Free amino acid imbalances in amniotic fluid in preeclampsia
Objective. To assess the role of imbalance in the amino acid composition of amniotic fluid in the development of preeclampsia (PE).Pogorelova T.N., Gunko V.O., Palieva N.V., Alliluev I.A., Kaushanskaya L.V., Larichkin A.V.
Subjects and methods. The investigation enrolled 34 women, whose pregnancy was complicated with PE (a study group) and 30 women with uncomplicated pregnancy (a control group). The levels of amino acids in the amniotic fluid were determined in the second and third trimesters of pregnancy, by using an automated amino acid analyzer.
Results. PE was found to develop in the presence of multidirectional changes in the content of amino acids involved in many metabolic processes required for normal fetoplacental function. Different trimesters of pregnancy differ in the magnitude of the deviations found in the composition of amino acids in the amniotic fluid. The most pronounced imbalance was observed in the second trimester. The possibility of damage to different metabolic pathways in the second and third trimesters is discussed.
Conclusion. The findings make it possible to expand our understanding of the biochemical mechanisms of the formation and development of PE and to elaborate criteria for predicting this pathology.
Keywords
Among the factors complicating pregnancy, the important role belongs to preeclampsia (PE) which currently continues to remain one of the leading causes of maternal and perinatal morbidity and mortality [1, 2]. The number of observations of the PE development in the world reaches 3–8% and has no tendency to decrease [3, 4]. The high frequency of various complications in PE is obviously related to the lack of accurate knowledge of the disease pathogenesis and reliable prognostic and diagnostic criteria for its development. The situation is complicated by the fact that this is a multifactorial pathology, and there are many theories and hypotheses of the PE pathogenesis, which do not allow their supporters to develop a unified scheme of optimal therapy. Most of the studies related to the biochemical aspects of the PE pathogenesis concern the issues of endothelial dysfunction, oxidative stress, angiogenesis and apoptosis [5]. At the same time, modification of such multifunctional molecules as proteins, which play a key role in all cellular reactions, remains poorly studied in these processes [6]. To maintain a normal protein profile requires a balance of structural components of protein molecules, which include proteinogenic amino acids. In addition to participating in the biosynthesis of proteins, amino acids serve as precursors of many bioactive compounds, they are involved in energy metabolism. Some of them perform independent functions, in particular, as regulators of the immune response, inducers of hormone synthesis, activators of proliferative processes intensively occurring in the placental complex, especially in early stages of gestation [7, 8]. Due to the important metabolic role of amino acids, as well as the need to search for methods of early preclinical diagnostics of PE, assessment of the amino acid spectrum in different parts of the mother-fetus system can be used as a predictor marker of this pathology.
The study of amniotic fluid provides important information about the course of pregnancy, the condition of the fetus and the prognosis of the newborn. It quickly reacts to changes in its composition to any deviations occurring in the mother, fetus and placenta, which is largely a source of supply of nutrients and energy components to the amniotic fluid [9, 10]. It should be noted that the metabolic processes between the mother and the fetus occur due to the main placental exchange and paraplacental exchange, in which the amniotic fluid plays an important role [11]. However, studies of the biochemical composition of this biologically active medium, especially the amino acid spectrum, are few and contradictory [12, 13], and there is no information about it in PE.
Therefore, the aim of the present work is to study the amino acid composition of the amniotic fluid in normal pregnancy and PE in order to clarify the role of the identified impairments in the development of this obstetric pathology.
Materials and Methods
The study included 64 women in two groups. The first (control) group included 30 healthy women with uncomplicated pregnancy and labor; the second (study) group consisted of 34 women whose pregnancy was complicated by moderate PE; in accordance with the international classification of diseases it is ICD-10, code O14.0. Criteria for the inclusion of patients into the study group were age from 20 to 35 years, clinical signs of PE. The examined women had the signs after 24-26 weeks of gestation, they had hypertension (pressure 140–160/90 mm Hg) and proteinuria (above 0.3 g /day, but less than 2 g /day). Pregnancy in these women completed with delivery on time (39–40 weeks). The criteria for inclusion into the control group were the normal course of pregnancy, full-term pregnancy and age from 20 to 35 years. The examined women were observed in the advisory clinic of the Rostov Research Institute of Obstetrics and Pediatrics under the program “Obstetric Monitoring”.
