Complement system dysregulation in patients with preeclampsia
Objective: To investigate the role of the complement system in the development and progression of preeclampsia.Sidorova I.S., Nikitina N.A., Ageev M.B., Kokin A.A., Kir'yanova M.A.
Materials and methods: A study group comprised 25 patients with preeclampsia (13 moderate, 12 severe) and a comparison group of 22 relatively healthy women with uncomplicated pregnancies. Serum levels of complement factors (C1q, C3, C5a, Factor B, Factor H, Factor I, Factor D) were determined before treatment and in the neonatal cord blood immediately after delivery using the Multiplex method (Merck complement panels, Germany).
Results: Patients with moderate preeclampsia had significantly elevated levels of C1q, C3, FB, FH, and a less marked increase in FI and FD. A paradoxical reduction in all these factors was seen in patients with severe preeclampsia. C5a levels increased with the progression of preeclampsia, culminating in severe preeclampsia. The changes of neonatal cord blood complement factors overlapped with maternal ones but were less pronounced, with a significant increase in C5a in the severe preeclampsia subgroup.
Conclusion: The high concentrations of C1q, C3, C5a, FB, FD in pregnant women with preeclampsia suggest complement activation through the classical and alternative pathways, accompanied by a compensatory increase in regulatory FH and FI to limit excessive complement activation. In severe preeclampsia, continuing complement activation is associated with consumption hypocomplementemia.
Keywords
Preeclampsia is one of the common pregnancy complications, but there is still no unanimity concerning its etiology and underlying pathophysiology. An international consensus of experts supports the hypothesis that preeclampsia is a primary placental disorder. However, there has been evidence that maternal somatic diseases (especially cardiovascular diseases) resulting in uterine and placental hypoperfusion are likely to cause secondary placental dysfunction and, therefore, preeclampsia [1].
The perinatal management of this pregnancy complication (magnesium sulfate, individualized antihypertensive therapy, infusion, etc.) has been developed mainly based on empirical observations and clinical and physiological analogies in the treatment of similar symptoms in other areas of clinical medicine. There is no good reason to expect a significant breakthrough in the treatment of preeclampsia, as there is no clear understanding of its underlying mechanism.
The immunological aspects of the development and progression of preeclampsia have been emphasized for many years. Since the fetal–placental unit is semi-allogeneic in relation to the mother, the development of immunopathological reactions is expected. During pregnancy, the development of immune tolerance mechanisms is crucial to prevent fetal rejection, which may manifest as early pregnancy loss, preterm birth, fetal growth restriction, or preeclampsia [2].
The immunological concept of preeclampsia suggests developing an excessive maternal systemic proinflammatory response to a semi-allogeneic fetal–placental unit [3, 4]. This systemic response results in generalized vascular endothelial injury, leading to multisystemic organ and system damage. The role of a triggering factor and the source of proinflammatory inducers belongs to the fetus and placenta.
It is known that during normal pregnancy, the success of trophoblast invasion, uterine spiral and radial arteries remodeling with the formation of an adequate placental blood supply depends on the maternal immune system. The adequate response of macrophages, natural killer (NK), dendritic cells (DC), T lymphocytes, including T regulatory cells (Treg) in the decidual membrane, is essential for the invasion of cytotrophoblast cells. The complement system also plays a vital role in these processes [5, 6]. It is a part of the innate immune system, consisting of more than 50 proteins circulating in plasma and bound to cell membranes. Activation of complement is possible by classical, alternative, or lectin pathways, which trigger a proteolytic cascade with the formation of the critical link, C3 convertase, and the final component, the membrane attack complex (MAC). The MAC inserts into membranes, damages cells, and activates proinflammatory pathways, opsonization, and phagocytosis, damaging pathogens and the pathogen's cells [7].
In addition to the lysis of damaged cells and pathogens, complement plays an essential role in developing anaphylactic reactions, inflammatory processes, tissue damage, and blood clotting.
It has been shown that healthy pregnancy is associated with a moderate activation of the complement system to protect the fetal-placental unit from pathogens and support placentation [8]. Over-activation of complement, or an imbalance between its activation and regulation, may be associated with adverse pregnancy outcomes, including pre-eclampsia.
However, the results of published studies are inconsistent and sometimes directly opposite. For example, Jia et al. (2019) observed increased serum concentrations of factor Bb in severe pre-eclampsia and a significant decrease in C1q, factor H, C3, and C4, compared with healthy pregnant women [9]. Other authors showed a substantial increase in Bb, C3a, C5a, and MAC in early and late pre-eclampsia [10].
It is essential for practical obstetrics to be able to assess abnormalities clinically; therefore, the normal range of complement components during healthy pregnancy and their threshold values for certain complications should be investigated.
In this regard, a deeper study of the complement system in healthy pregnancy and pre-eclampsia is promising because the correction of identified abnormalities may be a potential therapeutic target and an opportunity to prolong pregnancy.
This study aimed to investigate the clinical value of comparative analysis of complement components in pre-eclampsia and healthy pregnancy in diagnosing pre-eclampsia and determining its severity.
Materials and methods
This article reports preliminary findings regarding the complement components in pregnant women with preeclampsia, but the study will continue.
The Research Ethics Committee of the I.M. Sechenov First MSMU, Ministry of Health of Russia (Sechenov University) approved this study (Ref. No. 28-20 dated 07.10.2020). All participants provided signed informed consent to take part in the study.
The study comprised 47 women managed at the Maternity Hospital affiliated to the V.V. Veresaev Clinical Hospital of Moscow City Health Department. The study group included 25 patients with preeclampsia divided into subgroup I (n=13) with moderate and subgroup II (n=12) with severe preeclampsia. The control group initially consisted of 25 relatively healthy pregnant women without preeclampsia. Still, three patients were excluded from the study because they were subsequently diagnosed with respiratory or urinary tract infections (n=22). All patients were observed from admission to the maternity hospital to discharge, an average of 7.4 (5; 9) days.
The criteria for inclusion of patients in the study arm were written informed consent to participate in the study, a woman's reproductive age, the presence of pregnancy, and signs of preeclampsia of various severity.
Inclusion criteria for patients in the control group were patients' written informed consent to participate in the study, a woman's reproductive age, absence of extragenital and gynecological diseases, an uncomplicated reproductive history, uncomplicated pregnancy, and labor, and the postpartum period.
Criteria for not including patients in the study and control groups were any infections and inflammatory diseases.
Patient exclusion criteria were the patient's refusal to participate in the study, end of pregnancy before 22 weeks, fetal malformations, chromosomal and genetic abnormalities, and any infectious diseases.
