Diagnostic significance of proteome analysis of maternal plasma in fetal growth restriction
Volochaeva M.V., Tokareva A.O., Kononikhin A.S., Kukaev E.N., Tyutyunnik V.L., Kan N.E., Starodubtseva N.L.
Objective: The objective of the study was to determine diagnostic criteria for fetal growth restriction based on quantitative proteome analysis of maternal blood plasma.
Materials and methods: Case-control study included 50 pregnant women, who were into 5 groups. Group I consisted of pregnant women with early fetal growth restriction (<32 weeks) (n=10). Group II included pregnant women with late fetal growth restriction (≥32 weeks) (n=10). Group III and IV comprised the patients, who delivered before and after 32 weeks (n=10/n=10), respectively. Group V included pregnant women with small for gestational age fetuses (≥32 weeks) (n=10). Postnatal assessment of growth and weight parameters in newborns (n=50) was conducted according to INTERGROWTH-21st charts to confirm the antenatal diagnosis of fetal growth restriction and small for gestational age newborns, as well as to determine the normal body weight in the group of women with preterm birth (before and after 32 weeks). Quantitative analysis of 125 plasma proteins was performed using BAK 125 Human Plasma Proteomics Kit (MRM Proteomics Inc., Montreal, Canada) by high-performance liquid chromatography with tandem mass spectrometry (HPLC-MS/MS). Diagnostic models for fetal growth restriction and small for gestational age fetuses using logistic regression were developed after preliminary data processing.
Results: Based on the results of quantitative proteome analysis of maternal plasma proteins, three diagnostic models were developed. Model «1» (AUC=0.86), including alpha-2-macroglobulin as a variable, with 90% sensitivity and 90% specificity, enables to make the diagnosis of early fetal growth restriction. Model «2» (AUC=0.88), including the variables of proteins alpha-2-macroglobulin and apolipoprotein A-IV with 90% sensitivity and 80% specificity, enables to make the diagnosis of late fetal growth restriction. Model «3» (AUC=0.80), based on the variables of antithrombin-III and apolipoprotein C-I with 80% sensitivity and 80% specificity, enables to make the differential diagnosis of late fetal growth restriction and small for gestational age fetus.
Conclusion: The results of this study can be used in new approaches to diagnostic methods for different types of fetal growth restriction and small for gestational age fetus, as well as can be a starting point of future researches including potential therapeutic targets.
Authors' contributions: Volochaeva M.V., Tokareva A.O., Kononikhin A.S., Kukaev E.N., Tyutyunnik V.L., Kan N.E., Starodubtseva N.L. – the concept and design of the study, data collection and analysis, statistical data processing, writing the text of the manuscript, article editing.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: The work was carried out as part of experimental research «Improving management strategy and the timing of delivery for pregnant women with fetal growth restriction based on molecular genetic and metabolomic factors with subsequent introduction of modern diagnostic methods for the severity of this gestational complication», 121040600408-4.
Ethical Approval: The study was approved by the local Ethics Committee of the Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia.
Patient Consent for Publication: The patients signed informed consent for publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Volochaeva M.V., Tokareva A.O., Kononikhin A.S., Kukaev E.N., Tyutyunnik V.L., Kan N.E.,
Starodubtseva N.L. Diagnostic significance of proteome analysis of maternal plasma in fetal growth restriction.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2024; (4): 59-68 (in Russian)
https://dx.doi.org/10.18565/aig.2023.299
Keywords
Currently, fetal growth restriction is defined as a condition, when the fetus does not reach its growth potential as a result of placental dysfunction that has polyetiological character [1, 2]. Newborns with growth restriction or low birth weight have high rates of neonatal morbidity and mortality, as well as are at high risk of developing metabolic, cardiovascular, and neuropsychiatric diseases in adulthood [1]. Ultrasound assessment of Doppler parameters is the main imaging method for diagnosing fetal growth restriction at the stage of prenatal development. Accepted standards of clinical practice, that were developed by the international Delphi consensus, which are based on clinical, echographic and pathological Doppler characteristics, enable to diagnose early and late phenotypes of fetal growth restriction [3]. It should be noted that in 75% of cases, the diagnosis of fetal growth restriction or small for gestational age fetus remain unidentified up to the childbirth [4]. The gold standard for the diagnosis of fetal growth restriction is postnatal confirmation of birth weight and birth length [5, 6]. Currently, the differential diagnosis between low gestational weight fetus and fetal growth restriction still remains a challenging issue [7].