The material for the study was amniotic fluid taken from each woman in both groups twice. At 16-18 weeks gestation, the amniotic fluid was obtained by transabdominal amniocentesis. This procedure was carried out to exclude chromosomal abnormalities of the fetus (Order of the Ministry of Health of Russia dated 01.11.2012 No. 572n). All the fetuses of the women observed in the study had a normal chromosomal set. Repeated collection of amniotic fluid was carried out at 39-40 weeks gestation during the rupture of the membranes in the first stage of labor.
The content of free amino acids in the amniotic fluid was determined in the AAA-400 Automatic Amino Acid Analyzer (Microtechna, Czech Republic). Sample preparation and analysis were performed according to the instructions for the system according to the standard program using three sodium citrate buffer solutions of pH 3.25; 4.25; 5.28. The identification of amino acids, the calculation of peak areas and the determination of concentration were carried out according to the results of the analysis of the relevant standards (Sigma-Aldrich, USA) for calibrating the analyzer.
Statistical analysis was carried out using the licensed software package Statistica (version 6.0 of StatSoft Inc.). The evaluation of the data distribution using the Shapiro-Wilk test indicates a normal distribution. The data are presented as mean value (M), standard deviation (SD) and 95% confidence interval (95% CI). The statistical significance of differences between the compared indicators was determined by Student’s test (t-test) for independent samples, as well as by c2-test for comparison of categorical variables. Correlation analysis was performed using Pearson’s test and calculation of correlation coefficient r. The results were considered statistically significant at p<0.05.
Results
The compared groups of patients did not differ statistically in obstetric and somatic anamnesis (Table 1).
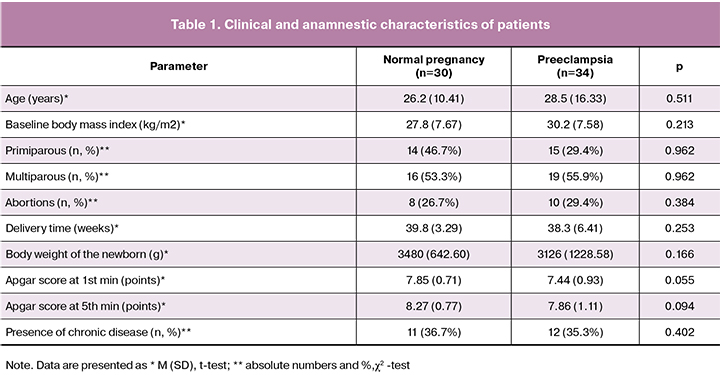
The results indicate that the development of PE is accompanied by the changes in the amino acid composition of amniotic fluid compared with similar indicators in normal pregnancy (Table 2, Figure 1).