The diagnosis of pre-eclampsia was made based on the criteria of the clinical guidelines "Preeclampsia. Eclampsia. Edema, proteinuria and hypertensive disorders in pregnancy, labor and the postpartum period" (2021). The examination, treatment, and delivery were performed in the framework of these guidelines. In addition, serum concentrations of 7 complement factors (C1q, C3, C5a, factor B-FB, factor H-FH, factor I-FI, and factor D-FD) were measured. Blood samples were taken at admission before the treatment initiation. An umbilical cord blood samples (1–2 ml) were taken immediately after delivery from the umbilical cord remnant. The choice of complement factors to be analyzed was determined by the need to investigate all pathways of its possible activation.
Blood samples were allowed to stand until clot formation then centrifuged at 1000 g for 15 min. The resulting supernatant was collected in Eppendorf tubes, transported to the laboratory, where samples were frozen and stored at -80°C until investigation.
Complement proteins were analyzed using the multiplex assay. Plasma samples were simultaneously analyzed for the seven complementary factors mentioned above using Human Complement panels from Merck (Millipore) (Germany) following the manufacturer's protocol. Complement protein concentrations were calculated from standard calibration curves for each protein.
The laboratory investigations were conducted at the Laboratory of Molecular Biomedicine of the Shemyakin and Ovchinnikov Institute of Bioorganic Chemistry (Head of Laboratory – Deputy Director for Science, Dr. Chem. Sci. A.A. Belogurov).
Statistical analysis
Statistical analysis was performed with IBM SPSS Statistics v22 (IBM Corp., USA) statistical software. The normality of the distribution was tested by the Shapiro–Wilk test. Variables not meeting normality assumptions are presented as Me (Q 25%; Q 75%), where Me is the median and Q 25% and Q 75% are the upper and lower quartiles. The Kruskal–Wallis test was used to compare numerical data between groups, followed by pairwise comparison using the Mann–Whitney U-test. When comparing three groups, Bonferroni correction was applied for multiple comparisons with Bonferroni's adjusted significance threshold of p<0.017. Categorical variables were compared using a two-sided Fisher's exact test. If Fisher's exact test could not be used (one of the proportions when comparing two samples was 0% or 100%), then Z-score for proportions with endpoints correction was used. Correlation analysis was conducted by calculating Spearman's rank correlation coefficients. The critical level of significance when testing statistical hypotheses was considered at p <0.05.
Results
Clinical characteristics of the patients
All participants were comparable regarding socioeconomic factors, unhealthy behaviors, and occupational hazards. The age of the pregnant women ranged from 18 to 41 years, being 30 (27;36) years in the study group and 32 (30;35) years in the control group (p=0.17). Five (22.7%) and 14/25 (56%) women in the control and the study group, respectively, were primigravida (p=0.01); 8/22 (36.4%) and 16/25 (64%) were primiparous (p=0.05), respectively.
Extragenital and gynecological diseases and the reproductive history of the patients are presented in Table 1.
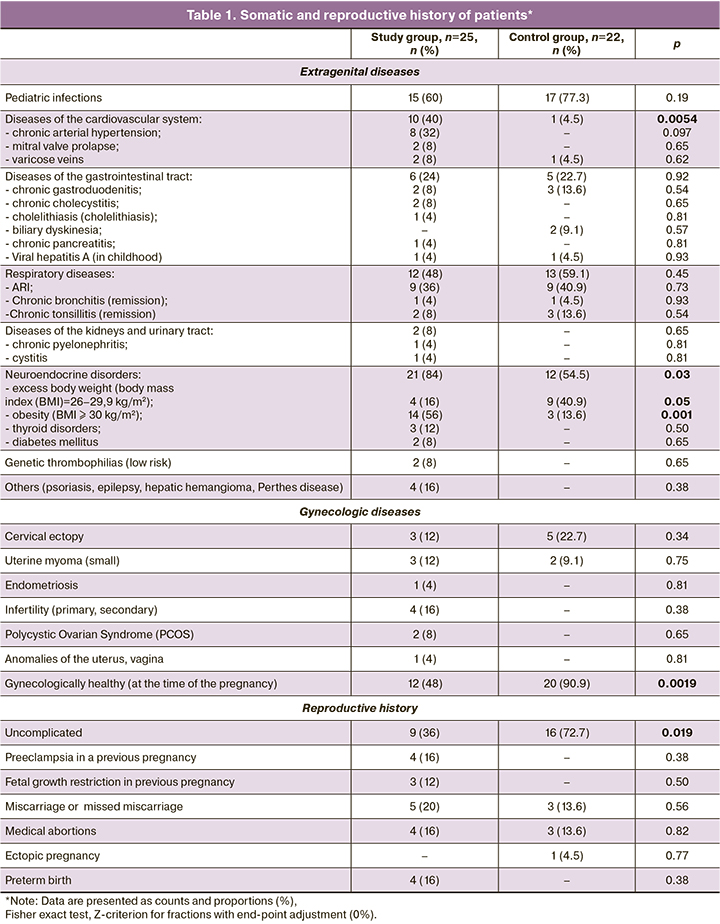
As shown in Table 1, the patients in the study group had significantly higher comorbidity rates, including cardiovascular diseases (mainly chronic arterial hypertension), renal and urinary tract, gastrointestinal tract (chronic cholecystitis, cholelithiasis, chronic pancreatitis), neuroendocrine disorders (obesity, diabetes, thyroid disease), genetic forms of thrombophilia, infertility, and PCOS.
Only 9/25 (36%) women in the study group had uncomplicated reproductive history, which was significantly different from the control group [16/22 (72.7%)] (p=0.009). In 12/25 (48%) patients of the study group had a history of preeclampsia (16%), fetal growth restriction (12%), recurrent pregnancy loss (20%), and preterm labor (16%).
The course of pregnancy and delivery characteristics in the study groups are presented in Table 2.