Thus, timely diagnosis of different phenotypes, finding significant differences between fetal growth restriction and low gestational weight fetus is one of the major objectives of modern obstetrics. One of possible ways to solve this issue is introduction of new “omics” technologies, including the use of metabolomics, which has scientific potential not only to study the pathogenesis of fetal growth restriction, but also discover new non-invasive biomarkers, which can be used in clinical practice.
Materials and methods
Case-control study included 50 pregnant women, who received prenatal care and gave birth at the National Medical Research Centre of Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov of Ministry of Health of Russia from 2019 to 2021. Five groups of patients were formed. Group I, group II and Group V were the main groups. Group III and group IV were the comparison groups. Group I consisted of pregnant women with early-onset fetal growth restriction (<32 weeks) (n=10). Group II included pregnant women with late-onset fetal growth restriction (≥32 weeks) (n=10). Group III and IV comprised the women, who gave birth before and after 32 weeks (n=10/n=10) to babies with normal normal birth weight and body length. Group V included pregnant women with small for gestational age fetuses (≥32 weeks) (n=10).
To ensure maximum accuracy of proteome identification, an individual selection of paired plasma samples from pregnant women in the main group/comparison group was performed (selection criterion was gestational age at the time of delivery).
Inclusion criteria comprised women aged 18–45 years, singleton pregnancy, prenatally diagnosed “fetal growth restriction”, “small for gestational age fetus” (according to the diagnostic values of ultrasound and Doppler parameters using Delphi method, as well as clinical recommendations of the Ministry of Health of Russia “Fetal growth restriction (fetal growth retardation) that requires to provide medical care to mother” [2], for the main groups, and fetal parameters appropriate to the gestational age, for the comparison groups.
Exclusion criteria were: multiple pregnancy, chromosomal pathology and fetal malformations, severe external genital pathology, hypertensive disorders in pregnancy, preeclampsia.
Postnatal assessment of growth and weight parameters in newborns (n=50) was conducted according to birthweight percentiles of INTERGROWTH-21st growth charts to confirm the antenatal diagnosis of fetal growth restriction and small for gestational age fetus, as well as to determine the normal body weight in the group of women with preterm birth before and after 32 weeks).
The conduction of research was approved by the local Ethics Committee. All patients have signed informed consent to participate in the study.
Venous blood samples were obtained from women in group I, II, V on the day of childbirth, and from women in group III and IV on the day of childbirth at gestational age that was appropriate to term birth in groups I, II, V. Venous blood samples (in EDTA tubes) were centrifuged at 300 g at 4°C for 20 minutes; supernatant was repeatedly centrifuged at 12000 g for 10 minutes. Then the samples were frozen and stored at -80°C. Quantitative analysis of 125 plasma proteins was performed using BAK 125 Human Plasma Proteomics Kit (MRM Proteomics Inc., Montreal, Canada) by high-performance liquid chromatography with tandem mass spectrometry (HPLC-MS/MS) with multiple reaction monitoring (MRM).
Probe preparation and subsequent LC-MS analysis according to the manufacturer’s protocol [8]. LC-MS data analysis was performed using QTRAP SCIEX6500+ System (SCIEX, Canada). Software program Skyline Quantitative Analysis was used for visual exploration of LC-MRM-MS based data. Calibration curves were generated using weighted linear regression 1/x2 and used to calculate the peptide concentrations (fmol/μL) in plasma samples.
Statistical analysis
Statistical analysis was performed using software programs Statistica 12.6, IBM SPSS Statistics 21. The Shapiro–Wilk test was used for assessment of compliance with the normal distribution of quantitative indicators. Normal distribution was described by arithmetic mean (M) and standard deviation (SD). When distribution differed from normal, the variables were presented as median (Me), the lower quartile and the upper quartile [Q1; Q3]. The categorical data were described by absolute values and percentage. Comparison of quantitative data (normally distributed data) between 3 or more groups was performed using one-way analysis of variance (ANOVA); post-hoc comparisons were performed using the Tukey test (when variances were equal), and the Games–Howell test (when variances were not equal), and the Kruskal–Wallis test and the Dunn test (for non-normal distribution). Comparison of quantitative data between 2 groups was performed using Student’s t-test (when distribution was normal), and Mann–Whitney U test (when distribution was non-normal).