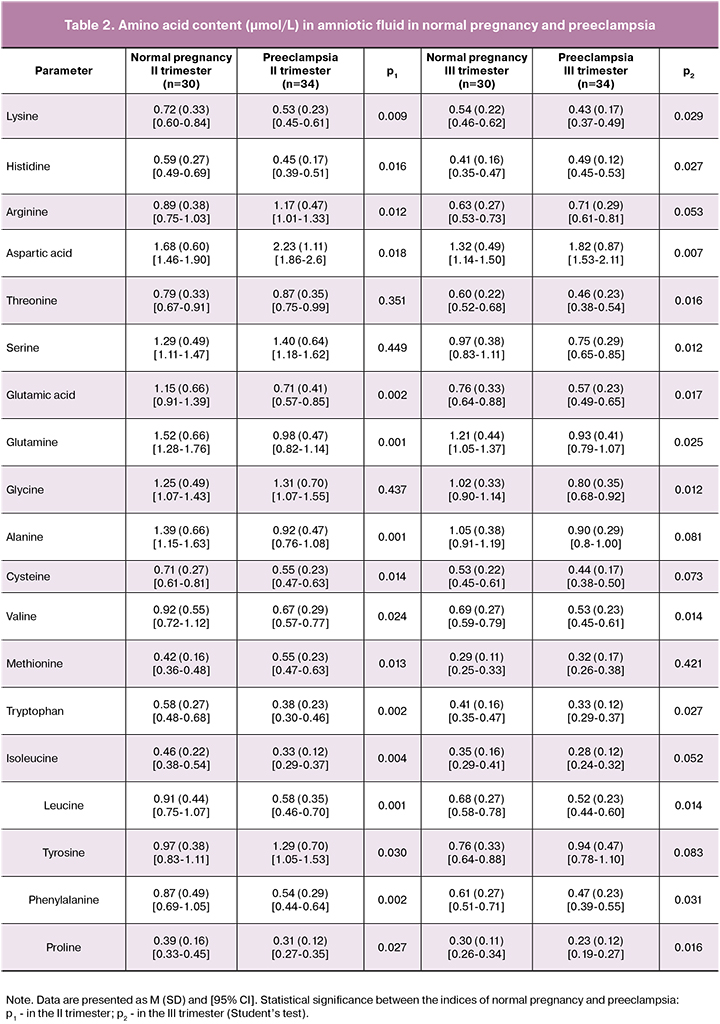
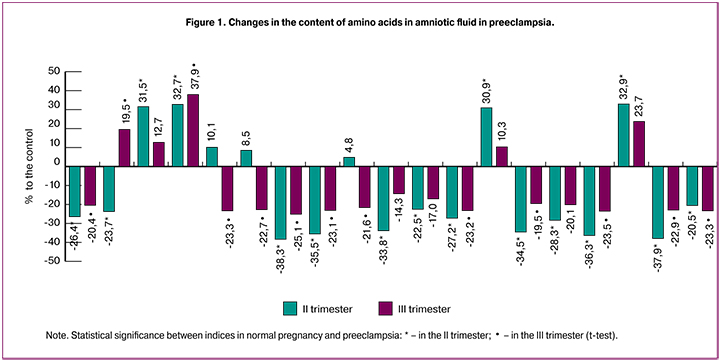
A significant modification of the content of the most amino acids takes place already in the II trimester, when the clinical signs of PE were absent. The most pronounced changes were found for glutamic acid and its amide, glutamine. The content of these compounds was reduced by 38.3% and 35.5%, respectively. The relationship between changes in these indicators is shown by correlation analysis. The correlation coefficient (r) between them is +0.87 (p = 0.042, 95% CI 0.84–0.9). The opposite direction of change is established for another dicarboxylic amino acid, aspartic, its amount increases in PE by 31.1%. The content of diamino acid, arginine (by 31.5%) was also increased, it was accompanied by the decrease in the content of two other diamino acids, lysine (by 26.4%) and histidine (by 23.7%). The values of most neutral amino acids: alanine, cysteine, valine, tryptophan, isoleucine, leucine, phenylalanine and proline in the amniotic fluid in the second trimester of pregnancy in PE are lower than those in the same period in normal pregnancy on average by 30.5%. An increased concentration is noted for methionine and tyrosine (by 30.9% and 32.9%, respectively). Significant differences are absent in the content of three essential amino acids: serine, glycine and threonine, which are metabolically related.
Such important indicator as the ratio of essential amino acids to non-essential ones is also decreasing. In normal pregnancy this coefficient in the second trimester is 0.45, and in PE it is 0.39.
At the end of pregnancy, deviations in the amino acid spectrum of amniotic fluid are less pronounced than in the second trimester. Thus, the content of glutamic acid and glutamine in the third trimester is lower in PE than in normal pregnancy, respectively by 25.1% and 23.1%. The amount of lysine was reduced by 20.4%, and the level of arginine did not change at all. There is an increase in the content of histidine by 19.5% in the third trimester in comparison with the second one. The degree of reduction in the content of proline, phenylalanine, valine is 23.3%, 22.9% and 23.2%, respectively. The absence of significant differences was found in the concentrations of neutral amino acids: alanine, cysteine, methionine, isoleucine and tyrosine. At the same time, serine, glycine and threonine obviously take a more active part in the development of the imbalance of amino acid composition in the third trimester of pregnancy in PE, the level of which decreases by 22.7%, 21.6% and 23.4%, respectively.
