Potential of ultrasound diagnostics in an experimental model of fetal growth restriction
Bespalova O.N., Blazhenko A.A., Kopteeva E.V., Pachulia O.V., Shelaeva E.V., Kogan I.Yu.
Objective: This study aimed to evaluate the effectiveness of an experimental model of fetal growth restriction (FGR) during the intrauterine stage, using ultrasound tracking of fetal biometric parameters and Doppler measurements in Oryctolagus cuniculus rabbits.
Materials and methods: The study included 16 female Oryctolagus cuniculus rabbits (eight for modeling early-onset FGR and eight for late-onset FGR). Ultrasound examinations were conducted on days 20th and 27th days of gestation, with assessment of the head circumference (HC), abdominal circumference (AC), femur length (FL), and pulsatility index (PI) of the umbilical cord artery.
Results: On the 20th day of gestation, the early-onset FGR experimental group exhibited the lowest values of HC (41.0 [IQR 39.5–42.1] mm), AC (48.8 [IQR 46.7–49.7] mm), and FL (4.7 [IQR 4.68–4.82] mm) compared to the control group (p<0.001). Additionally, fetuses from the experimental FGR group displayed an increase in PI in the umbilical cord artery compared with the control group (1.87 versus 1.56, respectively, p<0.001). A similar trend was observed on the 27th day of gestation. All fetal biometric parameters (HC, AC, and FL) were significantly reduced in early-onset FGR fetuses compared to both the control and late-onset FGR groups. Moreover, there was an increase in cord artery PI in early-onset FGR fetuses (both experimental (2.48, IQR 2.15–2.75) and control groups (2.44, IQR 2.30–2.56)) when compared to the late-onset FGR model (p<0.001). When modeling late-onset FGR, an increase in PI in the umbilical cord artery was observed in the experimental group compared to that in the control group (1.83 versus 1.57, respectively, p<0.001).
Conclusion: Selective ligation of uteroplacental vessels is an effective method for modeling FGR in rabbits, and ultrasound examination during the prenatal stage confirms the success of the operation and the presence of severe placental insufficiency.
Authors' contributions: Bespalova O.N., Blazhenko A.A., Kopteeva E.V. – conception and design of the study; Blazhenko A.A., Kopteeva E.V. – material collection and processing; Kopteeva E.V., Shelaeva E.V. – ultrasound examination; Kopteeva E.V., Blazhenko A.A. – statistical analysis; Kopteeva E.V., Blazhenko A.A., Pachulia O.V. – drafting of the manuscript; Pachulia O.V., Bespalova O.N., Kogan I.Yu. – editing of the manuscript.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the D.O. Ott Research Institute for OG&R № (Ref. No: 121 of 05.12.2022) and was conducted in accordance with the International Guidelines for Biomedical Research Involving Animals of the Society for the Study of Reproduction and the European Convention on Animal Experimentation.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Bespalova O.N., Blazhenko A.A., Kopteeva E.V., Pachulia O.V., Shelaeva E.V., Kogan I.Yu.
Potential of ultrasound diagnostics in an experimental model of fetal growth restriction.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2024; (6): 56-64 (in Russian)
https://dx.doi.org/10.18565/aig.2024.24
Keywords
Fetal growth restriction (FGR) is a leading cause of perinatal morbidity and mortality in developed countries, and is associated with impaired placental vascularization [1]. Currently, there are no effective treatments for FGR, and timely delivery is a key approach to prevent adverse perinatal outcomes in this group of patients [2].
Antenatal ultrasound surveillance is the main diagnostic tool for monitoring fetal growth and assessing fetal functional status using Doppler ultrasound [3].
Well-characterized experimental animal models of FGR are crucial to better understand the pathogenesis of FGR and develop new therapeutic strategies to correct this condition [4]. Rabbits (Oryctolagus cuniculus) are a convenient animal model for studying FGR because of their high reproductive potential and short gestation period of 30 days [5]. Their larger size compared to other laboratory rodents allows for the use of instrumental diagnostic methods, such as ultrasound imaging, providing accurate and objective information about fetal size and hemodynamic characteristics [6]. Additionally, the metabolic requirements and rate of embryonic genome activation at the early stages of development are similar in human and rabbit embryos [7, 8]. The placenta of rabbits (Oryctolagus cuniculus) has a hemochorial structure, which makes it more similar to the human placenta than to that of other animals [9].
There are various models of FGR formation in rabbits based on maternal nutritional restriction, surgical ablation or ligation of the uterine blood supply, or umbilical cord occlusion. However, it is obvious that no model completely reproduces the pathogenesis of FGR owing to the multifactorial nature of its causes [4, 10]. According to a review of the literature, some studies have demonstrated the success of using ultrasound examination (US examination) as an additional tool for assessing fetal growth and condition in animal models; therefore, its use in modeling FGR is of particular interest [9–12].
This study aimed to evaluate the effectiveness of an experimental model of fetal growth restriction during the intrauterine stage using ultrasound tracking of fetal biometric parameters and Doppler measurements in Oryctolagus cuniculus rabbits.