Comparison of percentages in multifield contingency tables was performed using the Pearson chi-square test. The differences between the compared values were statistically significant at p<0.05. Diagnostic models based on logistic regression were constructed after preliminary data processing for the classification tasks “early-onset fetal growth restriction/early control”, “late-onset fetal growth restriction/late control”, “late-onset fetal growth restriction/small for gestational age” [9, 10]. To create models based on logistic regression, a data set was generated that included linear values of protein concentrations, the result of pairwise multiplication of protein concentrations, and squared values of protein concentrations as independent variables. The groups of patients were defined as response variables: for the classification task “early-onset fetal growth restriction/early control” – 0 (early control), 1 (early-onset fetal growth restriction); for the task “late-onset fetal growth restriction/late control” – 0 (late control), 1 (late-onset fetal growth restriction); for the task “late-onset fetal growth restriction/small for gestational age” – 0 (small for gestational age fetus), 1 (late-onset fetal growth restriction). Potential variables for the model were preselected using the orthogonal projections to latent structures discriminant analysis (OPLS-DA) and VIP variable importance threshold greater than 1. Logistic regression was performed by stepwise variable selection to minimize Akaike information criterion (AIC) and ensure that marker coefficients had a non-zero probability below 0.05. Then the variables were eliminated step-by-step, starting with the variable with the highest non-zero probability, until none of marker coefficients had non-zero probability greater than 0.05. Model performance was evaluated using leave-one-out cross-validation, and the optimal threshold was determined by maximization of the sum of sensitivity and specificity. To assess the diagnostic value of the signs in predicting a certain outcome, ROC-curve analysis was used, with calculation of the area under the curve (AUC). When the AUC value was 0.9–1.0, informative value was assessed as “excellent quality”; the AUC value 0.8–0.9 denoted “very good quality”; the AUC value 0.7–0.8 denoted “good quality”; the AUC value 0.6–0.7 denoted “average quality”; and the AUC value 0.5–0.6 denoted “unsatisfactory quality”. The prognostic value of the constructed models was characterized by sensitivity (Se) and specifity (Sp).
Results
All patients participating in the study were comparable by the following clinical and anamnestic characteristics: age (р=0.39 according to the Games–Howell test), body mass index (BMI) (p=0.16 according to the Games–Howell test), the type of conception (p=0.43 according to Pearson’s chi-square test). No statistically significant differences were found in the frequency of somatic gynecological diseases. Also, there were no differences in the frequency of low-risk or high-risk thrombophilia-associated mutations (p=0.52 and p=0.54, respectively, according to Pearson’s chi-square test). There were no cases of intrauterine fetal demise in the study groups (Table 1).