Discussion
The revealed changes in the amino acid composition of amniotic fluid in the dynamics of pregnancy in PE can be caused by various reasons, and their imbalance, in turn, can lead to negative metabolic consequences. These reasons may include the impairment of transplacental transfer of amino acids, change of balance of anabolism and catabolism of proteins, modification of activity of the enzymes which catalyze reactions of an exchange of individual amino acids [14].
A significant change in the level of glutamic and aspartic acids, which are integral indicators of many enzymatic processes, is of critical importance. With their participation, the interaction of nitrogen and energy exchanges occurs; therefore, a change in their content undoubtedly affects metabolism in the fetoplacental system, as well as the efficiency of the tricarboxylic acid cycle (TCA). Multidirectional changes in the concentration of glutamate and aspartate may be explained by a decrease in the activity of aspartate aminotransferase which is one of the limiting factors in the exchange of aspartic acid. It results in accumulation of aspartic acid and, on the contrary, a decrease in the concentration of glutamic acid resulting from the reaction. The decrease in the level of glutamic acid may be accompanied by a decrease in the amount of glutamine during its amidation which is observed in PE. It is known that about 20% of fetal nitrogen is accounted for glutamine, which is more necessary for the growth of fetal cells than any other amino acid [15]. The nitrogen of the amide group of glutamine is important for the synthesis of nucleotides and hexosamines. In addition, during cyclization of glutamate, proline is produced, which is a part of collagen, an important component of the vascular wall. Modification of the structure of the latter, in combination with other factors, may be accompanied by the development of endothelial dysfunction [16]. In connection with the decrease in the amount of proline revealed by us, such consequences are quite probable.
In the second trimester of pregnancy, the amniotic fluid of women in the study group contains a smaller amount of alanine compared with the same value in the control group. The carbon chain of alanine constantly occurs during the processes of biological oxidation and the decrease in the intensity of the latter in complicated pregnancy may be accompanied by a restriction of the synthesis of this amino acid. As an important source of energy for fetal tissues, it also serves as a substrate of bioactive compounds such as carnosine, anserine, coenzyme A [14].
The deviations in the content of diamino acids, arginine and histidine, play an important role in the development of pregnancy complications [17]. The main reason for the increase in the level of arginine in the amniotic fluid in the second trimester in women of the study group may be a decrease in its use by the placenta as a source of a powerful vasodilator nitric oxide and, as a consequence, an impairment of placental hemodynamics.
The recent data testify the regulatory role of arginine in the expression of genes responsible for the synthesis of proteins (including enzymes) that affect the balance of pro- and antioxidant processes [18], which may be especially important in the development of PE. Obviously, a change in its content can cause an imbalance of these processes. As for histidine, since it has antioxidant properties [19], a decrease in its production also has negative consequences for the course of pregnancy and fetal development. Moreover, a decrease in histidine may be accompanied by an increase in histamine, its derivative, which has vasoconstrictive properties and impairs fetoplacental blood flow.
The development of PE is also affected by the reduction in the concentration of tryptophan and phenylalanine, which have significant antioxidant activity [12]. Tryptophan is considered to be an indicator of oxidative stress [20]. The decrease in the level of cysteine in the amniotic fluid in the third trimester of pregnancy in PE affects primarily the impairment of the glutathione synthesis, its restored form is an active antioxidant. The second way is the decrease in the participation of cysteine in the taurine synthesis; this amino acid is involved in antioxidant processes and has membrane protective properties [21]. The functional metabolic value of taurine in gestation is evidenced by the fact that its concentration in the placenta is two to three times higher than that of essential amino acids [22].