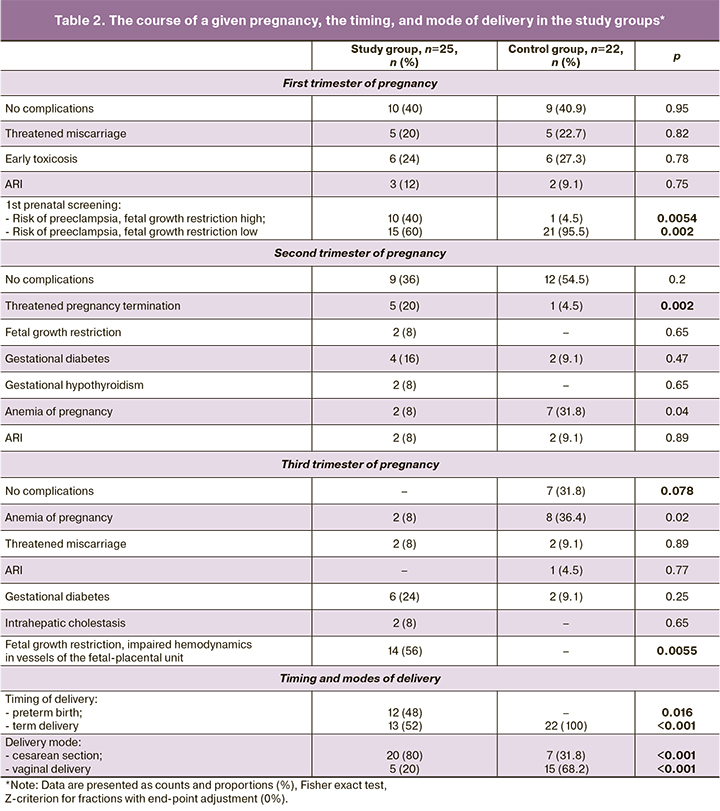
Based on the results of the 1st prenatal screening, 10/25 (40%) women in the study group had a high risk of preeclampsia and fetal growth restriction, which was significantly different from the control group [1/22 (4.5%)], (p=0.002).
All women in the control group delivered before term, 7/22 (31.8%) of them by cesarean section (due to the presence of a uterine scar, fetal malpositions, signs of a clinically narrow pelvis). Preterm delivery occurred in 12/25 (48%) women in the study group due to an increase in the severity of preeclampsia and/or deterioration of the fetus. Surgical delivery (cesarean section) was performed in 20/25 (80%) patients (due to the progression of preeclampsia, acute fetal hypoxia, need for rapid delivery if the birth canal was not ready, and uterine scar).
Moderate preeclampsia was diagnosed in 13/25 patients, severe in 12. The most frequent initial symptom was arterial hypertension in 19/25 (76%) and, less frequently, edema in 6/25 (24%).
Mean gestational age at the onset of moderate and severe preeclampsia was 34 (30; 35) and 31 (27; 36) weeks, respectively, (p=0.28). Its duration averaged 2 (2;4) weeks in moderate preeclampsia and 6.5 (5.5;8.5) weeks in severe preeclampsia (p=0.023).
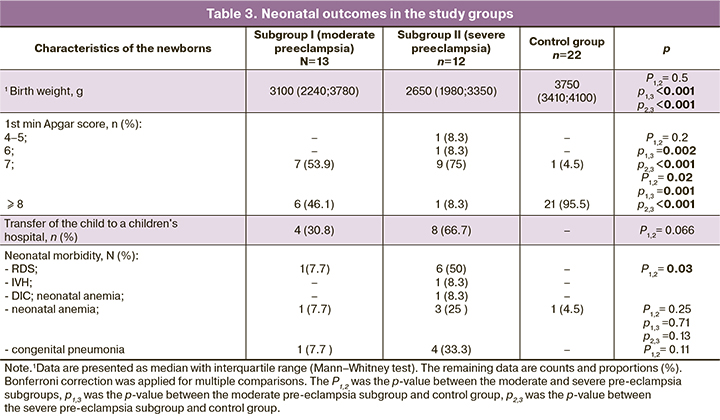
The neonatal outcomes of preeclampsia, particularly severe preeclampsia, were expected to be worse than those of the control group (Table 3). Neonates had significantly lower birth weight, and more than half were born with moderate asphyxia. Four/13 (30.8%) and 8/12 (66.7%) infants, respectively, from mothers with moderate and severe preeclampsia, were transferred for further treatment and follow-up to pediatric hospitals. The condition of neonates in the subgroups with preeclampsia was attributed to respiratory distress syndrome (RDS), pneumonia, intraventricular hemorrhage (IVH), DIC, and anemia. All neonates in the control group were discharged home on days 4–5 in a satisfactory condition.
Complement factor analysis
Concentrations of complement factor 7 in maternal serum and umbilical cord blood had considerable variability between the groups, suggesting a wide range of individual differences (Table 4).
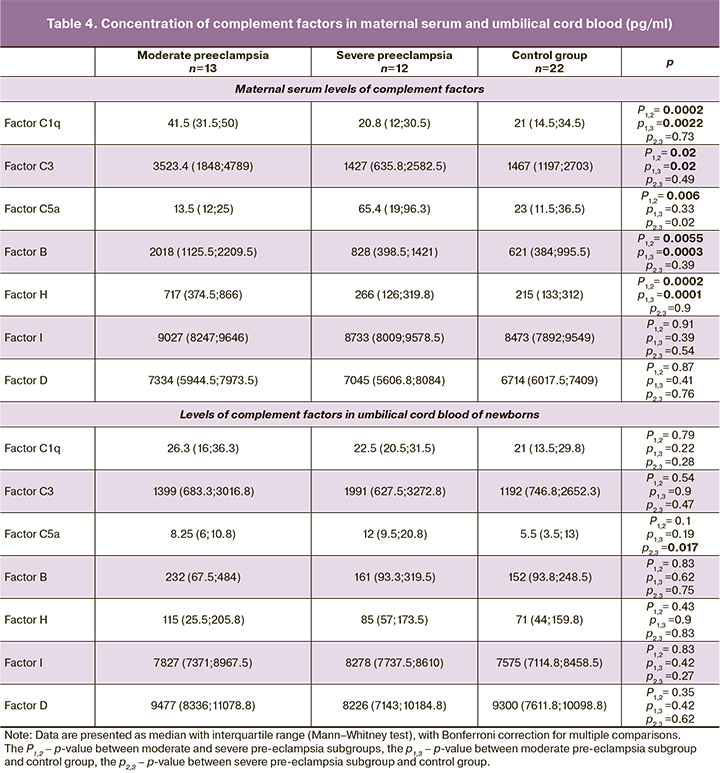
The development of moderate pre-eclampsia was associated with complement system activation with a significant increase in the levels of most factors compared to the control group: C1q to 41.5 (31.5; 50) versus 21 (14.5; 34.5) pg/ml (p=0.0022), C3 to 3523.4 (1848; 4789) versus 1467 (1197; 2703) pg/ml (p=0.017), FB to 2018 (1125.5; 2209.5) versus 621 (384; 995.5) pg/ml (p=0.0003), and FH to 717 (374.5; 866) versus 215 (133; 312) pg/ml (p=0.0001). There was also an increase in FI and FD concentrations, but these changes were less pronounced (p>0.017). There was no significant difference in C5a concentration between patients with moderate pre-eclampsia and the control group.
