Diagnostic value of anthropometric characteristics of obesity in women during the menopause transition
Objective: To investigate the diagnostic performance of body mass index (BMI) to identify excess adipose tissue (AT) and compare the diagnostic value of anthropometric characteristics in detecting visceral obesity in women during the menopause transition (MT). Materials and methods: A total of 125 women (mean age 47.0 (2.7) years) undergoing the MT without obesity (BMI<30 kg/m²) participated in the study. Clinical evaluation included hormonal and metabolic profile, body composition measured by dual-energy X-ray absorptiometry (DEXA), and anthropometric measurements. Results: 35% of women with normal BMI and waist circumference (WC) <80 cm had excess AT associated with twice higher prevalence of dyslipidemia and insulin resistance. BMI≥25 kg/m² had a sensitivity of 59.6%, a specificity of 93.7%, and positive (PPV) and negative (NPV) predictive values of 94.4 and 56.6% in detecting excess AT. The ROC analysis determined an optimal threshold of BMI>22.5 kg/m² with a sensitivity of 92.9%, specificity of 68.7%, PPV 84.1%, and NPV 84.6%. ROC analysis of all anthropometric indices showed diagnostic significance (p<0.001) for detecting visceral obesity; WC had the highest AUC of 0.950 [95% CI 0.875-0.987]. Conclusion: In women undergoing the MT, it is reasonable to use more accurate methods of assessing obesity, such as the DEXA. A BMI≥25 kg/m² is not sufficiently informative to detect excess AT in women during MT. The optimal threshold for identifying women with excessive AT during MT is BMI>22.5 kg/m². Among anthropometric indices, WC has the highest diagnostic accuracy for detecting visceral obesity.Yureneva S.V., Komedina V.I., Kuznetsov S.Yu.
Keywords
The results of large-scale studies conducted over the past 20 years indicated that menopause transition, regardless of chronological aging, is associated with changes in hormone profile, lipid metabolism, and transformation of body composition, resulting in an increased risk of cardiovascular disease, diabetes mellitus, and malignant neoplasms [1–3]. According to the SWAN study, with an 18-year follow-up from pre- to post-menopause, during the menopausal transition, two years before menopause, there was an accelerated accumulation of fat mass (450 g per year) and reduction of muscle mass (60 g per year), which continued until two years after the menopause [4]. Besides, there was a change in adipose tissue distribution with a predominant accumulation of visceral fat. During the menopause transition, visceral adipose tissue increases from 5–8% of total adipose tissue in premenopause to 15-20% in postmenopause [5, 6]. Fat depots of different locations are associated with varying risks of pathological processes associated with obesity. It is visceral obesity that conveys the most significant health risk [7].
Body mass index (BMI) is the most common clinical measure of normal body weight, overweight, and obesity, but it is not without its limitations. BMI indicates total body weight but does not entirely capture measurements of fat and muscle tissue distribution or body composition.
To assess obesity more accurately, most scientific communities recommend that waist circumference (WC) or waist /hip circumference (WC/HC) be measured in addition to BMI to provide an indication of the distribution of adipose tissue. WC ≥80 cm (as recommended by the International Diabetes Federation, IDF), WC/HC>0.85 (as recommended by WHO) are criteria for visceral obesity in women. The WC and WC/HC measurements are influenced not only by the volume of visceral but also by subcutaneous adipose tissue, which may negatively affect the method's accuracy, especially in the context of body composition transformation in women during the menopause transition. Therefore, the search for the best anthropometric measure of visceral obesity continues [8–10].
A key strategy during the menopause transition is early diagnosis of cardiometabolic risk factors, the most important of which are overweight and obesity. In this regard, the diagnostic performance of anthropometric methods for assessing obesity in women during the menopause transition is highly relevant.
The present study aimed to investigate the diagnostic performance of body mass index to identify excess adiposity and compare the diagnostic value of anthropometric characteristics in detecting visceral obesity in women during the menopause transition.