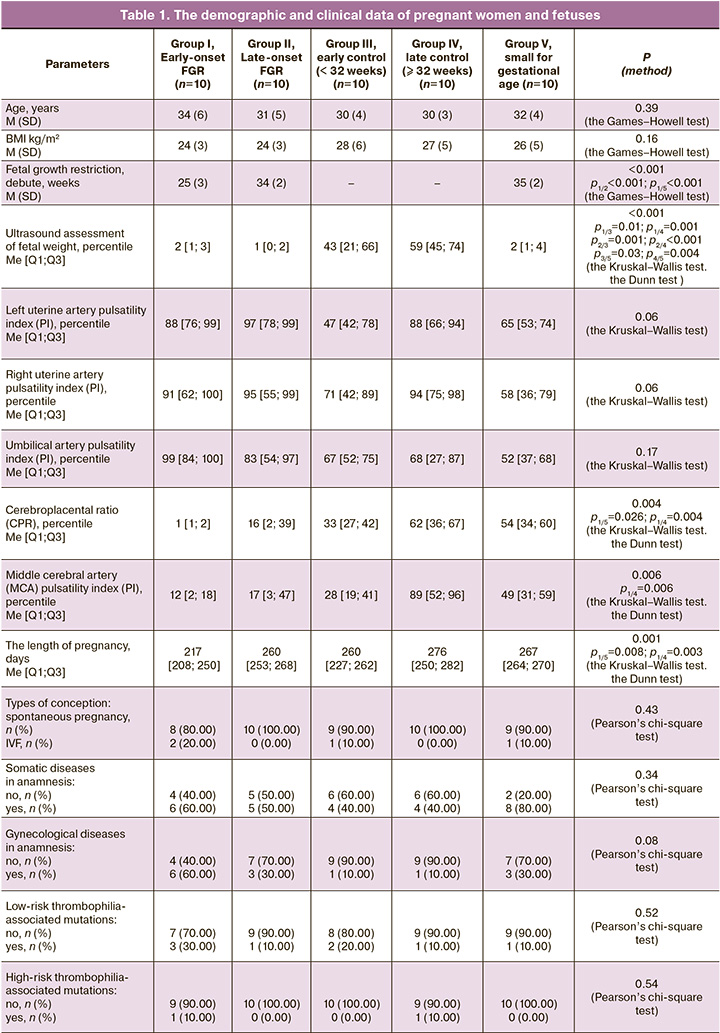
Analyzing the course of pregnancy, investigation of the changes in ultrasound and Doppler parameters was of particular interest. It should be noted, that the decline in estimated fetal weight (in percentiles), impaired blood flow according to Doppler measurements (PI values in uterine arteries, umbilical artery, MCA, cerebroplacental ratio) were most common and had more pronounced changes in the groups with fetal growth restriction (most often early-onset fetal growth restriction). According to a number of authors, despite the fact that ultrasound and Doppler assessment is the main diagnostic tool used in making the diagnosis, in some cases these methods do not help to detect fetal growth restriction antenatally [4–6]. It is noteworthy that even using a small sample in the study, in 10% of cases in the group of early-onset FGR, in 20% of cases of late-onset FGR and in 30% of cases in the group of low for gestational weight, Doppler and ultrasound signs were not detected antenatally. The obtained results confirm the literature data on the lack of sensitivity and specificity of functional methods for diagnosing fetal growth restriction and justify the need to search for new non-invasive criteria [2, 4, 5].
Quantitative analysis of 125 plasma proteins in pregnant women giving birth, was performed using BAK 125 Human Plasma Proteomics Kit (MRM Proteomics Inc., Montreal, Canada) by high-performance liquid chromatography with tandem mass spectrometry (HPLC-MS/MS) with multiple reaction monitoring (MRM) using internal standards. These proteins belong to the major proteins and other group of plasma proteins representing more than 99% of the total protein mass. Concentration difference of the studied plasma proteins covered 6 orders of magnitude. Most of these proteins are the markers of cardiovascular, oncological and neurodegenerative diseases [8, 11, 12].
Statistically significant elevated levels of adiponectin (ADIPOQ), alpha-2-macroglobulin (A2M), gelsolin (GSN), S100-A9 protein (S100A9), serum albumin (ALB) and pregnancy zone protein (PZP), and reduced levels of apolipoprotein B100 (APOB100), cathelicidin antimicrobial peptide (CAMP), complement component C7 (C7), tissue-type plasminogen activator (PLAT) were found in group I (early-onset fetal growth restriction) versus group III (comparison group < 32 weeks). In late-onset fetal growth restriction (group II), there were elevated levels of apolipoprotein A-IV (APOA4), biotinidase (BTD), fibrinogen gamma chain (FGC), gelsolin (GSN), glutathione peroxidase 3 (GPX3), plasminogen (PLG), and retinol-binding protein 4 were observed to be elevated (RBP4), as well as reduced cation-independent mannose-6-phosphate receptor (CI-MPR) in maternal plasma. Comparison between late-onset fetal growth restriction (group II) and “small for gestational age” (group V) showed that significant changes in the plasma proteome were associated with increased levels of gelsolin (GSN), serum paraoxonase/lactonase 3 (PON3), keratin, type I cytoskeletal 10 (KRT10), apolipoprotein C-I (APOC1), hemopexin (HPX) and transthyretin (TTR) (Table 2).