In severe pre-eclampsia, given the increased severity of the pathophysiological changes inherent in this pregnancy complication, we expected to see evidence of a more pronounced activation of the complement system. However, we found a dramatically opposite change in the levels of its proteins. Compared with moderate pre-eclampsia, there was a significant decrease in C1q to 20.8 (12; 30.5) pg/ml (p=0.0002), C3 to 1427 (635.8; 2582.5) pg/ml (p=0.02), FB to 828 (398.5; 1421) pg/ml (p=0.0055) and FH to 266 (126; 319.8) pg/ml (p=0.0002). There was also a decrease in FI and FD, but not statistically significant (p>0.017).
A slightly different pattern was observed in C5a dynamics. While there were no significant changes in C5F in mild pre-eclampsia, its concentration averaged 13.5 (12; 25) pg/ml, compared with 23 (11.5; 36.5) pg/ml in the control group (p=0.33), whereas in severe pre-eclampsia its levels were quite high, averaging 65.4 (19; 96.3) pg/ml, which was almost 5 times higher than those in moderate pre-eclampsia (p=0.006).
Changes in the levels of complement factors in the umbilical cord blood were not as pronounced as in maternal serum, but had much in common. In the subgroup of children from mothers with moderate pre-eclampsia, all of the complement proteins studied were increased to varying degrees relative to the control group: C1q to an average of 26.3 (16; 36.3) versus 21 (13.5; 29.8) pg/ml (p=0.22), C3 to 1399 (683.3; 3016.8) versus 1192 (746.8; 2652.3) pg/ml (p=0.9), C5a to 8.25 (6; 10.8) vs. 5.5 (3.5; 13) pg/ml (p=0.19), FB to 232 (67.5; 484) vs. 152 (93.8; 248.5) pg/ml (p=0.62), FH to 115 (25.5; 205.8) versus 71 (44; 159.8) pg/ml (p=0.9), FI to 7827 (7371; 8967.5) versus 7575 (7114.8; 8458.5) pg/ml (p=0.42), FD to 9477 (8336; 11078.8) versus 9300 (7611.8; 10098.8) pg/ml (p=0.42); however all these changes were not statistically significant.
In the subgroup with severe pre-eclampsia, the changes in C1q, FB, FH, and FD in children were unidirectional, with maternal levels declining compared to the subgroup with moderate pre-eclampsia (Table 4), but not up to the control group. However, C3, C5a, and FI in umbilical cord blood tended to be elevated in contrast to maternal levels. There were statistically significant changes in activated factor C5a in neonates in the subgroup with severe pre-eclampsia, its concentration in umbilical cord blood increasing to an average of 12 (9.5; 20.8) pg/ml (p=0.017) compared to 5.5 (3.5; 13) in the control group.
In general, levels of complement factors in umbilical cord blood were lower than maternal levels, except for FD.
Correlation analysis was conducted by calculating Spearman's rank correlation coefficients to examine the relationship between maternal and neonatal complement factor levels and their association with clinical data.
The most significant positive correlations were found between maternal C3 and C1q levels (r=0.694, p=0.0001, 95% CI 0.438–1.274), C3 and FB (r=0.83, p<0.001, 95% CI 0.78–1.616), C3 and FH (r=0.672, p<0.001, 95% CI 0.397–1.233), and between FB and FH levels (r=0.84, p<0.001, 95% CI 0.806–1.642), FD and FI (r=0.59, p=0.002, 95% CI 0.252–1.087). Similar parallels between the studied factors were observed in neonatal umbilical cord blood: between C3 and C1q (r=0.662, p=0.005, 95% CI 0.41–1.302), C3 and FB (r=0.70, p=0.0025, 95% CI 0.39–1.216), C3 and FH (r=0.768, p=0.0005, 95% CI 0.44–1.509), and between FB and FH (r=0.94, p<0.001, 95% CI 0.762–1.703), FD and FI (r=0.55, p=0.026, 95% CI 0.303–1.112).
There was a positive association between concentrations of maternal activated factor C5a and newborn C1q (r=0.454, p=0.05, 95% CI -0.054–1.033) and vice versa, between newborn C5a and maternal C1q levels (r=-0.47, p=0.05, 95% CI -1.058–0.030), FH (r=-0.63, p=0.009, 95% CI -1.281–-0.194), FD (r=-0.43, p=0.05, 95% CI -1.002– 0.085) and FI (r=-0.46, p=0.05, 95% CI -1.040–0.047).
Analysis of the relationship between serum complement factor levels in pregnant women and clinical data showed a significant negative correlation between the severity of preeclampsia and C1q (r=-0, 7, p=0.0001, 95% CI -1.285–-0.450), C3 (r=-0.43, p=0.03, 95% CI -0.881–-0.046), FB (r=-0.57, p=0.003, 95% CI -1.060–-0.224), FH (r=-0,76, p<0.001, 95% CI -1.402–-0.567), and a positive correlation with C5a (r=0.561, p=0.003, 95% CI 0.217–1.053). The course of preeclampsia from clinical presentation to delivery was also negatively correlated with maternal C1q levels (r=-0, 562, p=0.003, 95% CI -1.053–-0.217), FB (r=-0.509, p=0.009, 95% CI -0.979–-0.143), FH (r=-0.53, p=0.006, 95% CI -1.009–-0.173), and positively with newborn C5a (r=0.45, p=0.05, 95% CI 0.201–1.069).
The severity of arterial hypertension in pregnant women with preeclampsia was only significantly related with C5a (r=0.47, p=0.017, 95% CI 0.094–0.929), FB (r=-0.4, p=0.05, 95% CI -0.729–0.107) and FH (r=-0.51, p=0.009, 95% CI -0.814–0.022). There was also an association between proteinuria and C1q levels (r=-0.55, p=0.054, 95% CI -1.272–0.035), FH (r=-0.611, p=0.035, 95% CI -1.363–-0.056) in mother and C5a in neonate (r=0.5, p=0.05, 95% CI 0.234–1.152).
Analysis of the correlation between the changes in laboratory parameters in pregnant women in the study group and complement factors showed a significant correlation between hematocrit and maternal C5a (r=0.563, p=0.023, 95% CI 0.093–1.181), maternal creatinine and neonatal C1q (r=0, 518, p=0.04, 95% CI 0.087–1.279), between maternal lactate dehydrogenase and her C5a (r=0.745, p=0.0022, 95% CI -1.553–-0.371), between maternal lactate dehydrogenase and umbilical C1q levels (r=-0,895, p=0.0011, 95% CI -1.402–-0.476), C3 (r=-0.883, p=0.0016, 95% CI -1.322–-0.601), FB (r=-0.717, p=0.03, 95% CI -1.228–-0.336), FH (r=-0.783, p=0.0125, 95% CI -1.32–-0.448).