Materials and Methods
This single-center cross-sectional study enrolled 125 women aged 42 to 52 at the menopause transition (Stages -2, -1 of STRAW+10, Stages of Reproductive Aging Workshop, clinical and hormonal characterization of the stages of reproductive aging, 2011) with normal or excessive body weight (BMI 18.5–29.9 kg/m²), who were referred to the Department of Gynecological Endocrinology of the V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
Non-inclusion criteria were severe somatic and systemic autoimmune diseases; therapy with sex hormones less than 6 months before study inclusion; drugs affecting carbohydrate and lipid metabolism; and a history of the polycystic ovarian syndrome.
The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P. All participants provided signed informed consent to take part in the study.
Anthropometric assessments were conducted with the participants wearing light clothing and barefoot. BMI was calculated from the measured body weight and height: weight (kg)/height (m)². Overweight was defined when BMI was ≥25 kg/m², according to the WHO classification. WC was measured at the midpoint between the lower edge of the last palpable rib and the top of the iliac crest at the end of a normal exhalation. The HC was measured at the widest part of the buttocks. Based on these measurements, waist-to-hip ratio (WHR), waist-to-height ratio (WHtR), and waist/height0.5 (WHT.5R) were calculated [10]. Neck circumference (NC) was measured using the following landmarks: the lower edge of the thyroid cartilage on the front surface of the neck and the upper edge of the seventh cervical vertebra on the back [11].
Body composition was measured with dual-energy X-ray absorptiometry (DEXA) on a Lunar model 8743; GE Medical Systems (Madison, WI USA). The measurements included the percentage of total body fat and muscle tissue, the android/gynoid fat ratio (A/G ratio), visceral fat mass (using CoreScan software) [12]. A percentage of total adipose tissue >35% was considered excess adiposity [13]. An A/G ratio >0.85 indicated an android type of adipose tissue distribution [14, 15].
Blood pressure was measured using an automatic tonometer (Omron M2 Basic, Japan) and determined as the mean of two measurements.
Blood samples were collected after an 8–12 hour fasting. To estimate the level of sex hormones, blood was collected on days 2–4 of the menstrual cycle. The concentrations of sex hormones (follicle-stimulating hormone (FSH); estradiol; total testosterone) and serum insulin were determined by the electrochemiluminescence immunoassay. Sex hormone-binding globulin (hGHB) levels were determined by chemiluminescent immunoassay. Free testosterone index was calculated according to the formula: (Testosterone level)/(hGHB level) × 100.
Serum levels of leptin (Mediagnost, Germany), adiponectin (Mediagnost, Germany), and C-reactive protein were determined by enzyme immunoassay (Biomerica, Germany).
Biochemical tests were performed using photometric and turbidimetric methods and included cholesterol, triglycerides, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), apolipoprotein A1, apolipoprotein B, glucose, glycated hemoglobin, uric acid were determined. The atherogenicity coefficient was calculated according to the formula: (total cholesterol – HDL)/HDL. Insulin sensitivity by the HOMA insulin resistance index was determined using the following equations: HOMA-IR = (fasting glucose, mmol/L) × (fasting insulin, mU/L)/22.5.
The criterion for insulin resistance was HOMA-IR≥2.7 [16].
Statistical analysis
Statistical analyses were performed using Statistica 13.5.0 and MedCalc, version 20 software. The normality of the distribution was tested by the Shapiro–Wilk test. Quantitative variables showing normal distribution were expressed as means (M) and standard deviation (SD) and presented as M (SD); otherwise, the median (Me) with interquartile range (Q1; Q3) were reported. Categorical variables were reported as counts and proportions (%). To compare variables of samples with normal distribution, Student's t-test was used. Variables not meeting normality assumptions were compared with a nonparametric Mann–Whitney test. Categorical variables were compared using Fisher's exact test. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), percentage of correctly classified cases by a formula based on the patients enrolled and area under the ROC curve (AUC) were calculated to assess the diagnostic performance of BMI to detect excess body fat, defined at >35% total body fat. Sensitivity was determined as TP/(TP + FN) × 100%, where TP is the number of true positive results and FN is the number of false-negative results. Specificity was determined as TN/(TN+FP) × 100%, where TN is the number of true negative results and FP is the number of false-positive results. PPV was determined as TP/(TP+FP) × 100%, NPV as TN/(FN+TN) × 100%. The percentage of correctly classified cases was calculated as (TP + TN)/(TP + TN + FP + FN) × 100%. A ROC analysis was performed to determine the optimal BMI threshold for detecting excess adiposity, its sensitivity and specificity, PPV, NPV, and to determine the diagnostic value of anthropometric measures for detecting visceral obesity. Due to the lack of a known threshold value for the diagnosis of visceral obesity in women during the menopause transition, quartile values of visceral adipose tissue mass were determined based on DEXA data, the value of the upper quartile was assumed to be the threshold for the detection of visceral obesity. The critical level of significance when testing statistical hypotheses was considered at p<0.05.