Materials and methods
Rabbits Oryctolagus cuniculus with vaccination and health certificates obtained from the Stezar nursery were chosen as the experimental animals. This study included 16 female rabbits that were kept individually. The study was reviewed and approved by the local Research Ethics committee (Ref. No. 121 of 05.12.2022) and was conducted in accordance with the International Guidelines for Biomedical Research Involving Animals of the Society for the Study of Reproduction [7] and the European Convention on Animal Experimentation.
Sixteen female rabbits were divided into two groups: 1) eight animal units to create an early-onset FGR model and 2) eight animal units to create a late-onset FGR model. To create an experimental model, ligation of the vessels in the right horn of the uterus was performed. Fetuses that developed in the left horn (where vascular ligation was not performed) were included in the control group. Thus, both the FGR model and the «conditionally healthy» pregnancy model were reproduced in one female rabbit (Fig. 1). Female rabbits from the late-onset FGR group underwent vascular ligation on the 25th day of gestation. At the time of the first ultrasound assessment (20th day of gestation), the fetuses from these females constituted a «pure» control group (group III), because at this stage, the pregnancy developed without intervention. When conducting an US examination on the 27th day of gestation, this group represented a model of the formation of late-onset FGR (groups IV and V).
For ultrasound assessment, the following study groups were formed:
Group I was the experimental group with early-onset FGR (fetuses from the right horn, where vascular ligation was performed before pregnancy).
Group II was the control group for early-onset FGR (fetuses from the left horn where vascular ligation was not performed).
Group III was a «pure» control group (fetuses from females that did not undergo vascular ligation).
Group IV was the experimental group with late-onset FGR (fetuses from the right horn, where vascular ligation was performed on the 25th day of gestation).
Group V was the control group for late-onset FGR (fetuses from the left horn where vascular ligation was not performed).
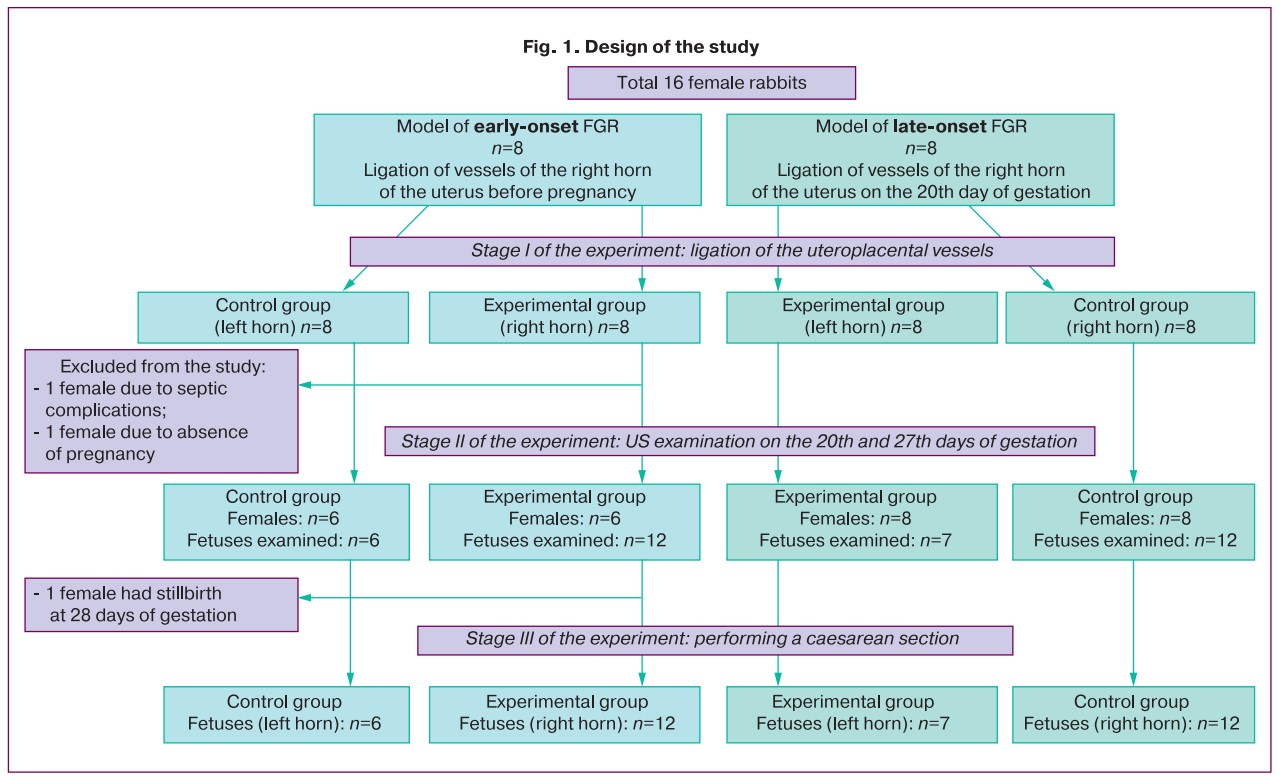
