Supplementary infertility factors in patients with intramural uterine fibroids
Relevance: Uterine fibroids (UFs) are the most common neoplasms affecting women of reproductive age and consist of smooth muscle cells. UFs are not unequivocally associated with abnormal fertility; however, their negative impact on reproductive function is undisputed. Our analysis of the literature allowed us to formulate the hypothesis that in infertile patients with intramural UFs without uterine cavity deformity, there are other factors in addition to the local factors that are associated with implantation failure. Objective: This study aimed to investigate clinical and anamnestic supplementary infertility factors in patients with intramural UFs. Materials and methods: The study analyzed medical records of 370 patients of reproductive age with UFs (FIGO type 3–6) who were managed between 2017 and 2020. The patients were divided into two groups. The study group comprised 240 infertile patients with intramural UFs. The control group included 130 fertile patients with intramural UFs. Among them, 70 patients were in the first trimester of pregnancy, and 60 women were diagnosed with UFs before or during pregnancy and gave birth less than 12 months ago. Statistical analysis was performed using SPSS (version 10.0.7) and Statistica (version 8.0) for Windows. Differences between the groups were considered statistically significant at p<0.001. Results: This study demonstrated for the first time the presence of supplementary factors associated with infertility in patients with intramural UFs. These factors include a history of spontaneous miscarriage, iron deficiency anemia, several endocrine diseases (insulin resistance, obesity), and a combination of UFs with proliferative diseases of the reproductive system (endometrial hyperplasia/polyp, endometriosis of the uterus, and pelvic peritoneum). Iron deficiency anemia, cardiovascular diseases (arterial hypertension and mitral valve prolapse), and a significant risk factor for their development, such as hypercholesterolemia, were significantly more frequent in the group of infertile patients with UFs. A strong association has also been found between infertility in patients with UFs, long and heavy menstruation, and chronic pelvic pain. Conclusion: This study revealed that patients with intramural UFs have a set of supplementary factors associated with infertility. It is likely that the concomitant gynecological and extragenital pathological changes identified in patients with intramural UFs can be classified as risk factors for infertility. Authors' contributions: Dubinskaya E.D., Kolesnikova S.N., Gasparov A.S. – conception and design of the study; Kolesnikova S.N., Alyoshkina E.V., Bashkirova E.S., Leffad M.L. – data collection and analysis; Kolesnikova S.N., Alyoshkina E.V. – manuscript drafting; Dubinskaya E.D., Kolesnikova S.N., Gasparov A.S. – manuscript editing. Conflicts of interest: The authors have no conflicts of interest to declare. Funding: There was no funding for this study. Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the Peoples' Friendship University of Russia. Patient Consent for Publication: All patients provided informed consent for the publication of their data. Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator. For citation: Dubinskaya E.D., Kolesnikova S.N., Alyoshkina E.V., Gasparov A.S., Bashkirova E.S., Leffad M.L. Supplementary infertility factors in patients with intramural uterine fibroids. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2023; (5): 75-82 (in Russian) https://dx.doi.org/10.18565/aig.2023.28Dubinskaya E.D., Kolesnikova S.N., Alyoshkina E.V., Gasparov A.S., Bashkirova E.S., Leffad M.L.
Keywords
Uterine fibroids (UFs) are the most common neoplasms affecting women of reproductive age and consist of smooth muscle cells [1, 2]. According to statistics, UFs are found in more than 30% of women of reproductive age and in 30–40% of women over 40 years of age [3].
One in ten women with infertility is known to have UFs. [4]. There is no clear link between UFs and abnormal fertility; however, there is no doubt that they have a negative impact on reproductive function. A meta-analysis by Pritts et al. showed a statistically significant reduction in the likelihood of implantation, pregnancy, and childbirth along with an increase in spontaneous miscarriages in patients with intramural fibroids [5]. In 2017. Christopoulos et al. also proved a reduced likelihood of pregnancy in assisted reproductive technology (ART) programs in patients with intramural UFs without cavity deformity [6]. Similar results have been reported by Sagi-Dain et al. [7].