Reducing the content of essential amino acids with branched side chains: valine, leucine and isoleucine in the second trimester of pregnancy in women of the study group also contributes to the development of PE, since they perform important functions in nitrogen and energy metabolism. These amino acids are used for synthesis of intermediate compounds of TCA and gluconeogenesis, regulate cell growth processes, influence insulin secretion and transplacental transition of other hydrophobic amino acids [22, 23].
Methionine, the concentration of which increases in PE, is of considerable interest for the assessment of amino acid composition disorders in amniotic fluid. It is a key source of methyl groups necessary for the synthesis of purines and methylation of DNA, proteins, biogenic amines, phospholipids. An important product in the chain of metabolic methionine transformations is homocysteine. It can be assumed that the increase in the level of methionine is accompanied by an increase in the amount of homocysteine, which is currently considered as a cytotoxic factor that plays an important role in the early stages of endothelial dysfunction, hyperhomocysteinemia, leading to impaired uteroplacental and fetoplacental blood flow [24, 25]. In addition to the above impairments in the amino acid composition, decrease in the number of essential and nonessential amino acids can lead to the disorder of protein synthesis.
In contrast to the second trimester of complicated pregnancy, the third trimester with the absence or less significant changes in the majority of amino acids showed a decrease in the concentration of serine, glycine and threonine. All three amino acids perform important functions, especially during pregnancy [14]. Serine, as well as methionine, is a participant of the so-called one-carbon metabolism, a source of methyl groups for the synthesis of purine nucleotides and other compounds. It is a precursor of phosphatidylcholine, sphingomyelin, glycine [26]. Impairment of the activity of enzymes of serine synthesis and metabolism is associated with various pathological conditions [27]. Threonine takes part in the formation of glycine and serine, the amount of glycine depends on the level of threonine [28]. In turn, glycine is also actively involved in metabolic regulation, synthesis of glutathione, heme, purines and porphyrins [29]. All three amino acids can be metabolized via pyruvate in acetyl coenzyme, thus linking them with TCA. A direct correlation is found between the change in the content of these amino acids. The correlation coefficient between serine and glycine is +0.85 (p=0.023, 95% CI 0.81–0.88), and between serine and threonine r=+0.84 (p=0.039, 95% CI 0.80–0.88). Reducing their content in PE, obviously, affects different aspects of metabolism, including energy, lipid, nucleotide. The impairment of these processes might result from the amino acid imbalance in the third trimester in PE than the change in pro-and antioxidant reactions, which is characteristic of the second trimester.
In general, assessing the modification of the amino acid spectrum in amniotic fluid in PE, it should be noted that the most significant impairments occur in the second trimester compared with the third trimester. This period of pregnancy is characterized by a high degree of cell differentiation, intensive proliferative, anabolic processes, reactions of angiogenesis and apoptosis, occurring primarily in the placenta and the fetus. According to our data, there is a higher level of free amino acids in women of the control group in the second trimester than in ones in the third trimester, which confirms the functional and metabolic significance of this trimester in the development of pregnancy. The impairments arising in this stage in the maternal body, fetus and placenta frequently cause subsequent complications during pregnancy [30]. Summing up the effects of changes in the amino acid composition of amniotic fluid in PE, it can be assumed that the most important impairments are the disorders of energy metabolism, the ratio of pro- and antioxidant balance, the rate of transplacental processes, methylation reactions of the most important biosubstrates, synthesis of nucleotides, proteins, phospholipids. Changes in all these processes make a significant contribution to the formation of dysfunction of the fetoplacental system and the creation of metabolic, structural and functional prerequisites for the development of PE.
The data obtained indicate a high reactivity of the metabolism of amniotic fluid in response to the development of a complicated pregnancy and, possibly, its active participation in the disorders in the placental complex.