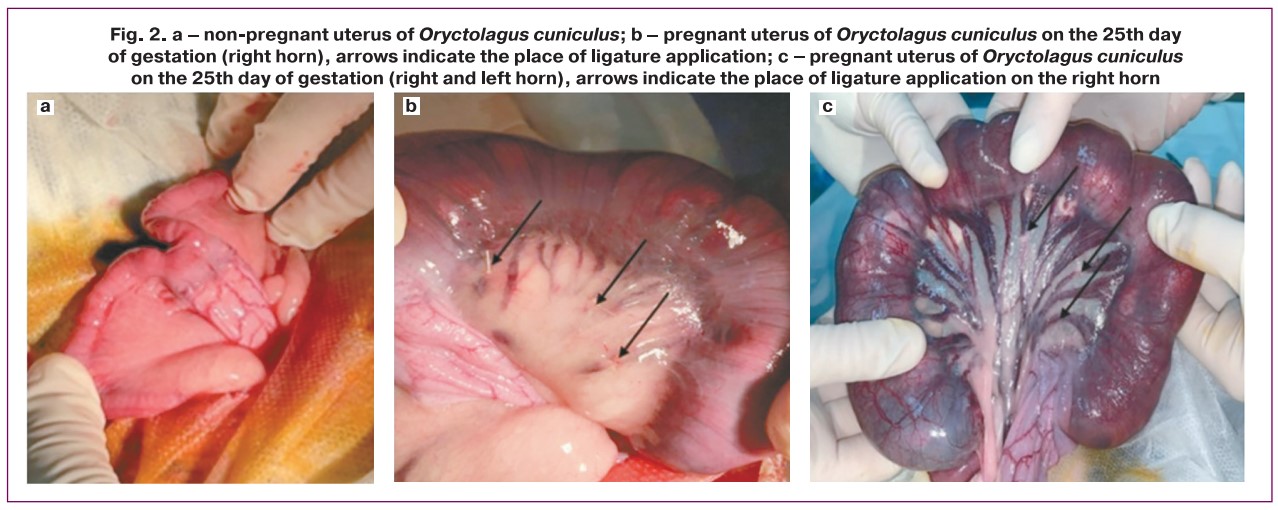
1) FGR modeling was performed surgically. To simulate early-onset FGR, ligation of uterine vessels was performed before pregnancy. The animals were monitored for one month before pregnancy. In the late-onset FGR model, ligation of the placental-uterine vessels was performed on the 25th day of rabbit pregnancy (Fig. 2). Pregnancy was dated from the moment of introduction to the male rabbit, and on the 14th day, a US examination was performed to confirm the presence of pregnancy. The uterine vessels were ligated only in the right horn of the uterus. The vessels of the left uterine horn were not ligated, and it was considered a «control».
2) The anesthesia procedure was approved by the local Research Ethics Committee.
3) Surgical procedure for simulating FGR.
After the anterior abdominal wall was shaved and aseptically treated, an incision of approximately 5 cm was made along the midline of the lower third of the abdomen. The tissues of the anterior abdominal wall were then opened layer-by-layer using sharp and blunt dissection. The uterus was completely exteriorized into the surgical field and lined with gauze pads moistened with warm saline solution. After identification of the uteroplacental vessels for each gestational sac, ligation 30–40% of these vessels were ligated, ligating only one horn and leaving the second horn intact. After completion of the manipulation, the uterus was carefully returned to the abdominal cavity and sutured in layers.
Ultrasound examination
US examination was performed on the 20th and 27th days of gestation in Oryctolagus cuniculus rabbits (total duration of pregnancy was 30 days). Before US examination, the rabbit anterior abdominal wall was prepared for optimal scanning. No anesthesia was used during the US examination, and the rabbits were held in the arms of a second researcher. This study was performed using a high-resolution linear transducer of 12–5 MHz. For scanning, two fetuses were selected from each horn of one female, which were located superficially and were accessible for inspection and reliable identification to avoid repeated measurements in one fetus. The first US examination was performed on the 10th day after mating to verify pregnancy and determine the number of fetal sacs.