Results
The mean age of the participants was 47.0 (2.2) years, BMI 24.5 (3.1) kg/m². Body composition parameters were as follows: total body fat percentage measured with DEXA was 36.2 (5.4) %, visceral adipose tissue was 494 (301; 682) g, and skeletal muscle mass was 61.8 (4.9)%. An excessive amount of adipose tissue according to BMI was found in 50/125 (40%) women, while when measured by DEXA, it was 1.5 times more common [80/125 (64%) women]. In 31/75 (41.3%) of patients with normal BMI and 22/63 (35%) of women with normal BMI and WC<80 cm, hidden obesity was identified based on a fat percentage >35% by DEXA. Body composition and metabolic profile of patients without and with hidden obesity are summarized in Table 1.
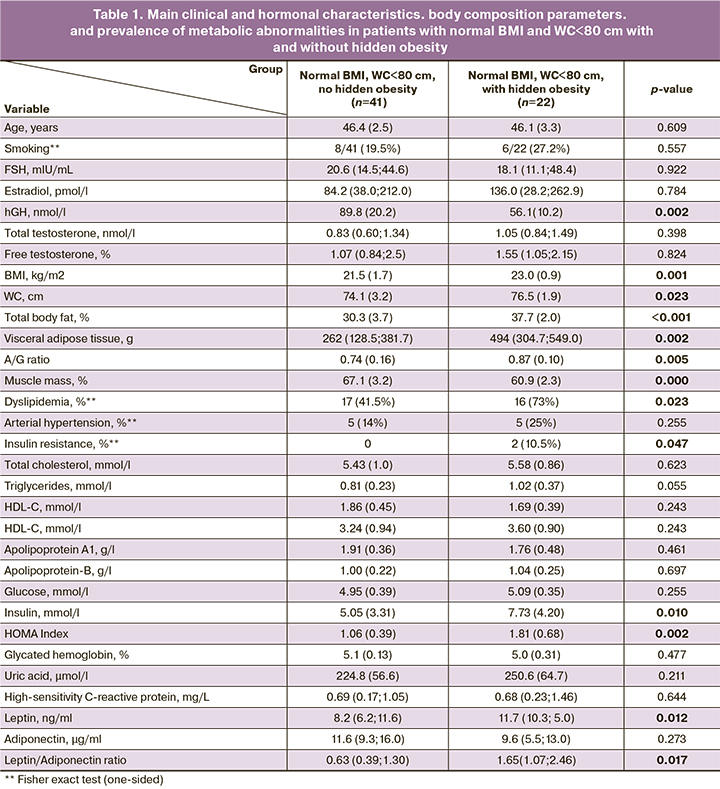
As seen in Table 1, women with and without hidden obesity had a mean BMI of 23.0 (0.9) and 21.5 (1.7) kg/m², with groups differing significantly in total body fat percentage measured by DEXA as 37.7 (2.0) and 30.3 (3.7)%, respectively. Women with hidden obesity had almost twice the visceral adipose tissue mass, an android-like distribution of adipose tissue, and lower muscle mass. Women with hidden obesity were almost twice as likely to have dyslipidemia and insulin resistance. They had 1.5 times higher leptin levels and 2.5 times higher leptin/adiponectin ratio than non-obese women.
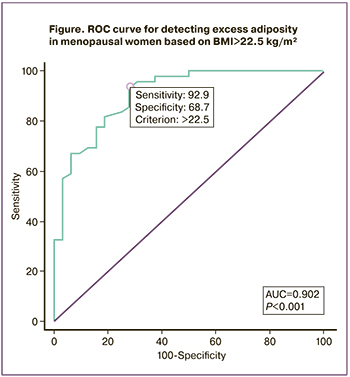
Given that one-third of women with a normal BMI and WC<80 cm had hidden obesity, associated with a higher incidence of metabolic abnormalities, the next step was to assess the diagnostic efficacy of the BMI≥25 kg/m² and determine, by ROC analysis, the optimal BMI threshold for detecting excess adiposity (>35%) in menopausal women (Table 2, Figure).