Concurrently, completely opposite results were reported in the literature. Klatsky et al. showed that there were no differences in pregnancy rates between patients with and without intramural UFs [8]. Similar data were obtained by other authors analyzing the results of artificial insemination in patients with interstitial UFs and unclear genesis of infertility [9].
Our analysis of the literature allowed us to formulate the hypothesis that in infertile patients with intramural UFs without uterine cavity deformities, there are other factors in addition to the local factors that are associated with implantation failure.
This study aimed to investigate clinical and anamnestic supplementary infertility factors in patients with intramural UFs.
Materials and methods
This retrospective study analyzed the medical records of 370 patients of reproductive age with UFs (FIGO type 3–6), who were managed between 2017 and 2020.
Patients were divided into two groups. The study group consisted of 240 infertile patients with intramural UF. The control group comprised 130 fertile patients with intramural UFs. Among them, 70 were in the first trimester of pregnancy, and 60 were diagnosed with UFs before or during pregnancy and gave birth less than 12 months ago.
The inclusion criteria for the study group were age 20–35 years, UFs (FIGO type 3–6), no pregnancy for more than 1 year of regular sexual intercourse, regular menstrual cycle, no use of hormonal drugs within the last 6 months, a history of failed IVF attempts, normal ovarian reserve (AMH≥1.2 ng/ml), no history of surgery for UFs, and informed consent to participate in the study.
Inclusion criteria for the control group were age 20–35 years, UFs (FIGO type 3–6), regular menstrual cycle, women who applied for routine care, outpatient medical supervision, and selection of contraception within 12 months of giving birth and who were diagnosed with UFs during pregnancy and/or before pregnancy, women in the first trimester of pregnancy, and informed consent to participate in the study.
The exclusion criteria for the study group were submucosal UFs (FIGO type 0–2) and subserosal UFs (FIGO type 7), large fibroids and uterus (> 12 weeks gestation) [10], presence of acute pelvic inflammatory diseases, tubal infertility verified at the time of the study, female infertility of cervical origin, male factor infertility, infertility associated with lack of ovulation, low ovarian reserve (AMH<1.2 ng/mL), ovarian tumors, history of surgery for UFs, widespread endometriosis verified by laparoscopy, cancer, hyperprolactinemia associated with macro- or microadenoma of the pituitary gland (according to MRI), and body mass deficiency (body mass index < 19.9 kg/m2).
The exclusion criteria for the control group were submucosal UFs (FIGO type 0-2) and subserosal UFs (FIGO type 7), large fibroids, and uterus (>12 weeks gestation) [10].
The location of UFs was determined using the FIGO classification [9] and the effect of large and small fibroids on fertility (according to the Russian Ministry of Health clinical guidelines on UFs (2020), large fibroids are considered larger than 12 weeks' gestation) [1]. Ultrasound diagnosis was performed using a transvaginal volumetric transducer on a Voluson E 10 Expert system. The presence of extragenital endometriosis was verified by laparoscopy or MRI, tubal factor infertility by laparoscopy, or hysterosalpingography. The sociodemographic and medical history data of the patients in the study groups were analyzed.
Statistical analysis
Statistical analysis was performed using SPSS (version 10.0.7) and Statistica (version 8.0) for Windows. Differences between the groups were considered statistically significant at p<0.001. The normality of the distribution was tested using the Kolmogorov-Smirnov test. Quantitative variables showing normal distribution were expressed as mean (M) and standard deviation (SD) and presented as M (SD); otherwise, the median (Me) with interquartile range (Q1; Q3) was reported. Categorical variables are presented as counts (n) and percentages (%). Differences between groups were assessed using the Student’s t-test when comparing continuous variables showing normal distribution and equality of variance (Levene's test). Otherwise, the nonparametric Mann–Whitney U test was used if the normality assumption was not met. All diseases were coded according to the International Classification of Diseases Revision 10 (ICD 10) and coded in binary form, where 1 was a disease and 0 was no disease. Categorical variables were compared using the chi-square test with maximum likelihood adjustment, Fisher's two-tailed exact test, or Z-score with end-point adjustment (in the case of a 0% or 100% comparison). In the analysis of the factors influencing female fertility, the relative risk with a 95% confidence interval (95% CI) was calculated.