Interestingly, there was no significant correlation between the levels of complement factors in umbilical cord blood and the newborn's condition (severity of asphyxia), the birth weight, the frequency of transfer to children's hospitals for further treatment and nursing. This fact, however, is understandable, given the less pronounced changes in the concentrations of complementary proteins in newborns compared with maternal ones. At the same time there was a significant association between maternal factor C3 levels (r=0.44, p=0.023, 95% CI 0.055–0.890), FB (r=0.43, p=0.03, 95% CI 0.041–0.877) and FH (r=0.404, p=0.045, 95% CI 0.010–0.846) and low Apgar scores (<5). The time of preeclampsia manifestation correlated positively with neonatal C3 and C5a levels (r=0.522, p=0.038, 95% CI 0.09–1.179 and r=0.44, p=0.058, 95% CI 0.071–1.09, respectively).
In summary, these results of our study show that:
1. The development of moderate preeclampsia is associated with:
- An increase in C1q factor, indicating activation of the complement system through the classical pathway;
- An increase in C3, a critical link in the activation of all pathways of the complement system;
- An increase in factors FB and FD, markers of activation of the complement system through an alternative pathway;
- Increase in factors FH and FI – regulatory (inhibitory) proteins responsible for limiting excessive complement activation.
2. Severe preeclampsia is associated with a paradoxical decrease in all complement factors except C5a, which is essentially consumption hypocomplementemia. The increase in C5a concentration confirms the ongoing process of complement system activation and indirectly indicates the formation of IAC and the continuing damage to maternal cells and tissues.
3. The dynamics of complement system proteins in newborns' umbilical cord blood largely coincide with maternal, but they are less pronounced. There was a significant increase in factor C5a in patients with severe preeclampsia. There was no significant correlation between the newborn's condition and the level of their complement factors.
Discussion
Published research evidence suggests that even in a healthy pregnancy, there is a moderate activation of the complement system accompanied by an increase in its regulatory factors such as FH, CD46, CD55, and CD59 [11–14]. It is thought that this process is induced by apoptotic cells or free fetal DNA, which inevitably enter the maternal bloodstream during placentation [13, 15].
However, any deviation from the boundaries of normal complement system activation and regulation can lead to adverse pregnancy outcomes, such as miscarriage, preterm delivery, preeclampsia, and fetal growth restriction [16, 17].
To examine the role of the complement system in the development of preeclampsia and the diagnostic value of changes in the levels of its proteins and regulators, we determined the concentrations of seven complement system factors (C1q, C3, C5a, FB, FH, FD, FI) in healthy pregnant women, pregnant women with preeclampsia, and in the cord blood of their newborns.
The results were entirely unexpected. There was a general trend of change in the proteins in each subgroup of women and their neonates.
Thus, moderate preeclampsia was associated with a significant increase in serum concentrations of factors C1q, C3, FB, FH, and, to a lesser extent, FD, FI, which suggests activating at least two complement pathways (classical and alternative).
There were the opposite changes in severe preeclampsia: a fairly steep decrease in all proteins except C5a, which was increased to maximal values. Such a decrease in complement factors is probably due to their consumption in a long activation cascade as preeclampsia progressed (consumption hypocomplementemia), which may be a diagnostic marker for the severity of this pregnancy complication. A similar situation is seen in immune complex-mediated diseases, where excessive consumption of complement factors is followed by their gradual depletion and the development of hypocomplementemia.
The C1q factorб, the first glycoprotein in the cascade of the classical complement activation pathway, is widely expressed in the decidual membrane, extravillous cytotrophoblast, and plays an essential role in the placental formation and pregnancy development [18].
An increase in C1q concentration in moderate preeclampsia indicates the activation of the complement system through the classical pathway, which is usually induced by the antigen-antibody complex and confirms the development of immunopathological reactions in this pregnancy complication. Similar dynamics of C1q in preeclampsia have been described by other authors [19].
C1q is also crucial for the complete migration of invasive cytotrophoblast and spiral artery remodeling. Apart from the liver, C1q is synthesized by decidual macrophages and some cells of the uterine decidua, as well as by the extravillous trophoblast, facilitating its invasion into the endo- and myometrium [16, 20]. However, no C4 deposits have been found in the decidual membrane at the site of the C1q location [13, 15], indicating that C1q in healthy pregnancy does not initiate local complement activation in the maternal-fetal interface.
The accumulating evidence for C1q suggests that further studies are needed to understand its role in pregnancy and the pathophysiology of preeclampsia.
The published data on the activation of the alternative complement pathway are patchy, but most studies have analyzed only one or two factors. We, therefore, set out to assess the most important markers and regulators.
An important feature of the alternative complement pathway is its minimal but constant activation [21], which is ensured by spontaneous hydrolysis of the C3 component to form the C3b fragment. The latter can bind both to pathogens and the body's cells. After binding to FB, an unstable C3-convertase is formed on the cell surface, cleaving C3 into C3a and C3b fragments. Upon binding to C3-convertase C3b, C5-convertase, an enzymatic complex that cleaves the C5 component of complement with the formation of C5a and C5b followed by MAK assembly, is formed.
FB is considered a significant marker for activating the complement system via the alternative pathway and is cleaved by factor D to form the C3-convertase of the alternative pathway (C3bBb), which helps to trigger a further complement cascade up to the MAC terminal complex. FD (adipsin), synthesized by hepatocytes and adipocytes, is thus also an activator of the alternative complement pathway.
The main regulatory proteins (inhibitors) of the alternative complement activation pathway are FH and FI, circulating in plasma. FH blocks the formation of C3-convertase and directly accelerates its breakdown. FI cleaves C3b to an inactive fragment in the presence of cofactors.
C5a is a potent anaphylatoxin, increases the proinflammatory state, participates in immediate hypersensitivity reactions, increases vascular permeability of the microcirculation, causes vasoconstriction – in effect, supports the basic mechanisms of pathogenesis of preeclampsia.
Our data show that pregnant women with moderate preeclampsia have high levels of FB and FD, suggesting excessive activation of the alternative complement pathway. An increase in FB and FD was also positively correlated with an increase in FH and FI in the same group of women, which may be explained by compensatory activation of complement regulatory proteins. Similar results have been obtained by some other authors [9, 22]. According to Lynch A.M. et al., an increase in FB is detectable early in pregnancy in patients with preeclampsia. The authors suggest that it should be used as an early preeclampsia predictor [23].
The markedly significant decrease in FB and FH concentrations in lengthy and severe preeclampsia indicates the potential for their use as markers of complement activation in an alternative pathway and as criteria for assessing the severity of preeclampsia.
The trend in C3 levels in preeclampsia had the same pattern as that described above. Low C3 concentrations are generally found in genetic defects of complement factors. They have also been described in 80 percent of patients with the atypical hemolytic-uremic syndrome in the presence of a C3 gene mutation [24]. However, when severe preeclampsia develops, C3consumption due to prolonged activation of the complement system may lead to a decline in C3 levels.