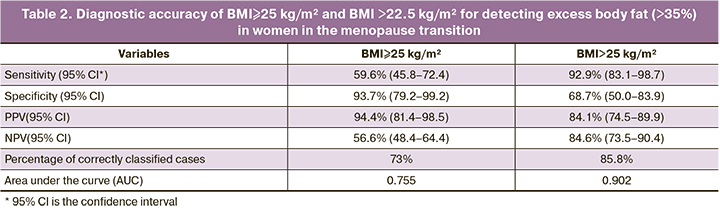
According to Table 2, BMI≥25 kg/m² had a low sensitivity of 59.6% and an NPV of 56.6% for detecting excess adipose tissue (>35%) in women in the menopause transition. The threshold BMI>22.5 kg/m² has a higher sensitivity of 92.9% and NPV of 84.6%, and its use increased the proportion of correctly classified patients to 85.8%, compared with 73% for BMI≥25 kg/m².
The diagnostic value of anthropometric variables (BMI, WC, WC/height, WC/height0.5, WC/HC, and NC) for visceral obesity in women in the menopause transition was assessed by ROC analysis and comparison of the area under the ROC curve (AUC) (Table 3). The criterion for visceral obesity was visceral adipose tissue mass >682 g (the value of the upper quartile of the visceral adipose mass).
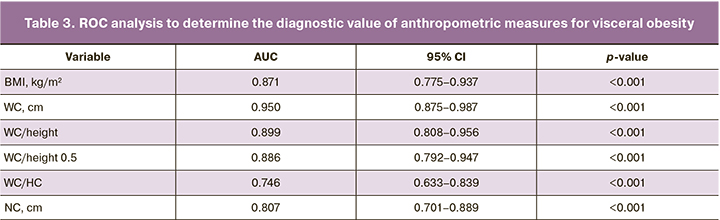
According to Table 3, all anthropometric measures had a diagnostic value for detecting visceral obesity (p<0.001). WC had the highest AUC= 0.950 [95% CI 0.875–0.987], WC/height ratio had AUC=0.899 [95% CI 0.808–0.956] and WC/HC had the lowest AUC=0.746 [95% CI 0.633–0.839].
Discussion
The study findings suggest that one-third (35%) of women with normal BMI and WC<80 cm during the menopause transition have excess adiposity, so-called hidden obesity. In women with hidden obesity, body composition parameters, such as an android type of adipose tissue distribution, increased visceral fat mass and lower muscle mass, indicate metabolically unhealthy normal weight phenotype. Women with hidden obesity had a higher prevalence of dyslipidemia (41.5% versus 73%), insulin resistance (0 versus 10.5%), higher leptin levels, and a leptin/adiponectin ratio. Leptin and adiponectin are adipokines secreted by adipose tissue having opposite effects on insulin secretion anti-inflammatory and pro-inflammatory cytokines. According to research evidence, high leptin and low adiponectin levels are independent predictors of increased risk for metabolic syndrome and type 2 diabetes; the leptin/adiponectin ratio has a higher predictive value for metabolic disorders than each of the adipokines alone [17, 18]. The observed increased incidence of metabolic abnormalities in patients with hidden obesity suggests the need to consider it as a risk factor for cardiometabolic disease and use the most accurate methods for the early detection of obesity in women during the menopause transition.
The study demonstrated a low diagnostic accuracy of BMI≥25 kg/m² for detecting excess adipose tissue in women in the menopause transition due to low sensitivity of 59.6%. This means that among women with excess adiposity, only 59.6% would be correctly classified and the remaining 40.4% would be false-negative, i.e., identified as not having excess adiposity. However, a BMI≥25 kg/m² threshold has a high specificity of 93.7%, meaning that among women without excess adiposity, 93.7% would be classified correctly, and the remaining 6.3% would be false-positive; that is, they would be identified as having excess adipose tissue. BMI≥25 kg/m² has a low NPV of 56.6%, reflecting the probability of not having excess adiposity in women with a normal BMI<25 kg/m². Our results are consistent with previous studies demonstrating the low diagnostic accuracy of BMI in older women [19, 20].