Results
The sociodemographic and clinical characteristics of the patients in both groups are shown in Table 1.
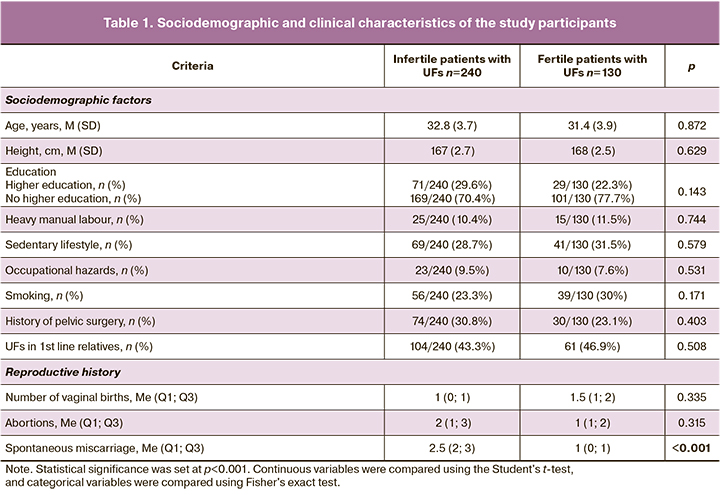
The data showed that the patients in both groups did not differ in terms of age. Every second patient in both groups had an uncomplicated family history of UFs. The vast majority of the patients in both groups had higher education, a healthy lifestyle, and no occupational hazards. The proportion of smokers did not differ between the groups and was 56/240 (23.3%) and 39/130 (30%) patients, respectively.
A history of spontaneous miscarriage was significantly more common in the group of infertile patients with UFs than in the group of fertile women with UFs (2.5 (2; 3) vs. 1 (0; 1), p<0.001).
The comorbidity analysis of the patients in the study groups is presented in Table 2.
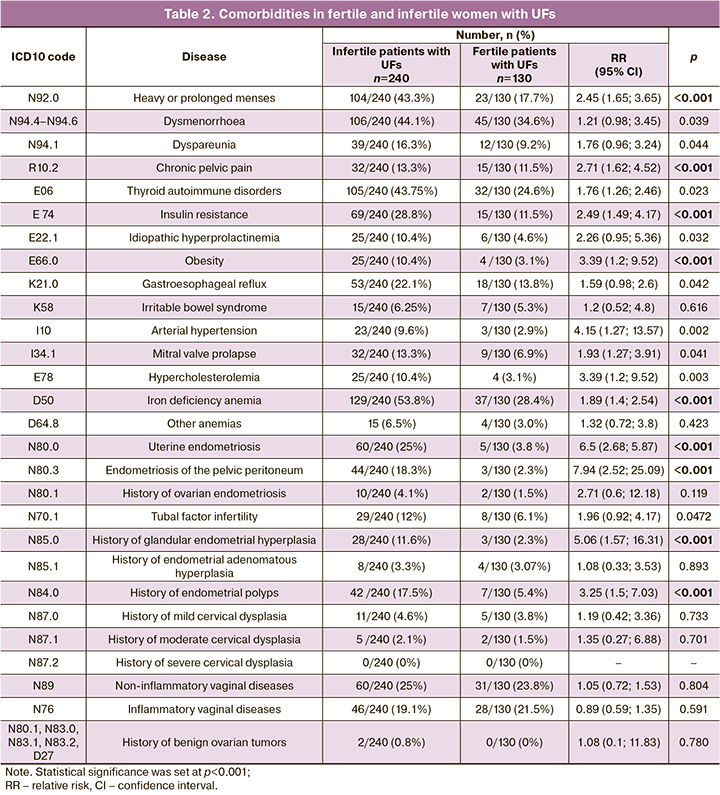
Comparative analysis of the data showed that endocrine pathology (obesity, insulin resistance, autoimmune thyroid disease, and hyperprolactinemia) was more than 2-fold more common in the study group of infertile women with UFs than among fertile patients with UFs.