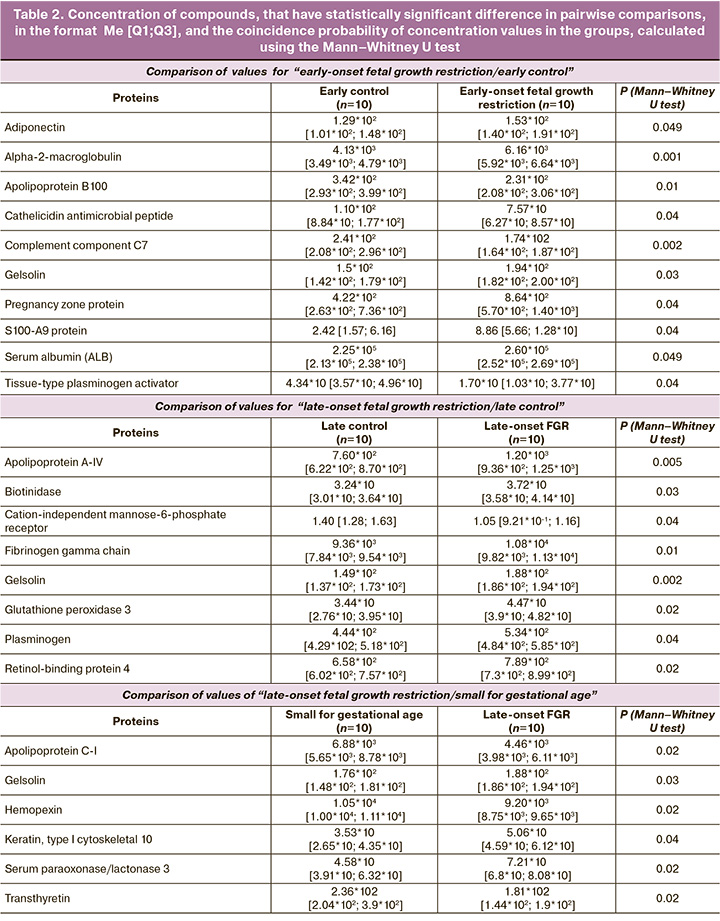
Based on the results of quantitative proteome analysis of 125 maternal plasma proteins three diagnostic models were developed using logistic regression (Figure, Table 3).
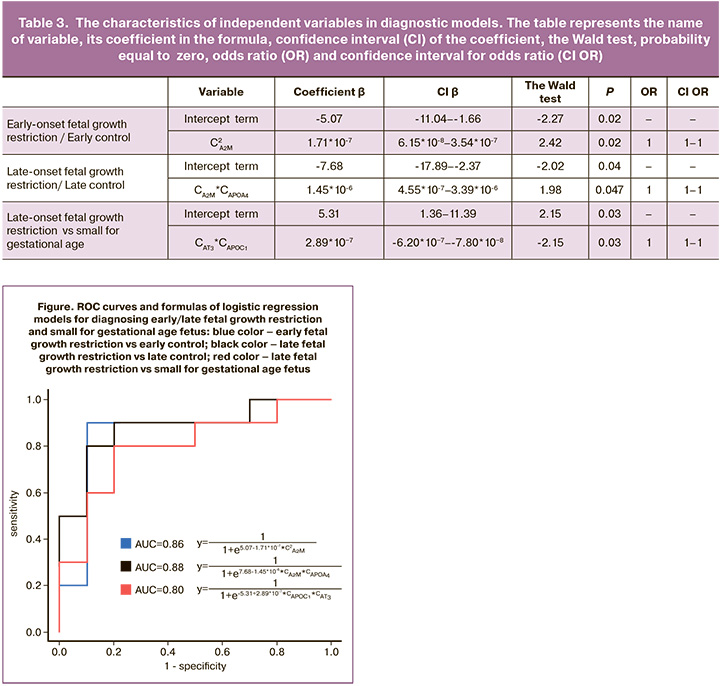
The formula for Model 1 (AUC=0.86) for diagnosing early-onset fetal growth restriction, including alpha-2-macroglobulin as a variable, is the following: . Sensitivity of Model 1 was 90% and specificity was 90%.