ROC analysis identified an optimal BMI threshold of >22.5 kg/m² with a sensitivity of 92.9%, specificity of 68.7%, PPV of 84.1%, and NPV of 84.6%. Lowering the BMI threshold resulted in increased sensitivity, allowing more women with excess adipose tissue to be identified; however, specificity became lower, leading to more false-positive results. Given the cardiometabolic risks associated with obesity, it makes more sense to effectively identify overweight patients, even though false-positive results are likely to increase.
The criterion used for the diagnosis of overweight in Mongoloid women is BMI≥23 kg/m² [21]. In the Russian Federation, about 9% of the population belong to different types of Mongoloid race and a mixed Mongoloid–Caucasoid race [22]. It can be assumed that this factor may have influenced the results. In studies of postmenopausal women, the optimal BMI for the detection of obesity was between 24.9 and 27.05 kg/m² [19, 20]. Our results support the need for a differential BMI classification for specific female populations.
According to our study, WC was the best diagnostic value for detecting visceral obesity in normal and overweight women (BMI<30 kg/cm²) during the menopause transition. The WC/height ratio was less accurate as an anthropometric index, and the WC/HC index was the least accurate. Published research evidence on the diagnostic ability of anthropometric indices to detect visceral obesity in women varies under the influence of different factors, such as age, BMI, and race [9, 10, 23, 24]. The WC/height ratio has been reported by several studies to have high diagnostic accuracy for detecting visceral obesity, and a meta-analysis involving more than 300 000 people in several ethnic groups has demonstrated the superiority of the WC/height ratio over WC and BMI for detecting risk factors for cardiometabolic diseases [25].
Conclusion
The study findings suggest that menopausal women with normal BMI and WC<80 cm develop hidden obesity due to body composition transformation, which is associated with an increased incidence of metabolic abnormalities. BMI had low diagnostic accuracy for detecting excess adiposity in women during the menopause transition. In this group of patients, it is reasonable to use more accurate methods of assessing obesity, such as the DEXA, to ensure that obesity is not missed and measures to prevent cardio-metabolic disease are taken in a timely manner. As the DEXA is not widely available, the study determined an optimal threshold of BMI>22.5 kg/m² to identify women with excess adiposity during the menopause transition. Among anthropometric indices, WC has the highest diagnostic accuracy for detecting visceral obesity in women during the menopause transition.
References
- Slopien R., Wender-Ozegowska E., Rogowicz-Frontczak A., Meczekalski B., Zozulinska-Ziolkiewicz D., Jaremek J.D. et al. Menopause and diabetes: EMAS clinical guide. Maturitas. 2018; 117: 6-10. https://dx.doi.org/10.1016/j.maturitas.2018.08.009.
- El Khoudary S.R., Aggarwal B., Beckie T.M., Hodis H.N., Johnson A.E., Langer R.D. et al. Menopause Transition and Cardiovascular Disease Risk: Implications for Timing of Early Prevention: A Scientific Statement from the American Heart Association. Circulation. 2020: 506-32. https://dx.doi.org/10.1161/CIR.0000000000000912.
- Kase N.G., Gretz Friedman E., Brodman M., Kang C., Gallagher E.J., LeRoith D. The midlife transition and the risk of cardiovascular disease and cancer Part I: magnitude and mechanisms. American Journal of Obstetrics and Gynecology 2020; 223: 820-33. https://dx.doi.org/10.1016/j.ajog.2020.05.051.
- Greendale G.A., Sternfeld B., Huang M.H., Han W., Karvonen-Gutierrez C., Ruppert K. et al. Changes in body composition and weight during the menopause transition. JCI Insight. 2019; 4. https://dx.doi.org/10.1172/jci.insight.124865.
- Karvonen-Gutierrez C., Kim C. Association of Mid-Life Changes in Body Size, Body Composition and Obesity Status with the Menopausal Transition. Healthcare. 2016; 4: 42. https://dx.doi.org/10.3390/healthcare4030042.