Chronic pelvic pain was also strongly associated with infertility in patients with UFs. Uterine endometriosis was diagnosed 6 times more frequently in infertile patients with UFs than in fertile women with UFs. Proliferative diseases of the female reproductive system (endometrial hyperplasia/polyp and endometriosis of the pelvic peritoneum) were also significantly more frequent in infertile patients with UFs.
Discussion
This study demonstrated for the first time the presence of supplementary factors associated with infertility in intramural UFs. These include symptoms of UFs (prolonged and heavy menstruation and chronic pelvic pain), a history of spontaneous miscarriage, iron deficiency anemia, a number of endocrine diseases (insulin resistance, obesity), and a combination of UFs with proliferative diseases of the reproductive system (hyperplastic processes/endometrial polyps, uterine and pelvic peritoneal endometriosis).
The study showed that women with UF and no deformities in the uterine cavity can be both fertile and infertile; patients with low ovarian reserve and concomitant infertility factors (male, endocrine, and tubal-peritoneal at the time of the study) were excluded from the study.
An analysis of the findings and an attempt to systematize them led to the formulation of the following concept. It is known that a significant number of immunological, neoplastic, infectious diseases, and even the development of atherosclerosis are associated with a pathological inflammatory process that contributes to perpetual tissue damage, which leads to the formation of chronic diseases [11]. Many researchers have proven that the chronic inflammatory process plays a significant role in the formation of benign diseases of the female genital sphere, including UFs [12]. There is evidence of an increased number of CD68+ macrophages in UFs compared to the unchanged myometrium, confirming the role of local inflammation in the disease [13, 14]. Systemic manifestations of inflammation have also been proven in myomas, and an increase in the levels of serum tumor necrosis factor-⍺, including a tumor size-dependent increase, has been established [15]. Unfortunately, to date, it is not clear where and how pathological inflammation develops at the systemic or local level or is formed in parallel [16]. Similar pro-inflammatory changes have been observed in endometriosis [17].
Many authors have also shown the presence of chronic inflammatory processes in adipose tissue of obese patients [18]. Our findings suggest that diseases and conditions with an inflammatory component in their pathogenesis, such as obesity and insulin resistance, adenomyosis, menorrhagia, dyspareunia, and a history of spontaneous miscarriage, are associated with infertility in patients with intramural UFs.
Increased body mass index is associated with an increased likelihood of gynecological diseases, including UFs [19]. Elevated leptin levels are associated with UFs, endometriosis, and arterial hypertension [20]. It is also known that leptin itself impairs steroidogenesis and directly affects embryo quality, thus reducing the likelihood of implantation [21]. A vicious circle emerges where all changes are interrelated and affect each other indefinitely, supporting the pathological process.
The results of this study showed a 3-fold higher incidence of arterial hypertension in infertile patients with intramural UFs than in the control group. However, there is an increased chance of UFs formation in the presence of hypertensive disorders [22]. Furthermore, polymorphisms of genes that regulate vascular tone and arterial blood flow through angiotensin-converting enzymes and angiotensin receptors are also associated with fibroids [23]. On the one hand, hypertension is known to promote smooth muscle cell damage through mechanical stress involving the blood supply to the myometrium, inducing proliferation and growth of fibroids. Additionally, locally elevated blood pressure induces a pro-inflammatory shift and dysregulates extracellular matrix synthesis [24].
In 1983, Walters et al. showed that UFs are associated with prolactin expression in the endometrium and myometrium [25]. Several cases of hyperprolactinemia in UFs have been confirmed by modern researchers, with decreased levels after myomectomy [26]. Serum prolactin levels are known to correlate with UF size [27]. However, the present study did not reveal statistically significant differences in the presence of hyperprolactinemia that was not associated with pituitary macroadenomas or microadenomas between the groups.