Conclusion
Summarizing the obtained data, it can be concluded that the PE development is accompanied by the significant changes in the content of amino acids in the amniotic fluid. Amino acid imbalance is obviously an important cause and, at the same time, a reflection of homeostasis impairment in the mother-placenta-fetus system. Various trimesters of pregnancy differ in varying degrees of revealed deviations in the spectrum of amino acids. The most pronounced changes in a larger number of amino acids occur in the second trimester compared with the third trimester. The directionality of changes in the content of some amino acids in PE in the third trimester differs from that in the second trimester, which indicates the possibility of disorders of different metabolic pathways in these stages of pregnancy. The results of this study allow us to expand our understanding of the biochemical mechanisms of formation and further development of PE.
References
- Ходжаева З.С., Коган Е.А., Клименченко Н.И., Акатьева А.С., Сафонова А.Д., Холин А.М., Вавина О.В., Сухих Г.Т. Клинико-патогенетические особенности ранней и поздней преэклампсии. Акушерство и гинекология. 2015; 1: 12-7. [Khodzhaeva Z.S., Kogan Y.A., Klimenchenko N.I., Akatyeva A.S., Safonova A.D., Kholin A.M. et al. Clinical and pathogenetic features of early and late preeclampsia. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2015; 1: 12-17. (in Russian)]
- Мирошина Е.Д., Тютюнник Н.В., Храмченко Н.В., Харченко Д.К., Кан Н.Е.Диагностика преэклампсии на современном этапе (обзор литературы). Проблемы репродукции. 2017; 23(1): 96-102. [Miroshina E.D., Tyutyunnik N.V., Khramchenko N.V., Kharchenko D.K., Kan N.E. Modern diagnostic methods of preeclampsia (a review). Problemy reprodukcii/ Reproduction problems. 2017; 23(1): 96-102. (in Russian)] doi: 10.17116/repro201723196-102/
- Ghulmiyyah L., Sibai B. Maternal mortality from preeclampsia/eclampsia. Semin. Perinatol. 2012; 36(1): 56-9. doi: 10.1053/j.semperi.2011.09.011.
- Сидорова И.С., Никитина Н.А. Предиктивный, превентивный подход к ведению беременных группы риска развития преэклампсии. Российский вестник акушера-гинеколога. 2014; 14(5): 44-9. [Sidorova I.S., Nikitina N.A. A predictive and preventive approach to managing pregnant women at risk of preeclampsia. Rossijskij vestnik akushera-ginekologa/ Russian journal of obstetrician-gynecologist. 2014; (5): 44-49. (in Russian)]
- Anderson U.D., Olsson M.G., Kristensen K.H., Åkerström B., Hansson S.R. Review: Biochemical markers to predict preeclampsia. Placenta. 2012; 33(Suppl.): S42-7. doi: 10.1016/j.placenta.2011.11.021.
- Погорелова Т.Н., Гунько В.О., Линде В.А. Протеомный профиль плаценты при физиологической беременности и беременности, осложненной преэклампсией. Акушерство и гинекология. 2013; 7: 24-9. [Pogorelova T.N., Gunko V.O., Linde V.A. Proteome profile of the placenta in physiological pregnancy and pregnancy complicated by preeclampsia. Obstetrics and gynecology. 2013; 7: 24-9. (in Russian)]
- D’Mello J.P.F., ed. Amino acids in human nutrition and health. Wallingford: CAB International; 2012.
- Bröer S., Bröer A. Amino acid homeostasis and signalling in mammalian cells and organisms. Biochem. J. 2017; 474(12): 1935-63. doi: 10.1042/BCJ20160822.
- Tong X.L., Wang L., Gao T.B., Qin Y.G., Qi Y.Q., Xu Y.P. Potential function of amniotic fluid in fetal development---novel insights by comparing the composition of human amniotic fluid with umbilical cord and maternal serum at mid and late gestation. J. Chin. Med. Assoc. 2009; 72(7): 368-73. doi: 10.1016/S1726-4901(09)70389-2.
- Tong X. Amniotic fluid may act as a transporting pathway for signaling molecules and stem cells during the embryonic development of amniotes. J. Chin. Med. Assoc. 2013; 76(11): 606-10. doi: 10.1016/j.jcma.2013.07.006.