Since, according to our data, an increase in FB, FD, and C3 levels occurs in parallel with an increase in the concentrations of the regulatory proteins FH and FI, we believe that this mechanism allows the excessive activation of complement by the alternative pathway at the C3 level to be restrained for some time. A decrease in the activity of the main inhibitors of the alternative complement pathway is a poor prognostic sign because it blocks the primary regulatory mechanism limiting excessive activation of the complement cascade, which can lead to damage to own cells and tissues (renal tubules, hepatocytes, endotheliocytes, alveolocytes, placental trophoblast, etc.), chronic activation of platelets, leukocytes, neutrophils and endothelial cells with microthrombosis and occlusion of small vessels, edema, and destruction of endotheliocytes (systemic thrombotic microangiopathy), and finally - to multiple organ dysfunction.
Gradually increasing serum C5a levels in patients with preeclampsia suggests the activation of the terminal pathway of the complement system, which is consistent with the findings of other authors [25]. An increase in C5a in pregnant women was directly correlated with the severity of preeclampsia. It was found not only in blood but also in the umbilical cord blood of the neonate. This phenomenon has also been described by Denny K.J. et al. (2013) [26].
Evidence for the crucial role of anaphylotoxin C5a in aberrant placentation in preeclampsia has also now been published. Ma Y. et al. (2018) described the interaction of C5a with its receptor C5aR on trophoblast cells. They showed that C5a directly inhibits extravillous trophoblast migration and induces its development in an antiangiogenic phenotype by mediating an imbalance of soluble fms-like tyrosine kinase-1 (sFlt-1) and placental growth factor (PIGF) [27]. Therefore, C5a may be an important therapeutic target, especially in early and severe preeclampsia associated with superficial placentation and insufficient invasive cytotrophoblast.
There is also evidence of the complement system activation by the lectin pathway in preeclampsia, with altered concentrations of its markers [28]. The exact role of the lectin pathway in the development of preeclampsia is not yet clear. Still, high maternal plasma concentrations of its markers indicate the involvement of this mechanism in the pathophysiology of preeclampsia.
Maternal changes in complement protein levels in the newborn's umbilical cord blood suggest the possibility of transplacental transmission of both pro-inflammatory factors and complement activation products. Studies have been published on the critical role of complement activation in fetal organ damage (in particular the heart and brain) and the development of neurocognitive and mental disorders in postnatal life [29–31].
Thus, over-activation of complement in preeclampsia can be induced by both classical, alternative, and lectin pathways, complicating the therapeutic options for correcting any of these links. However, there is an active search for therapeutic agents that can regulate functional complement activity.
In particular, it has been reported that low doses of acetylsalicylic acid, when administered in time, can influence trophoblastic invasion by suppressing the expression of C3 and factor B in the placenta [32]. New aspects of low molecular weight heparin use in preeclampsia have been described: its positive effect on the maternal vasculature and endothelial function by increasing PIGF levels and suppressing complement activation by inhibiting C5a has been shown experimentally [33, 34]. Studies have also shown the effect of the nonsteroidal anti-inflammatory cyclooxygenase-2 inhibitor celecoxib on restoring anti-angiogenic balance and complement system regulation [35]. Targeted therapeutic agents affecting some or other links of the complement system - monoclonal C5 inhibitor (eculizumab), monoclonal antibodies to FH, C3- and C5-convertase inhibitors, C5 inhibitor, C5 antagonists and C3a, etc. – are actively investigated [36–39].
Conclusion
Published data and our studies suggest an essential role of the complement system in the pathogenesis of preeclampsia as one of the links in the mechanism of immunological tolerance failure.
Given the identified complement activation in the classical pathway induced by antigen-antibody complexes, the search for potential antigens is necessary. The timing of preeclampsia (22 weeks and later, according to research evidence) implies a certain degree of maturation of the higher structures of the fetal central nervous system, allowing it to survive extra-early birth. It is logical to assume the role of fetal cerebral proteins in forming immune complexes and generalized immune damage to maternal microcirculatory vascular endothelium. In our study, a simultaneous study of neuro-specific proteins in the same serum samples was planned, but we did not obtain the results.
Excessive activation of complement by the alternative pathway is characteristic of thrombotic microangiopathy with severe multi-organ lesions, a variant of which is severe preeclampsia. The comorbid background of the patients is an aggravating factor in this case, which was also confirmed in our study. If the condition worsens after delivery in these women, the first thing to be suspected is activating an alternative complement pathway.
An essential aspect of the problem is that excessive activation and dysregulation of complement in preeclampsia significantly increases the risk of various abnormalities in children.
Existing treatment options for preeclampsia are mostly symptomatic and therefore ineffective, so further research on the complement system as a potential target in treating and preventing this pregnancy complication is promising.
References
- Melchiorre K., Giorgione V., Thilaganathan B. The placenta and preeclampsia: villain or victim? Am. J. Obstet. Gynecol. 2021 Mar 24; S0002-9378(20)31198-4. https://dx.doi.org/10.1016/j.ajog.2020.10.024.
- Collier A.Y., Smith L.A., Karumanchi S.A. Review of the immune mechanisms of preeclampsia and the potential of immune modulating therapy. Hum. Immunol. 2021; 82(5): 362-70. https://dx.doi.org/10.1016/j.humimm.2021.01.004.
- Harmon A.C., Cornelius D.C., Amaral L.M., Faulkner J.L., Cunningham M.W. Jr, Wallace K., LaMarca B. The role of inflammation in the pathology of preeclampsia. Clin. Sci. (Lond). 2016; 130(6): 409-19. https://dx.doi.org/10.1042/CS20150702.
- Regal J.F., Burwick R.M., Fleming S.D. The complement system and preeclampsia. Curr. Hypertens. Rep. 2017; 19(11): 87. https://dx.doi.org/10.1007/s11906-017-0784-4.
- Girardi G., Lingo J.J., Fleming S.D., Regal J.F. Essential role of complement in pregnancy: from implantation to parturition and beyond. Front. Immunol. 2020; 11: 1681. https://dx.doi.org/10.3389/fimmu.2020.01681.
- Pierik E., Prins J.R., van Goor H., Dekker G.A., Daha M.R., Seelen M.A.J., Scherjon S.A. Dysregulation of complement activation and placental dysfunction: a potential target to treat preeclampsia? Front. Immunol. 2020; 10: 3098. https://dx.doi.org/10.3389/fimmu.2019.03098.
- Ling M., Murali M. Analysis of the complement system in the clinical immunology laboratory. Clin. Lab. Med. 2019; 39(4): 579-90. https://dx.doi.org/10.1016/j.cll.2019.07.006.