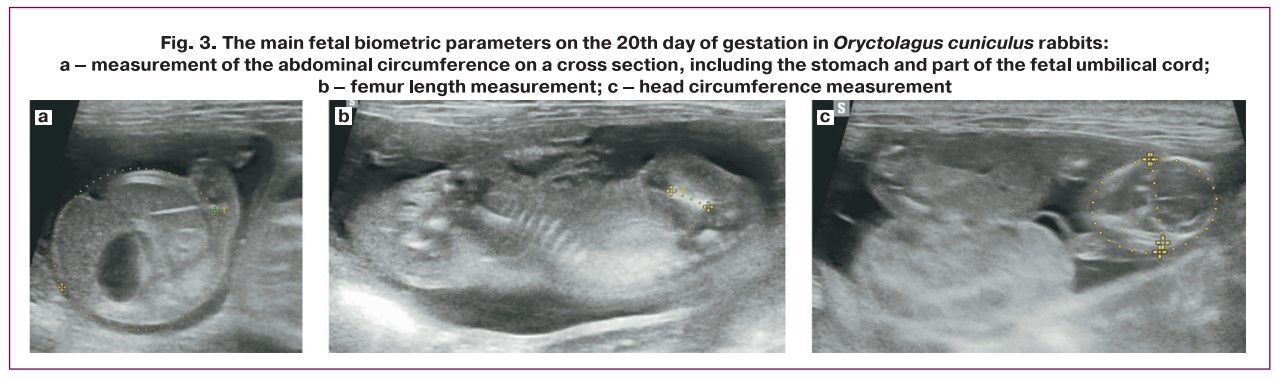
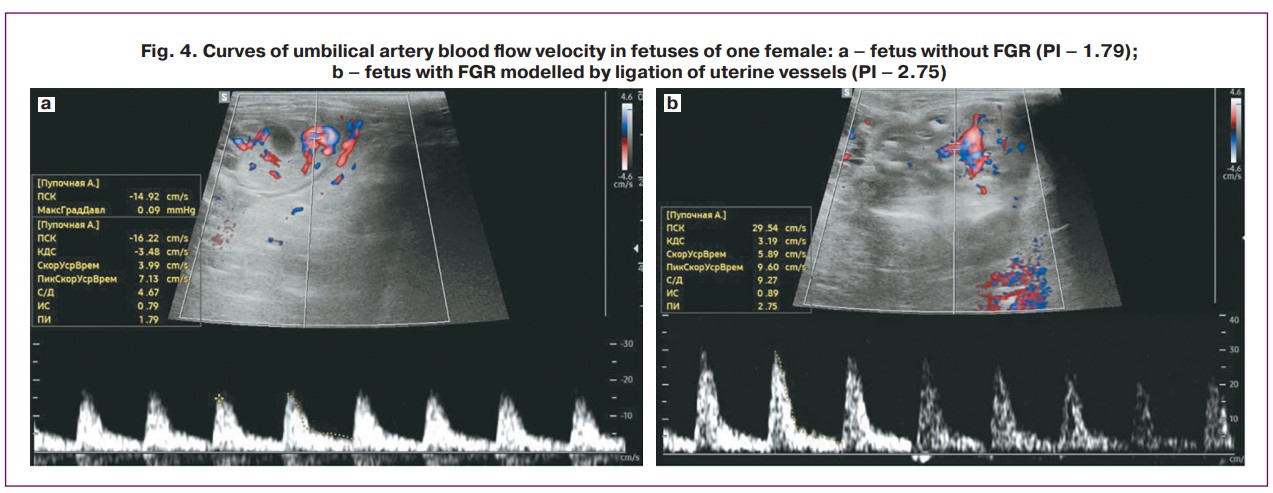
Ultrasound fetal biometry was performed according to a method previously described by Coombs P. et al. [3]. To measure head circumference (HC), the coronal plane was chosen, where the falx and thalamus were used to determine the level at which the maximum biparietal dimension could be obtained (Fig. 3a). Abdominal circumference (AC) was measured in the axial plane of the abdomen at the level of the stomach and umbilical vein, and the outer contour was determined using an ellipse (Fig. 3b). Femur length (FL) was measured when the maximum length of the femoral metaphysis was imaged (Fig. 3c). The pulsatility index (PI) of the umbilical cord artery was also assessed using spectral Doppler mode (Fig. 4).
Statistical analysis
The distribution of continuous variables was tested for normality using the Kolmogorov-Smirnov test. Continuous variables were not normally distributed and were reported as median (Me) and interquartile range (IQR)=(Q1–Q3). The statistical significance of between-group differences in continuous variables was tested using the Mann–Whitney U test. Kruskal–Wallis test was used to compare numerical data between three or more groups, followed by Dunn’s multiple comparison test with Holm’s correction. The hypothesis regarding the equality of mean values in the study groups was rejected at a significance level of p<0.05. Statistical analysis was performed using the Jamovi software version 2.4.8.
Results
Performance of the experimental model
When modeling early-onset FGR, which included ligation of uterine vessels on a non-pregnant uterus, two females were excluded from the study: one due to the development of septic complications and the second due to the absence of pregnancy. The third female was excluded from the final phase of the study because of stillbirth at 28 days of gestation.