- Погорелова Т.Н., Крукиер И.И., Линде В.А. Биохимия амниотической жидкости. LAP LAMBERT Academic Publishing; 2012. 132с. [Pogorelova T.N., Krukier I.I., Linde V.A. Biochemistry of amniotic fluid. LAP LAMBERT Academic Publishing; 2012. 132 p. (in Russian)]
- Orczyk-Pawilowicz M., Jawien E., Deja S., Hirnle L., Zabek A., Mlynarz P. Metabolomics of human amniotic fluid and maternal plasma during normal pregnancy. PLoS One. 2016; 11(4): e0152740. doi: 10.1371/journal.pone.0152740.
- Fotiou M., Michaelidou A.M., Masoura S., Menexes G., Koulourida V., Biliaderis C.G. et al. Second trimester amniotic fluid uric acid, potassium, and cysteine to methionine ratio levels as possible signs of early preeclampsia: A case report. Taiwan J. Obstet. Gynecol. 2016; 55(6): 874-6. doi: 10.1016/j.tjog.2016.09.001.
- Wu G. Functional amino acids in growth, reproduction, and health. Adv. Nutr. 2010; 1(1): 31-7. doi: 10.3945/an.110.1008.
- Wu X., Xie C., Zhang Y., Fan Z., Yin Y., Blachier F. Glutamate-glutamine cycle and exchange in the placenta-fetus unit during late pregnancy. Amino Acids. 2015; 47(1): 45-53. doi: 10.1007/s00726-014-1861-5.
- Wadsack C., Desoye G., Hiden U. The feto-placental endothelium in pregnancy pathologies. Wien. Med. Wochenschr. 2012; 162(9-10): 220-4. doi: 10.1007/s10354-012-0075-2.
- Khalil A., Hardman L., O’Brien P. The role of arginine, homoarginine and nitric oxide in pregnancy. Amino Acids. 2015; 47(9): 1715-27. doi: 10.1007/s00726-015-2014-1.
- Lei X., Feng C., Liu C., Wu G., Meininger C.J., Wang F. et al. Regulation of protein expression by L-arginine in endothelial cells. Front. Biosci. (Schol. Ed.). 2011; 3: 655-61.
- Vera-Aviles M., Vantana E., Kardinasari E., Koh N.L., Latunde-Dada G.O. Protective role of histidine supplementation against oxidative stress damage in the management of anemia of chronic kidney disease. Pharmaceuticals (Basel). 2018; 11(4). pii: E111. doi: 10.3390/ph11040111.
- Xu K., Liu H., Bai M., Gao J., Wu X., Yin Y. Redox properties of tryptophan metabolism and the concept of tryptophan use in pregnancy. Int. J. Mol. Sci. 2017; 18(7). pii: E1595. doi: 10.3390/ijms18071595.
- Lambert I.H., Kristensen D.M., Holm J.B., Mortensen O.H. Physiological role of taurine--from organism to organelle. Acta Physiol. (Oxf.) 2015; 213(1): 191-212. doi: 10.1111/apha.12365.
- Desforges M., Parsons L., Westwood M., Sibley C.P., Greenwood S.L. Taurine transport in human placental trophoblast is important for regulation of cell differentiation and survival. Cell Death Dis. 2013; 4: e559. doi: 10.1038/cddis.2013.81.
- Tanaka K., Sakai K., Matsushima M., Matsuzawa Y., Izawa T., Nagashima T. et al. Branched-chain amino acids regulate insulin-like growth factor-binding protein 1 (IGFBP1) production by decidua and influence trophoblast migration through IGFBP1. Mol. Hum. Reprod. 2016; 22(8): 890-9. doi: 10.1093/molehr/gaw032.