- Юренева С.В., Комедина В.И., Кузнецов С.Ю. Прибавка массы тела у женщин в перименопаузе: методы оценки композиционного состава тела и тактика ведения. Акушерство и гинекология. 2020; 2: 56-61. [Yureneva S.V., Komedina V.I., Kuznetsov S.Yu. Weight gain in perimenopausal women: methods for assessing the body composition and maintenance tactics. Akusherstvo i Ginekologiia/Obstetrics and Gynecology. 2020; 2: 56-61. (in Russian)]. https://dx. doi.org/10.18565/aig.2020.2.56-61.
- Leeners B., Geary N., Tobler P.N., Asarian L. Ovarian hormones and obesity. Human Reproduction Update 2017; 23: 300-21. https://dx.doi.org/10.1093/humupd/dmw045.
- Corrêa M.M., Thumé E., de Oliveira E.R.A., Tomasi E. Performance of the waist-to-height ratio in identifying obesity and predicting non-communicable diseases in the elderly population: A systematic literature review. Archives of Gerontology and Geriatrics. 2016; 65: 174-82. https://dx.doi.org/10.1016/j.archger.2016.03.021.
- Zhao L., Huang G., Xia F., Li Q., Han B., Chen Y. et al. Neck circumference as an independent indicator of visceral obesity in a Chinese population. Lipids in Health and Disease. 2018; 17: 85. https://dx.doi.org/10.1186/s12944-018-0739-z.
- Swainson M.G., Batterham A.M., Tsakirides C., Rutherford Z.H., Hind K. Prediction of whole-body fat percentage and visceral adipose tissue mass from five anthropometric variables. PLoS One. 2017; 12. https://dx.doi.org/10.1371/journal.pone.0177175.
- Zhang Y., Wu H., Xu Y., Qin H., Lan C., Wang W. The correlation between neck circumference and risk factors in patients with hypertension: What matters. Medicine. 2020; 99: e22998. https://dx.doi.org/10.1097/MD.0000000000022998.
- Messina C., Albano D., Gitto S., Tofanelli L., Bazzocchi A., Ulivieri F.M. et al. Body composition with dual energy X-ray absorptiometry: From basics to new tools. Quantitative Imaging in Medicine and Surgery. 2020; 10: 1687-98. https://dx.doi.org/10.21037/QIMS.2020.03.02.
- Dickey R.A., Bartuska D., Bray G.W., Callaway C.W., Davidson E.T., Feld S. AACE/ACE position statement on the prevention, diagnosis, and treatment of obesity (1998 Revision). Endocr Pract. 1998 ;4: 297-350.
- Kelly T.L., Wilson K.E., Heymsfield S.B. Dual energy X-ray absorptiometry body composition reference values from NHANES. PLoS One. 2009; 4. https://dx.doi.org/10.1371/journal.pone.0007038.
- Petak S., Barbu C.G., Yu E.W., Fielding R., Mulligan K., Sabowitz B. et al. The Official Positions of the International Society for Clinical Densitometry: Body Composition Analysis Reporting. Journal of Clinical Densitometry 2013; 16: 508-19. https://dx.doi.org/10.1016/j.jocd.2013.08.018.
- Tang Q., Li X., Song P., Xu L. Optimal cut-off values for the homeostasis model assessment of insulin resistance (HOMA-IR) and pre-diabetes screening: Developments in research and prospects for the future. Drug Discoveries & Therapeutics. 2015; 9: 380-5. https://dx.doi.org/10.5582/ddt.2015.01207.
- Thorand B., Zierer A., Baumert J., Meisinger C., Herder C., Koenig W. Associations between leptin and the leptin/adiponectin ratio and incident Type 2 diabetes in middle-aged men and women: Results from the MONICA/KORA Augsburg Study 1984–2002. Diabetic Medicine. 2010; 27(9): 1004-11. https://dx.doi.org/10.1111/j.1464-5491.2010.03043.x.
- Lee K.W., Shin D. Prospective Associations of Serum Adiponectin, Leptin, and Leptin-Adiponectin Ratio with Incidence of Metabolic Syndrome: The Korean Genome and Epidemiology Study. International Journal of Environmental Research and Public Health. 2020; 17: 3287. https://dx.doi.org/10.3390/ijerph17093287.