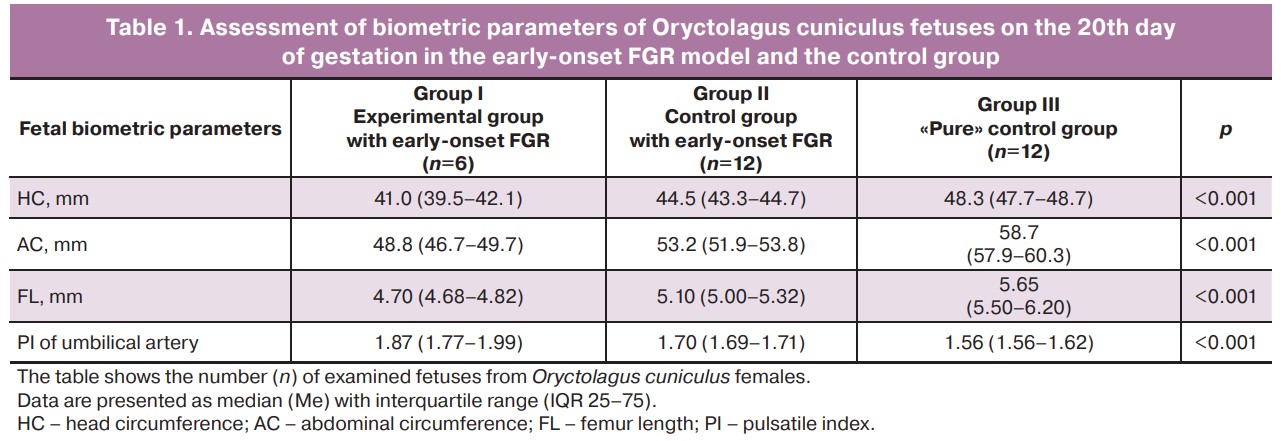
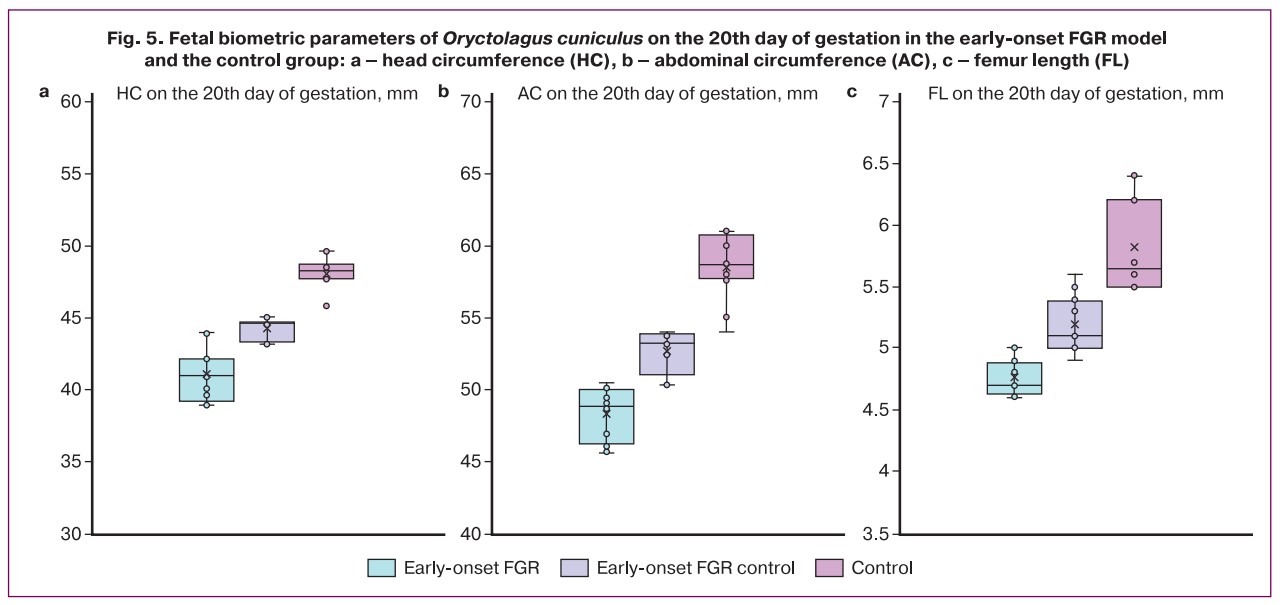
Assessment of fetal biometric parameters of Oryctolagus cuniculus fetuses on the 20th day of gestation
When analyzing the biometric parameters of rabbit fetuses on the 20th day of gestation in the early-onset FGR model (ligation of the uterine vessels of one horn before pregnancy) and the control group, the main parameters differed significantly between the study groups. The lowest values of HC (41.0 (IQR 39.5–42.1) mm), AC (48.8 (IQR 46.7–49.7) mm), FL (4.7 (IQR 4.68–4) .82) mm) were observed in the early-onset FGR experimental group. It was also found that fetuses from the early-onset FGR control group had higher fetal biometric parameters than those from the experimental group, but lower parameters than those from the control group (p<0.001, Table 1, Fig. 5). At the same time, fetuses from the experimental FGR group showed an increase in PI in the umbilical cord artery compared with the control group (1.87 versus 1.56, respectively, p<0.001).