In the context of the statistically significant increase in the combination of proliferative diseases of female genital organs and infertility in patients with UFs identified in this study, it is also appropriate to mention the well-studied nuclear antigen of proliferating cells, a protein and proteome that determines life processes inside the cell. Its increased expression has been associated with UFs, endometriosis, endometrial hyperplastic processes and infertility [28].
It is possible that overall genome instability and abnormalities in the same genes contribute to a particular pattern of changes in the macroorganism, manifesting as a complex of supplementary factors in patients with UFs, leading to infertility.
Conclusion
This study showed that patients with intramural UFs have a set of supplementary factors associated with infertility. It is likely that the concomitant gynecological and extragenital pathological changes identified in patients with intramural UFs are risk factors for infertility. The contribution of each of these factors to the genesis of infertility in UFs remains to be clarified.
References
- Министерство здравоохранения Российской Федерации. Клинические рекомендации. Миома матки. 2020. [Ministry of Health of the Russian Federation. Clinical guidelines. Uterine myoma. 2020. (in Russian)].
- Пономаренко И.В., Чурносов М.И. Современные представления об этиопатогенезе и факторах риска лейомиомы матки. Акушерство и гинекология. 2018; 8: 27-32. [Ponomarenko I.V., Churnosov M.I. Current views on the etiopathogenesis and risk factors of uterine leiomyoma. Obstetrics and Gynecology. 2018; (8): 27-32. (in Russian)]. https://dx.doi.org/10.18565/aig.2018.8.27-32.
- Stewart A., Cookson C.L., Gandolfo R. A., Schulze-Rath R. Epidemiology of uterine fibroids: a systematic review. BJOG. 2017; 124(10): 1501-12.https://dx.doi.org/10.1111/1471-0528.14640.
- Ikhena D.E., Bulun S.E. Literature review on the ole of uterine fibroids in endometrial function. Reprod. Sci. 2018; 25(5): 635-43.https://dx.doi.org/10.1177/1933719117725827.
- Pritts E.A., Parker W.H., Olive D.L. Fibroids and infertility: an updated systematic review of the evidence. Fertil. Steril. 2009; 91(4): 1215-23.https://dx.doi.org/10.1016/j.fertnstert.2008.01.051.
- Christopoulos G., Vlismas A., Salim R., Islam R., Trew G., Lavery S. Fibroids that do not distort the uterine cavity and IVF success rates: an observational study using extensive matching criteria. BJOG. 2017; 124(4): 615-21.https://dx.doi.org/10.1111/1471-0528.14362.
- Sagi-Dain L., Ojha K., Bider D., Levron J., Zinchenko V., Walster S. et al. Pregnancy outcomes in oocyte recipients with fibroids not impinging uterine cavity. Arch. Gynecol. Obstet. 2017; 295(2): 497-502.https://dx.doi.org/10.1007/s00404-016-4273-9.
- Klatsky P.C., Lane D.E., Ryan I.P., Fujimoto V.Y. The effect of fibroids without cavity involvement on ART outcomes independent of ovarian age. Hum. Reprod. 2007; 22(2): 521-6. https://dx.doi.org/10.1093/humrep/del370.
- Styer A.K., Jin S., Liu D., Wang B., Polotsky A.J., Christianson M.S. et al. Association of uterine fibroids and pregnancy outcomes after ovarian stimulation-intrauterine insemination for unexplained infertility. Fertil. Steril. 2017; 107(3): 756-62.e3. https://dx.doi.org/10.1016/j.fertnstert.2016.12.012.
- Munro M.G., Critchley H.O., Broder M.S., Fraser I.S.; FIGO Working Group on Menstrual Disorders. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int. J. Gynaecol. Obstet. 2011; 113(1): 3-13. https://dx.doi.org/10.1016/j.ijgo.2010.11.011.
- Chen L., Deng H., Cui H., Fang J., Zuo Z., Deng J. et al. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2018; 9(6): 7204-18. https://dx.doi.org/10.18632/oncotarget.23208.