- Burwick R.M., Feinberg B.B. Complement activation and regulation in preeclampsia and HELLP syndrome. Am. J. Obstet. Gynecol. 2020 Sep 25; S0002-9378(20)31129-7. https://dx.doi.org/10.1016/j.ajog.2020.09.038.
- Jia K., Ma L., Wu S., Yang W. Serum levels of complement factors C1q, Bb, and H in normal pregnancy and severe pre-eclampsia. Med. Sci. Monit. 2019; 25: 7087-93. https://dx.doi.org/10.12659/MSM.915777.
- He Y., Xu B., Song D., Yu F., Chen Q., Zhao M. Expression of the complement system's activation factors in plasma of patients with early/late-onset severe pre-eclampsia. Am. J. Reprod. Immunol. 2016; 76(3): 205-11. https://dx.doi.org/10.1111/aji.12541.
- Richani K., Soto E., Romero R., Espinoza J., Chaiworapongsa T., Nien J.K. et al. Normal pregnancy is characterized by systemic activation of the complement system. J. Matern. Fetal Neonatal Med. 2005; 17(4): 239-45. https://dx.doi.org/10.1080/14767050500072722.
- Derzsy Z., Prohászka Z., Rigó J. Jr, Füst G., Molvarec A. Activation of the complement system in normal pregnancy and preeclampsia. Mol. Immunol. 2010; 47(7-8): 1500-6. https://dx.doi.org/10.1016/j.molimm.2010.01.021.
- He Y.D., Xu B.N., Song D., Wang Y.Q., Yu F., Chen Q., Zhao M.H. Normal range of complement components during pregnancy: A prospective study. Am. J. Reprod. Immunol. 2020; 83(2): e13202. https://dx.doi.org/10.1111/aji.13202.
- Ueda M., Sato Y., Horie A., Tani H., Miyazaki Y., Okunomiya A. et al. Endovascular trophoblast expresses CD59 to evade complement-dependent cytotoxicity. Mol. Cell. Endocrinol. 2019; 490: 57-67. https://dx.doi.org/10.1016/j.mce.2019.04.006.
- Girardi G. Complement activation, a threat to pregnancy. Semin. Immunopathol. 2018; 40(1): 103-11. https://dx.doi.org/10.1007/s00281-017-0645-x.
- Regal J.F., Gilbert J.S., Burwick R.M. The complement system and adverse pregnancy outcomes. Mol. Immunol. 2015; 67(1): 56-70. https://dx.doi.org/10.1016/j.molimm.2015.02.030.
- Стрижаков А.Н., Тимохина Е.В., Федюнина И.А., Игнатко И.В., Асланов А.Г., Богомазова И.М. Почему преэклампсия трансформируется в HELLP-синдром? Роль системы комплемента. Акушерство и гинекология. 2020; 5: 52-7. [Strizhakov A.N., Timokhina E.V., Fedyunina I.A., Ignatko I.V., Aslanov A.G., Bogomazova I.M. Why does preeclampsia transform into hellp syndrome? the role of the complement system. Obstetrics and Gynecology. 2020; 5: 52-7. (in Russian)]. https://dx.doi.org/10.18565/aig.2020.5.52-57.
- Agostinis C., Tedesco F., Bulla R. Alternative functions of the complement protein C1q at embryo implantation site. J. Reprod. Immunol. 2017; 119: 74-80. https://dx.doi.org/10.1016/j.jri.2016.09.001.
- Agostinis C., Stampalija T., Tannetta D., Loganes C., Vecchi Brumatti L., De Seta F. et al. Complement component C1q as potential diagnostic but not predictive marker of preeclampsia. Am. J. Reprod. Immunol. 2016; 76(6):475-81. https://dx.doi.org/10.1111/aji.12586.
- Agostinis C., Bulla R., Tripodo C., Gismondi A., Stabile H., Bossi F. et al. An alternative role of C1q in cell migration and tissue remodeling: contribution to trophoblast invasion and placental development. J. Immunol. 2010; 185(7): 4420-9. https://dx.doi.org/10.4049/jimmunol.0903215.
- Elvington M., Liszewski M.K., Atkinson J.P. Evolution of the complement system: from defense of the single cell to guardian of the intravascular space. Immunol. Rev. 2016; 274(1): 9-15. https://dx.doi.org/10.1111/imr.12474.
- Hoffman M.C., Rumer K.K., Kramer A., Lynch A.M., Winn V.D. Maternal and fetal alternative complement pathway activation in early severe preeclampsia. Am. J. Reprod. Immunol. 2014; 71(1): 55-60. https://dx.doi.org/10.1111/aji.12162.
- Lynch A.M., Murphy J.R., Byers T., Gibbs R.S., Neville M.C., Giclas P.C. et al. Alternative complement pathway activation fragment Bb in early pregnancy as a predictor of preeclampsia. Am. J. Obstet. Gynecol. 2008; 198(4): 385.e1-9. https://dx.doi.org/10.1016/j.ajog.2007.10.793.
- Loirat C., Frémeaux-Bacchi V. Atypical hemolytic uremic syndrome. Orphanet. J. Rare Dis. 2011; 6: 60. https://dx.doi.org/10.1186/1750-1172-6-60.
- Burwick R.M., Fichorova R.N., Dawood H.Y., Yamamoto H.S., Feinberg B.B. Urinary excretion of C5b-9 in severe preeclampsia: tipping the balance of complement activation in pregnancy. Hypertension. 2013; 62(6): 1040-5. https://dx.doi.org/10.1161/HYPERTENSIONAHA.113.01420.
- Denny K.J., Coulthard L.G., Finnell R.H., Callaway L.K., Taylor S.M., Woodruff T.M. Elevated complement factor C5a in maternal and umbilical cord plasma in preeclampsia. J. Reprod. Immunol. 2013; 97(2): 211-6. https://dx.doi.org/10.1016/j.jri.2012.11.006.
- Ma Y., Kong L.R., Ge Q., Lu Y.Y., Hong M.N., Zhang Y. et al. Complement 5a-mediated trophoblasts dysfunction is involved in the development of pre-eclampsia. J. Cell. Mol. Med. 2018; 22(2): 1034-46. https://dx.doi.org/10.1111/jcmm.13466.
- Larsen J.B., Andersen A.S., Hvas C.L., Thiel S., Lassen M.R., Hvas A.M., Hansen A.T. Lectin pathway proteins of the complement system in normotensive pregnancy and pre-eclampsia. Am. J. Reprod. Immunol. 2019; 81(4): e13092. https://dx.doi.org/10.1111/aji.13092.