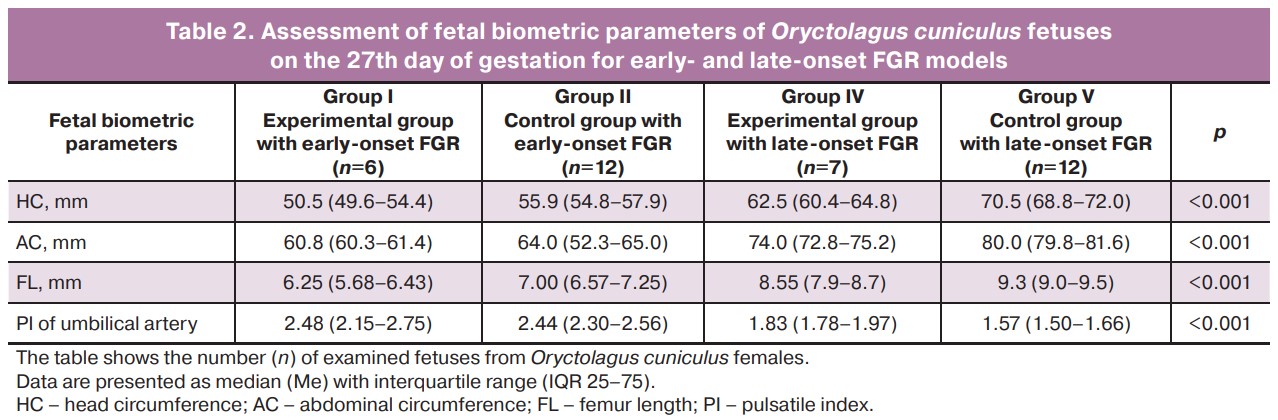
Assessment of fetal biometric parameters of Oryctolagus cuniculus fetuses on the 27th day of gestation
When analyzing the fetal biometric parameters of fetuses on the 27th day of gestation for the model of early-onset FGR (ligation of the uterine vessels of one horn before pregnancy) and late-onset FGR (ligation of the uterine vessels of one horn on the 25th day of gestation), it was found that the lowest HC values (50.5 (IQR 49.6–54.4) mm), AC (60.8 (IQR 60.3–61.4) mm) and FL (6.25 (IQR 5.68–6.43) mm) were observed in the early-onset FGR experimental group and were significantly different compared to other groups (p<0.001, Table 2, Fig. 6). In the late-onset FGR model, significant differences were also revealed: all fetal parameters (HC, AC, FL) were significantly reduced in fetuses from the horn where vascular ligation was performed compared to fetuses from the control horn (p<0.001), and fetuses from the early-onset FGR control group had higher fetal biometric parameters than fetuses from the experimental group but lower than those from the late-onset FGR model (p<0.001, Table 2, Fig. 6). There was an increase in cord artery PI in fetuses with early-onset FGR (both experimental (2.48, IQR 2.15–2.75) and control groups (2.44, IQR 2.30–2.56), compared with the model of late-onset FGR (p<0.001). When modeling late-onset FGR, there was an increase in PI in the umbilical cord artery in the experimental group compared to that in the control group (1.83 versus 1.57, respectively, p<0.001).
Estimation of weight of newborn Oryctolagus cuniculus
Thirty-seven live fetuses were obtained from 13 female rabbits. Insufficient fetal growth in FGR is confirmed by a decrease in body weight at birth. Significant differences were observed between the study groups when modelling early- and late-onset FGR (p<0.001). The median fetal weight in the control group with early-onset FGR was 29.5 (IQR 27.3–33.3) g, and in the experimental group it was 26.5 (IQR 20.5–28.8) g. When modeling late-onset FGR in the control group median birth weight was 49.5 (IQR 48.8–52.3) g, in the late-onset FGR experimental group it was 34.0 (IQR 33.0–35.5) g.
Estimation of weight of Oryctolagus cuniculus placentas at birth
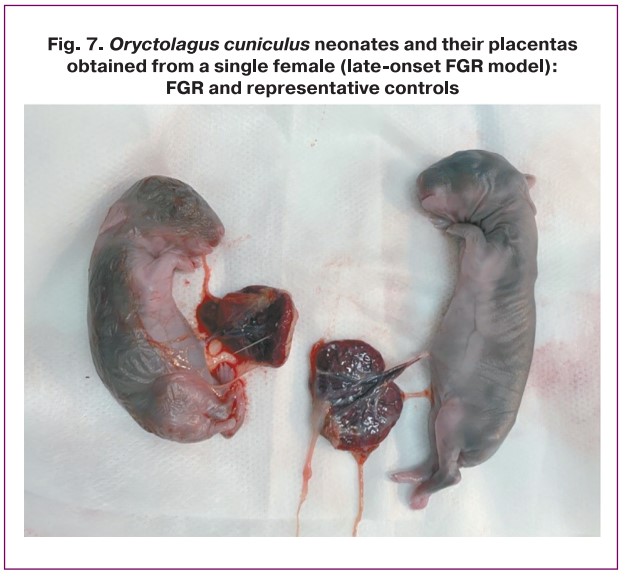
The weights of the placentas in the study groups also showed significant differences (p=0.007). When modeling early-onset FGR, placental weights did not differ between the experimental and control groups (7.5 (IQR 5.5–8.0) g and 7.0 (IQR 6.0–8.3) g, respectively; p>0.05). When modeling late-onset FGR, the weight of placentas in the experimental and control groups was significantly different (4.0 (IQR 3.0–4.5) g and 7.0 (IQR 7.0–8.0) g, respectively, p=0.028) (Table 3, Fig. 7).