- Коненков В.И., Королева Е.Г., Орлов Н.Б., Прокофьев В.Ф., Шевченко А.В., Новиков А.М., Дергачева Т.И., Останин А.А. Противовоспалительная активность цитокинов сыворотки крови (IL-4, IL-10, IL-13) и природного антагониста рецептора IL-1β (IL-1ra) у женщин с миомой матки. Акушерство и гинекология. 2018; 10: 80-5. [Konenkov V.I., Koroleva E.G., Orlov N.B., Prokof’ev V.F., Shevchenko A.V., Novikov A.M., Dergacheva T.I., Ostanin A.A. Anti-inflammatory activity of serum cytokines (IL-4, IL-10, IL-13) and the natural IL-1β receptor antagonist (IL-1Ra) in women with uterine myoma. Obstetrics and Gynecology. 2018; (10): 80-5. (in Russian)]. https://dx.doi.org/10.18565/aig.2018.10.80-85.
- Protic O., Toti P., Islam M.S., Occhini R., Giannubilo S.R., Catherino W.H. et al. Possible involvement of inflammatory/reparative processes in the development of uterine fibroids. Cell Tissue Res. 2016; 364(2): 415-27.https://dx.doi.org/10.1007/s00441-015-2324-3.
- Yang Q., Ali M., El Andaloussi A., Al-Hendy A. The emerging spectrum of early life exposure-related inflammation and epigenetic therapy. Cancer Stud. Mol. Med. 2018; 4(1): 13-23. https://dx.doi.org/10.17140/CSMMOJ-4-125.
- Ciebiera M., Włodarczyk M., Wrzosek M., Wojtyła C., Błażej M., Nowicka G. et al. TNF-alpha serum levels are elevated in women with clinically symptomatic uterine fibroids. Int. J. Immunopathol. Pharmacol. 2018; 32: 2058738418789805. https://dx.doi.org/10.1177/2058738418779461.
- AlAshqar A., Reschke L., Kirschen G.W., Borahay M.A. Role of inflammation in benign gynecologic disorders: from pathogenesis to novel therapies. Biol. Reprod. 2021; 105(1): 7-31. https://dx.doi.org/10.1093/biolre/ioab054.
- García-Gómez E., Vázquez-Martínez E.R., Reyes-Mayoral C., Cruz-Orozco O.P., Camacho-Arroyo I., Cerbón M. Regulation of inflammation pathways and inflammasome by sex steroid hormones in endometriosis. Front. Endocrinol. (Lausanne). 2019; 10: 935. https://dx.doi.org/10.3389/fendo.2019.00935.
- Kawai T., Autieri M.V., Scalia R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol. Cell Physiol. 2021; 320(3): C375-91. https://dx.doi.org/10.1152/ajpcell.00379.2020.
- Gallagher C.S., Makinen N., Harris H.R., Rahmioglu N., Uimari O., Cook J.P. et al. Genome-wide association and epidemiological analyses reveal common genetic origins between uterine leiomyomata and endometriosis. Nat. Commun. 2019; 10(1): 485. https://dx.doi.org/10.1038/s41467-019-12536-4.
- Plowden T.C., Zarek S.M., Rafique S., Sjaarda L.A., Schisterman E.F., Silver R.M. et al. Preconception leptin levels and pregnancy outcomes: a prospective cohort study. Obes. Sci. Pract. 2020; 6(2): 181-8. https://dx.doi.org/10.1002/osp4.399.
- Broughton D.E., Moley K.H. Obesity and female infertility: potential mediators of obesity's impact. Fertil. Steril. 2017; 107(4): 840-7. https://dx.doi.org/10.1016/j.fertnstert.2017.01.017.
- Pan L., Fu Z., Yin P., Chen D. Pre-existing medical disorders as risk factors for preeclampsia: an exploratory case-control study. Hypertens. Pregnancy. 2019; 38(4): 245-51. https://dx.doi.org/10.1080/10641955.2019.1667381.