- van der Linde D., Konings E.E., Slager M.A., Witsenburg M., Helbing W.A., Takkenberg J.J., Roos-Hesselink J.W. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J. Am. Coll. Cardiol. 2011; 58(21): 2241-7. https://dx.doi.org/10.1016/j.jacc.2011.08.025.
- Woodruff T.M., Ager R.R., Tenner A.J., Noakes P.G., Taylor S.M. The role of the complement system and the activation fragment C5a in the central nervous system. Neuromolecular Med. 2010; 12(2): 179-92. https://dx.doi.org/10.1007/s12017-009-8085-y.
- Lahti-Pulkkinen M., Girchenko P., Tuovinen S., Sammallahti S., Reynolds R.M., Lahti J. et al. Maternal hypertensive pregnancy disorders and mental disorders in children. Hypertension. 2020; 75(6): 1429-38. https://dx.doi.org/10.1161/HYPERTENSIONAHA.119.14140.
- Ducat A., Vargas A., Doridot L., Bagattin A., Lerner J., Vilotte J.L. et al. Low-dose aspirin protective effects are correlated with deregulation of HNF factor expression in the preeclamptic placentas from mice and humans. Cell Death Discov. 2019; 5: 94. https://dx.doi.org/10.1038/s41420-019-0170-x.
- Wat J.M., Hawrylyshyn K., Baczyk D., Greig I.R., Kingdom J.C. Effects of glycol-split low molecular weight heparin on placental, endothelial, and anti-inflammatory pathways relevant to preeclampsia. Biol. Reprod. 2018; 99(5): 1082-90. https://dx.doi.org/10.1093/biolre/ioy127.
- McLaughlin K., Scholten R.R., Parker J.D., Ferrazzi E., Kingdom J.C.P. Low molecular weight heparin for the prevention of severe preeclampsia: where next? Br. J. Clin. Pharmacol. 2018; 84(4): 673-8. https://dx.doi.org/10.1111/bcp.13483.
- Sones J.L., Cha J., Woods A.K., Bartos A., Heyward C.Y., Lob H.E. et al. Decidual Cox2 inhibition improves fetal and maternal outcomes in a preeclampsia-like mouse model. JCI Insight. 2016; 1(3): e75351. https://dx.doi.org/10.1172/jci.insight.75351.
- Risitano A.M., Ricklin D., Huang Y., Reis E.S., Chen H., Ricci P. et al. Peptide inhibitors of C3 activation as a novel strategy of complement inhibition for the treatment of paroxysmal nocturnal hemoglobinuria. Blood. 2014; 123(13): 2094-101. https://dx.doi.org/10.1182/blood-2013-11-536573.
- Martel C., Granger C.B., Ghitescu M., Stebbins A., Fortier A., Armstrong P.W. et al. Pexelizumab fails to inhibit assembly of the terminal complement complex in patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Insight from a substudy of the Assessment of Pexelizumab in Acute Myocardial Infarction (APEX-AMI) trial. Am. Heart J. 2012; 164(1): 43-51. https://dx.doi.org/10.1016/j.ahj.2012.04.007.
- Lillegard K.E., Loeks-Johnson A.C., Opacich J.W., Peterson J.M., Bauer A.J., Elmquist B.J. et al. Differential effects of complement activation products c3a and c5a on cardiovascular function in hypertensive pregnant rats. J. Pharmacol. Exp. Ther. 2014; 351(2): 344-51. https://dx.doi.org/10.1124/jpet.114.218123.
- Pouw R.B., Brouwer M.C., de Gast M., van Beek A.E., van den Heuvel L.P., Schmidt C.Q. et al. Potentiation of complement regulator factor H protects human endothelial cells from complement attack in aHUS sera. Blood Adv. 2019; 3(4): 621-32. https://dx.doi.org/10.1182/bloodadvances.2018025692.
Received 14.12.2021
Accepted 19.01.2022
About the Authors
Iraida S. Sidorova, Dr. Med. Sci., Professor, Academician of the RAS, Merited Scholar of the Russian Federation, Department of Obstetrics and Gynecology № 1,N.V. Sklifosovsky Institute of Clinical Medicine, I.M. Sechenov First MSMU, Ministry of Health of Russia (Sechenov University), sidorovais@yandex.ru,
119991, Russia, Moscow, Trubetskaya str., 8 bld. 2.
Natalya A. Nikitina, Dr. Med. Sci., Professor at the Department of Obstetrics and Gynecology № 1, N.V. Sklifosovsky Institute of Clinical Medicine, I.M. Sechenov First MSMU, Ministry of Health of Russia (Sechenov University), natnikitina@list.ru, 119991, Russia, Moscow, Trubetskaya str., 8 bld. 2.
Mikhail B. Ageev, Ph.D., Teaching Assistant at the Department of Obstetrics and Gynecology №1, N.V. Sklifosovsky Institute of Clinical Medicine, I.M. Sechenov First MSMU,
Ministry of Health of Russia (Sechenov University), mikhaageev@yandex.ru, 119991, Russia, Moscow, Trubetskaya str., 8 bld. 2.
Albert A. Kokin, Head of the Department of Anesthesiology and Intensive Care, Maternity Hospital affiliated to the V.V. Veresaev Clinical Hospital of Moscow City Health Department, Alberkokin@yandex.ru, 127247, Russia, Moscow, 800th anniversary of Moscow str., 22.
Marina A. Kir'yanova, Postgraduate Student at the Department of Obstetrics and Gynecology №1, N.V. Sklifosovsky Institute of Clinical Medicine, I.M. Sechenov First MSMU,
Ministry of Health of Russia (Sechenov University), 119991, Russia, Moscow, Trubetskaya str., 8 bld. 2.
Authors' contributions: Sidorova I.S., Nikitina N.A. – conception and design of the study; Nikitina N.A., Kokin A.A., Kir’yanova M.A. – data collection and analysis; Nikitina N.A., Kokin A.A. – statistical analysis; Nikitina N.A., Ageev M.B. – manuscript drafting; Sidorova I.S. – manuscript editing.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: The study was supported by the Russian Science Foundation (grant no. 17-74-30019 “Structural and kinetic features of antigen presentation as a key to understanding the mechanisms of induction of autoimmune pathologies and lymphomogenesis”).
Ethical approval: The study was reviewed and approved by the Research Ethics Committee of the I.M. Sechenov First Moscow State Medical University, Ministry of Health of the Russian Federation (Sechenov University) (Ref. No: 28-20/07.10.2020).
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Sidorova I.S., Nikitina N.A., Ageev M.B., Kokin A.A., Kir'yanova M.A.
Complement system dysregulation in patients with preeclampsia.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2022; 2: 46-58 (in Russian)
https://dx.doi.org/10.18565/aig.2022.2.46-58