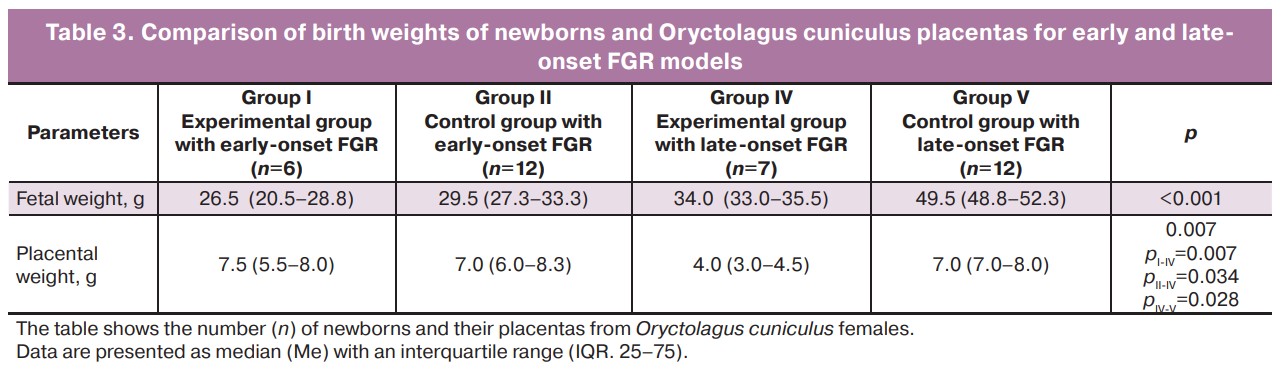
Discussion
In our study, to verify the modeling of early- and late-onset FGR, pregnant females of Oryctolagus cuniculus underwent US examination on days 20 and 27 of gestation. When analyzing fetal biometric parameters on the 20th day of gestation, there was a significant decrease in fetal biometric parameters (HC, AC, and FL) in the experimental group compared to the control group of early-onset FGR modeling; however, the indicators in both groups were reduced compared to the control group (p<0.001). A similar trend persisted during the US examination on the 27th day of gestation: fetal biometric parameters continued to lag in the experimental group of early-onset FGR compared to the control group of early-onset FGR. When modeling late-onset FGR on the 25th day of gestation, a significant difference in fetal biometric parameters was noted in the experimental group. The HC, AC, and FL were significantly higher in the late-onset FGR model than in the early-onset FGR model. The greatest increase in umbilical artery PI was observed in the early-onset FGR model; when modeling late-onset FGR, the umbilical cord artery PI was higher in the experimental group.
Coombs P. et al. (2020) [3] demonstrated the possibility of using ultrasound biometry of Oryctolagus cuniculus fetuses to assess growth during pregnancy in a rabbit model, defining standards for measuring key parameters (biparietal size, HC, AC and FL). In addition, Chavatte-Palmer P. et al. (2008) [9] and DeKoninck P. et al. (2014) [11] documented fetal growth curves in rabbits.
In our study, when modeling early-onset FGR, where ligation of the uterine vessels in one of the uterine horns occurred before pregnancy, fetuses from both the experimental and control groups had lower fetal biometric parameters and birth weights compared to the late-onset FGR group. Such results can be explained by an overall decrease in uterine perfusion and the formation of collateral arteries during pre-pregnancy vascular ligation, which results in inadequate nutrient delivery to all fetuses of a female. At the same time, in the simulation of late-onset FGR, fetuses from the control horns were able to fully realize their growth potential, and fetuses from the experimental group began to slow down their growth rates on day 27 of gestation; however, their size exceeded that of fetuses from the early-onset FGR group. There was a significant decrease in placental weight in late-onset FGR compared to early-onset FGR, which is probably explained by adaptation mechanisms during the initial disturbance of perfusion by surgical ligation of the artery.
In our study, fetuses with FGR had a higher umbilical artery PI than fetuses from the control group. Lopez-Tello J. et al. (2015) [5] used a food-restricted FGR model in rabbits. On day 21, maternal food restriction resulted in a significant reduction in fetal size. At birth, the fetal biometric dimensions of newborn Oryctolagus cuniculus from the study group were significantly smaller (crown-rump length, biparietal diameter, and transverse thoracic diameter) and had a lower weight compared to the control group. Feeding restriction led to an increase in umbilical artery PI, and fetuses with FGR showed compensatory effects characterized by a decrease in the cerebroplacental ratio compared to the control group [5]. Hodges et al. (2013) [10] used a surgical model of FGR in Oryctolagus cuniculus and showed that placental insufficiency is manifested by the absence or reversal of end-diastolic blood flow in the umbilical artery, which is consistent with sonographic data during the formation of FGR in humans [10].
Herrera E.A. et al. [12] showed similar results in an experiment conducted on guinea pigs. Uterine artery occlusion induced a decrease in fetal AC growth rate (0.205 cm day (-1)) compared to the control (0.241 cm day (-1), p<0.001). The PI of the umbilical cord arteries on the 10th and 20th days after surgery in fetuses with FGR was significantly higher than that in the control group (p<0.01). These effects were associated with a decrease in the relative luminal area of the placental arteries (21.3±2.2% vs. 33.2±2.7%, P<0.01) in the FGR gilts. Uterine artery intervention reduced the weight of the fetus (~30%), placenta (~20%), and liver (~50%) compared with the control group [12].
The study had limitations regarding the use of US examination in pregnant females with Oryctolagus cuniculus. With a large number of fetuses (more than four) in one female, it was impossible to qualitatively identify the fetuses, which made it difficult to observe them in the late stages of gestation. Another important limitation of this study is that the uterine artery ligation technique is often associated with high rates of fetal death, especially when it is performed early in pregnancy and 40–50% of the vessels are ligated [4].
Conclusion
Selective ligation of uteroplacental vessels is an effective method for modeling early- and late-onset FGR in rabbits. Ultrasound examination during the prenatal stage confirms the success of the operation and the presence of severe placental insufficiency.
References
- Burton G.J., Jauniaux E. Pathophysiology of placental-derived fetal growth restriction. Am. J. Obstet. Gynecol. 2018; 218(2S): S745-S761. https://dx.doi.org/10.1016/J.AJOG.2017.11.577.
