik D., Fignon A., Stale H., Berson M.., Bodard S. et al. Assessment of the fetal PO2 changes by cerebral and umbilical Doppler on lamb fetuses during acute hypoxia. Ultrasound Med. Biol. 1995; 21(7): 861-70. https://dx.doi.org/10.1016/0301-5629(95)00025-m
- Flood K., Unterscheider J., Daly S., Geary M.P., Kennelly M.M., McAuliffe F.M. et al. The role of brain sparing in the prediction of adverse outcomes in intrauterine growth restriction: results of the multicenter PORTO Study. Am. J. Obstet. Gynecol. 2014; 211(3): 288.e281-5. https://dx.doi.org/10.1016/j.ajog.2014.05.008
- González V.C., Herraiz I., Quezada M.S., Gómez-Arriaga P.I., Gómez-Montes E., Galindo A. Fetal biometry and Doppler study for the assessment of perinatal outcome in stage i late-onset fetal growth restriction. Fetal. Diagn. Ther. 2018; 44(4): 264-70. https://dx.doi.org/10.1159/000485124
- Gramellini D., Folli M.C., Raboni S., Vadora E., Merialdi A. Cerebral-umbilical Doppler ratio as a predictor of adverse perinatal outcome. Obstet. Gynecol. 1992; 79(3): 416-20. https://dx.doi.org/10.1097/00006250-199203000-00018
- Wolf H., Stampalija T., Monasta L., Lees C.C. Ratio of umbilical and cerebral artery pulsatility indices in assessment of fetal risk: numerator and denominator matter. Ultrasound Obstet. Gynecol. 2020; 56(2): 163-5. https://dx.doi.org/10.1002/uog.22004
- Stumpfe F.M., Mayr A., Schneider M.O., Kehl S., Stübs F., Antoniadis S. et al. Cerebroplacental versus umbilicocerebral ratio-analyzing the predictive value regarding adverse perinatal outcomes in low- and high-risk fetuses at term. Medicina (Kaunas). 2023; 59(8): 1385. https://dx.doi.org/10.3390/medicina59081385
- Kalafat E., Ozturk E., Kalaylioglu Z., Akkaya A.D., Khalil A. Re: Ratio of umbilical and cerebral artery pulsatility indices in assessment of fetal risk: numerator and denominator matter. Ultrasound Obstet. Gynecol. 2020; 56(2): 290-2. https://dx.doi.org/10.1002/uog.22139
- Di Mascio D., Herraiz I., Villalain C., Buca D., Morales-Rosello J., Loscalzo G. et al. Comparison between cerebroplacental ratio and umbilicocerebral ratio in predicting adverse perinatal outcome in pregnancies complicated by late fetal growth restriction: a multicenter, retrospective study. Fetal. Diagn. Ther. 2021; 48(6): 448-56. https://dx.doi.org/10.1159/000516443
Received 02.07.2025
Accepted 14.08.2025
About the Authors
Elizaveta V. Stoliarova, PhD student, 1st Obstetric Department of Pregnancy Pathology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia,117997, Russia, Moscow, Ac. Oparin str., 4, ev_stolyarova@oparina4.ru, https://orcid.org/0009-0001-2049-3119
Alexey M. Kholin, PhD, Head of the Department of Telemedicine, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia,
117997, Russia, Moscow, Ac. Oparin str., 4, a_kholin@oparina4.ru, https://orcid.org/0000-0002-4068-9805
Zulfiya S. Khodzhaeva, Dr. Med. Sci, Professor, Deputy Director, Institute of Obstetrics, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia,
117997, Russia, Moscow, Ac. Oparin str., 4, z_khodzhaeva@oparina4.ru, https://orcid.org/0000-0001-8159-3714
Aleksandr I. Gus, Dr. Med. Sci., Chief Researcher at the Department of Ultrasound and Functional Diagnostics, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4; Head of the Department of Ultrasound Diagnostics, Medical Institute of Patrice Lumumba Peoples’ Friendship University of Russia, a_gus@oparina4.ru, https://orcid.org/0000-0003-1377-3128