- Арутюнян А.В., Пустыгина А.В., Милютина Ю.П., Залозняя И.В., Козина Л.С. Молекулярные маркеры окислительного стресса у потомства при экспериментальной гипергомоцистеинемии. Молекулярная медицина. 2015; 5: 41-6. [Arutjunyan A.V., Pustygina A.V., Milyutina Yu.P., Zaloznyaya I.V, Kozina L.S. Prenatal hyperhomocysteinemia and oxidative stress profile in the rat offspring. Molekulyarnaya medicina/ Molecular medicine. 2015; 5: 41-46. (in Russian)]
- Dasarathy J., Gruca L.L., Bennett C., Parimi P.S., Duenas C., Marczewski S. et al. Methionine metabolism in human pregnancy. Am. J. Clin. Nutr. 2010; 91(2): 357-65. doi: 10.3945/ajcn.2009.28457.
- El-Hattab A.W. Serine biosynthesis and transport defects. Mol. Genet. Metab. 2016; 118(3): 153-9. doi: 10.1016/j.ymgme.2016.04.010.
- Metcalf J.S., Dunlop R.A., Powell J.T., Banack S.A., Cox P.A. L-Serine: a naturally-occurring amino acid with therapeutic potential. Neurotox. Res. 2018; 33(1): 213-21. doi: 10.1007/s12640-017-9814-x.
- Avagliano L., Garò C., Marconi A.M. Placental amino acids transport in intrauterine growth restriction. J. Pregnancy. 2012; 2012: 972562. doi: 10.1155/2012/972562.
- Razak M.A., Begum P.S., Viswanath B., Rajagopal S. Multifarious beneficial effect of nonessential amino acid, glycine: a review. Oxid. Med. Cell. Longev. 2017; 2017: 1716701. doi: 10.1155/2017/1716701.
- Bogavac M., Lakic N., Simin N., Nikolic A., Sudji J., Bozin B. Biomarkers of oxidative stress in amniotic fluid and complications in pregnancy. J. Matern. Fetal Neonatal. Med. 2012; 25(1): 104-8. doi: 10.3109/14767058.2011.560625.
Received 29.06.2018
Accepted 21.09.2018
About the Authors
Pogorelova, Tatiana N., Doctor of Biological Science, professor, chief researcher, Scientific-Research Institute of Obstetrics and Pediatrics of Rostov State Medical University. 344012, Russia, Rostov-on-Don, Mechnikova str. 43. E-mail: tnp.rniiap@yandex.ru. ORCID ID https://orcid.org/0000-0002-0400-0652Gunko,Victorya O., PhD, senior researcher, Scientific-Research Institute of Obstetrics and Pediatrics of Rostov State Medical University.
344012, Rostov-on-Don, st. Mechnikova, 43. E-mail: rniiap@yandex.ru. ORCID ID https://orcid.org/0000-0001-8607-9052
Palieva Natalya Viktorovna, MD, chief researcher, Scientific-Research Institute of Obstetrics and Pediatrics of Rostov State Medical University.
344012, Russia, Rostov-on-Don, Mechnikova str. 43. E-mail: nat-palieva@yandex.ru. ORCID ID https://orcid.org/0000-0003-2278-5198
Аlliluev, Ilya А., junior researcher of Scientific-Research Institute of Obstetrics and Pediatrics of Rostov State Medical University.
344012, Russia, Rostov-on-Don, Mechnikova str. 43. E-mail: rniiap@yandex.ru. ORCID ID https://orcid.org/0000-0001-7654-0650
Kaushanskaya, Lyudmila V., chief researcher, Scientific-Research Institute of Obstetrics and Pediatrics of Rostov State Medical University.
344012, Russia, Rostov-on-Don, Mechnikova str. 43. E-mail: kaushan60@mail.ru. ORCID ID https://orcid.org/0000-0001-8574-6394
Larichkin, Аndrej V., junior researcher, Scientific-Research Institute of Obstetrics and Pediatrics of Rostov State Medical University.
344012, Russia, Rostov-on-Don, Mechnikova str. 43. E-mail: rniiap@yandex.ru
For citations: Pogorelova T.N., Gunko V.O., Palieva N.V., Alliluev I.A., Kaushanskaya L.V., Larichkin A.V. Free amino acid imbalances in amniotic fluid in preeclampsia. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2019; (2): 60-7. (in Russian)
http://dx.doi.org/10.18565/aig.2019.2.60-67