- Bertholdt C., Dap M., Pillot R., Chavatte-Palmer P., Morel O., Beaumont M. Assessment of placental perfusion using contrast-enhanced ultrasound: a longitudinal study in pregnant rabbit. Theriogenology. 2022; 187: 135-40. https://dx.doi.org/10.1016/J.THERIOGENOLOGY.2022.05.002.
- Coombs P., Walton S.L., Maduwegedera D., Flower R.L., Denton K.M. Fetal growth and well-being in a study of maternal hypertension in rabbits. Anat. Rec. (Hoboken). 2020; 303(10): 2646-56. https://dx.doi.org/10.1002/AR.24344.
- Lopez-Tello J., Arias-Alvarez M., Gonzalez-Bulnes A., Sferuzzi-Perri A.N. Models of intrauterine growth restriction and fetal programming in rabbits. Mol. Reprod. Dev. 2019; 86(12): 1781-809. https://dx.doi.org//10.1002/MRD.23271.
- López-Tello J., Barbero A., González-Bulnes A., Astiz S., Rodríguez M., Formoso-Rafferty N. et al. Characterization of early changes in fetoplacental hemodynamics in a diet-induced rabbit model of IUGR. J. Dev. Orig. Health Dis. 2015; 6(5): 454-61. https://dx.doi.org/10.1017/S2040174415001385.
- Mourier E., Tarrade A., Duan J., Richard C., Bertholdt C., Beaumont M. et al. Non-invasive evaluation of placental blood flow: lessons from animal models. Reproduction. 2017; 153(3): R85-R96. https://dx.doi.org/10.1530/REP-16-0428.
- Liu Z., Foote R.H. Effects of amino acids on the development of in-vitro matured/in-vitro fertilization bovine embryos in a simple protein-free medium. Hum. Reprod. 1995; 10(11): 2985-91. https://dx.doi.org/10.1093/oxfordjournals.humrep.a135834.
- Christians E., Boiani M., Garagna S., Dessy C., Redi C.A., Renard J.P. et al. Gene expression and chromatin organization during mouse oocyte growth. Dev. Biol. 1999; 207(1): 76-85. https://dx.doi.org/10.1006/DBIO.1998.9157.
- Chavatte-Palmer P., Laigre P., Simonoff E., Chesné P., Challah-Jacques M., Renard J.P. In utero characterisation of fetal growth by ultrasound scanning in the rabbit. Theriogenology. 2008; 69(7): 859-69. https://dx.doi.org/10.1016/J.THERIOGENOLOGY.2007.12.013.
- Hodges R., Endo M., La Gerche A., Eixarch E., DeKoninck P., Ferferieva V. et al. Fetal echocardiography and pulsed-wave Doppler ultrasound in a rabbit model of intrauterine growth restriction. J. Vis. Exp. 2013; (76): 50392. https://dx.doi.org/10.3791/50392.
- DeKoninck P., Endo M., Sandaite I., Richter J., De Catte L., Van Calster B. et al. A pictorial essay on fetal rabbit anatomy using micro-ultrasound and magnetic resonance imaging. Prenat. Diagn. 2014; 34(1): 84-9. https://dx.doi.org/10.1002/PD.4259.
- Herrera E.A., Alegría R., Farias M., Díaz-López F., Hernández C., Uauy R. et al. Assessment of in vivo fetal growth and placental vascular function in a novel intrauterine growth restriction model of progressive uterine artery occlusion in guinea pigs. J. Physiol. 2016; 594(6): 1553-61. https://dx.doi.org/10.1113/JP271467.
Received 02.02.2024
Accepted 29.05.2024
About the Authors
Olesya N. Bespalova, Dr. Med. Sci., Deputy Director for Science, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology, 199034, Russia,St. Petersburg, Mendeleevskaya line, 3, shiggerra@mail.ru, https://orcid.org/0000-0002-6542-5953
Alexandra A. Blazhenko, PhD, Junior Researcher at the Department of Pharmacology, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology,
199034, Russia, St. Petersburg, Mendeleevskaya line, 3, alexandrablazhenko@gmail.com, https://orcid.org/0000-0002-8079-0991
Ekaterina V. Kopteeva, Junior Researcher at the Department of Obstetrics, Division of Maternal-Fetal Medicine, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology, 199034, Russia, St. Petersburg, Mendeleevskaya line, 3, ekaterina_kopteeva@bk.ru, https://orcid.org/0000-0002-9328-8909
Olga V. Pachulia, PhD, Scientific Secretary, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology, 199034, Russia, St. Petersburg, Mendeleevskaya line, 3, for.olga.kosyakova@gmail.com, https://orcid.org/0000-0003-4116-0222
Elizaveta V. Shelayeva, PhD, Senior Researcher at the Department of Obstetrics, Division of Maternal-Fetal Medicine, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology, 199034, Russia, St. Petersburg, Mendeleevskaya line, 3, eshelaeva@yandex.ru
Igor Yu. Kogan, Dr. Med. Sci., Professor, Corresponding Member of RAS, Director of D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology,
199034, Russia, St. Petersburg, Mendeleevskaya line, 3, ikogan@mail.ru, https://orcid.org/0000-0002-7351-6900
Corresponding author: Alexandra A. Blazhenko, alexandrablazhenko@gmail.com