- Kesha Varzi F., Tetmoori B., Farzaneh F., Mokhtari M., Najafi D., Salimi S. Association of ACE I/D and AGTR1 A1166C gene polymorphisms and risk of uterine leiomyoma: a case-control study. Asian Pac. J. Cancer Prev. 2019; 20(9): 2595-9. https://dx.doi.org/10.31557/APJCP.2019.20.9.2595.
- Haan Y.C., Diemer F.S., Van Der Woude L., Van Montfrans G.A., Oehlers G.P., Brewster L.M. The risk of hypertension and cardiovascular disease in women with uterine fibroids. J. Clin. Hypertens. (Greenwich). 2018; 20(4): 718-26.https://dx.doi.org/10.1111/jch.13253.
- Walters C.A., Daly D.C., Chapitis J., Kuslis S.T., Prior J.C., Kusmik W.F., RiddickI D.H. Human myometrium: a new potential source of prolactin. Am. J. Obstet. Gynecol. 1983; 147(6): 639-44. https://dx.doi.org/10.1016/0002-9378(83)90441-6.
- Barry L., Pather S., Gargya A., Marren A. Prolactin-secreting leiomyoma causing hyperprolactinaemia unresponsive to dopamine agonist therapy and resolution following myomectomy. Case Rep. Endocrinol. 2021; 2021: 5553187.https://dx.doi.org/10.1155/2021/5553187.
- Burke W.T., Penn D.L., Castlen J.P., Donoho D.A., Repetti C.S., Iuliano S. et al. Prolactinomas and nonfunctioning adenomas: preoperative diagnosis of tumor type using serum prolactin and tumor size. J. Neurosurg. 2019 Jun 14:1-8. https://dx.doi.org/10.3171/2019.3.JNS19121.
- Pan J., Zhang J. Research progress of PCNA in reproductive system diseases. Evid. Based Complement. Alternat. Med. 2021; 2021: 2391917.https://dx.doi.org/10.1155/2021/2391917.
Received 01.02.2023
Accepted 07.04.2023
About the Authors
Ekaterina D. Dubinskaya, Dr. Med. Sci., Professor at the Department of Obstetrics, Gynecology and Perinatology, RUDN University, +7(903)117-55-58,eka-dubinskaya@yandex.ru, https://orcid.org/0000-0002-8311-0381, 8 Miklukho-Maklaya str., Moscow, 117198, Russia.
Svetlana N. Kolesnikova, PhD, Associate Professor at the Department of Obstetrics, Gynecology and Pediatrics, Reaviz Medical University, +7(916)500-10-99,
ksnmed@mail.ru, https://orcid.org/0000-0001-9575-02741, 2-2 Krasnobogatyrskaya str., Moscow, 107564, Russia.
Elizaveta V. Alyoshkina, Teaching Assistant at the Department of Obstetrics, Gynecology and Reproductive Medicine, Faculty of Postgraduate Education, RUDN University,
+7(926)768-44-27, alyoshkina.ev@yandex.ru, https://orcid.org/0000-0001-5339-1285, 5-2 General Antonov str., Moscow, 117342, Russia.
Alexander S. Gasparov, Dr. Med. Sci., Professor, Professor at the Department of Obstetrics, Gynecology and Perinatology, RUDN University, +7(903)117-55-58,
eka-dubinskaya@yandex.ru, https://orcid.org/0000-0001-6301-1880, 8 Miklukho-Maklaya str., Moscow, 117198, Russia.
Ekaterina S. Bashkirova, Teaching Assistant at the Department of Obstetrics, Gynecology and Reproductive Medicine, Faculty of Postgraduate Education, RUDN University, +7(926)768-44-27, alyoshkina.ev@yandex.ru, https://orcid.org/0000-0002-6633-5095, 5-2 General Antonov str., Moscow, 117342, Russia.
Mohamed L. Leffad, PhD Student at the Department of Obstetrics, Gynecology and Perinatology, RUDN University, +7(985)846-18-89, lemin.leffad@gmail.com,
https://orcid.org/0000-0001-6816-3314, 8 Miklukho-Maklaya str., Moscow, 117198, Russia.
Corresponding author: Svetlana N. Kolesnikova, ksnmed@mail.ru