- Batsis J.A., Mackenzie T.A., Bartels S.J., Sahakyan K.R., Somers V.K., Lopez-Jimenez F. Diagnostic accuracy of body mass index to identify obesity in older adults: NHANES 1999–2004. International Journal of Obesity. 2016; 40: 761-7. https://dx.doi.org/10.1038/ijo.2015.243.
- Banack H.R., Wactawski-Wende J., Hovey K.M., Stokes A. Is BMI a valid measure of obesity in postmenopausal women? Menopause. 2018; 25: 307-13. https://dx.doi.org/10.1097/GME.0000000000000989.
- Stegenga H., Haines A., Jones K., Wilding J. Identification, assessment, and management of overweight and obesity: summary of updated NICE guidance. BMJ. 2014; 349: g6608–g6608. https://dx.doi.org/10.1136/bmj.g6608.
- Дедов И.И., Шестакова М.В., Мельниченко Г.А., Мазурина Н.В., Андреева Е.Н., Бондаренко И.З., Гусова З.Р. и др. Междисциплинарные клинические рекомендации «Лечение ожирения и коморбидных заболеваний». Ожирение и метаболизм. 2021; 18: 5-99. [Dedov I.I., Shestakova M.V., Melnichenko G.A., Mazurina N.V., Andreeva E.N., Bondarenko I.Z., Gusova Z.R. et al. Interdisciplinary clinical practice guidelines “Management of obesity and its comorbidities.” Obesity and Metabolism. 2021;18: 5-99. (in Russian)]. https://dx.doi.org/10.14341/omet12714.
- Pintér Z., Molnár A., Szász A., Kiss G., Orbán K., Varga C. et al. Reliability of anthropometric parameters in the prediction of the visceral fat area among adult women. Anthropologischer Anzeiger. 2013; 70: 147-64. https://dx.doi.org/10.1127/0003-5548/2012/0238.
- Segura-Fragoso A., Rodríguez-Padial L., Alonso-Moreno F.J., Villarín-Castro A., Rojas-Martelo G.A., Rodríguez-Roca G.C. et al. Anthropometric measurements of general and central obesity and discriminative capacity on cardiovascular risk: RICARTO study. Semergen. 2019; 45(5): 323-32. https://dx.doi.org/10.1016/j.semerg.2019.02.013.
- Ashwell M., Gunn P., Gibson S. Waist-to-height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: systematic review and meta-analysis. Obesity Reviews. 2012; 13: 275-86. https://dx.doi.org/10.1111/j.1467-789X.2011.00952.x.
Received 22.12.2021
Accepted 25.01.2022
About the Authors
Svetlana V. Yureneva, Dr. Med. Sci., Professor at the Department of Obstetrics and Gynecology of the Department of Vocational Education, Leading Researcher at the Department of Gynecologic Endocrinology, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, syureneva@gmail.com, 117997, Russian Federation, Moscow, Oparin str., 4.Veronika I. Komedina, Ph.D. Student at the Department of Gynecologic Endocrinology, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, komedina.veronika@gmail.com, 117997, Russian Federation, Moscow, Oparin str., 4.
Sergey Yu. Kuznetsov, Ph.D., Radiologist, Obstetrician-Gynecologist at the Department of Gynecologic Endocrinology, V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, kuznetsov.s@list.ru, 117997, Russian Federation, Moscow, Oparin str., 4.
Corresponding author: Veronika I. Komedina, komedina.veronika@gmail.com
Authors' contributions: Komedina V.I., Yureneva S.V. – conception and design of the study, drafting of the manuscript; Komedina V.I., Yureneva S.V., Kuznetsov S.Yu. – data collection and analysis; Komedina V.I. – statistical analysis; Yureneva S.V., Kuznetsov S.Yu. – manuscript editing.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Yureneva S.V., Komedina V.I., Kuznetsov S.Yu. Diagnostic value of anthropometric characteristics of obesity in women during the menopause transition.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2022; 2: 72-79 (in Russian)
https://dx.doi.org/10.18565/aig.2022.2.72-79