The formula for Model 2 (AUC=0.88) for diagnosing late-onset fetal growth restriction, including alpha-2-macroglobulin and apolipoprotein A-IV as variables, is the following: . Sensitivity of Model 2 was 90% and specificity was 80%.
Model 3 (AUC=0.80) was created for the differential diagnosis between late-onset fetal growth restriction and small for gestational age fetus. The model included antithrombin-III and apolipoprotein C-I as variables. The formula for Model 3 is the following: . Sensitivity of Model 3 was 80% and specificity was 80%.
Discussion
Fetal growth restriction is one of the most studied issues in present-day obstetrics. The search for non-invasive or minimally invasive diagnostic methods and possible prediction of this complication is of particular interest. According to Sovio U. et al. [13], uterine fundal height measurement using a tape measure during pregnancy and referral of asthenic women and/or the women having risk factors for fetal growth restriction/low for gestational age fetus, for ultrasound examination, enables to suspect and detect low for gestational age fetus only in 20% of cases, and sensitivity of ultrasound assessment of fetal biometric parameters in the third trimester of pregnancy is 52–57% [14]. According to [15, 16], introduction into practice the evaluation of cerebroplacental ratio could improve the differential diagnosis between fetal growth restriction and low for gestational age fetus. However, multicenter prospective study by Morales-Roselló J. et al. [17] showed that in the group of low risk (normal pregnancy), the effectiveness of evaluation of cerebroplacental ratio for the diagnosis of fetal growth restriction/low for gestational age fetus was moderate. The foregoing led to conduct the studies on finding new clinical and laboratory criteria for fetal growth restriction. Despite a large number of studies in the field of transcriptomics (microRNA), epigenetics, lipidomics and other omics areas of research, the impact of discovered and studied genes and biologically active molecules on the diagnosis of fetal growth restriction has not been sufficiently explored.
Currently, more than 2000 human microRNAs targeting more than 60% of genes encoding human proteins have been identified. [18, 19]. However, microRNAs do not have specificity, since they can regulate the activity of not only several genes, but also messenger RNAs [20]. The use of data obtained from epigenetic studies is quite promising in perinatal medicine as prognostic markers for fetal growth restriction. However, as in the case of microRNAs, it is currently difficult to detect specific molecules among multiple molecules leading to this pathology [20]. Many efforts have been made for early detection or prediction of fetal growth restriction in early pregnancy with determination of β-human chorionic gonadotropin (β-hCG), pregnancy-associated plasma protein A (PAPP-A), placental growth factor (PlGF) and soluble fms-like tyrosine kinase-1 (sFlt-1). Unfortunately, when these biomarkers are used in isolation, their value in predicting fetal growth restriction is quite low. Many researchers reported that a comprehensive determination of maternal serum markers (β-hCG, PAPP-A, PlGF, sFlt-1) in combination with Doppler measurements in high-risk groups (fetal growth restriction and preeclampsia), increases the effectiveness of predicting adverse outcomes [22–24]. In 2012, information about the metabolic processes that are involved in the pathogenesis of fetal growth restriction appeared for the first time [25]. Despite relatively limited data, metabolomic profiling suggests accuracy in distinguishing between fetal growth restriction and small for gestational age fetuses [26, 27]. Previous studies reported clear differences between the groups with and without fetal growth restriction, and there is no data on the differences between late-onset fetal growth restriction and small for gestational age fetuses. Also it is important to note clinical heterogeneity of pregnant women and newborns included in the study, as well as analytical approaches (qualitative proteomics) [24]. There are strengths and weaknesses in our study. The homogeneity of the groups should be noted as a strong aspect of the research. All pregnant women were included in the study exclusively prospectively with strict adherence to inclusion/exclusion criteria. However, it is necessary to take into account that a small sample size was used in the study, and verification of the results obtained by us requires larger cohorts. For the first time, quantitative proteome analysis of maternal plasma proteins was performed in this study for different types of growth restriction and low for gestational age fetuses and compared with comparison groups without fetal growth restriction. The results of the study showed that impairments in the main group (fetal growth restriction) versus the comparison groups, occur in lipid metabolism and immune reactions, that is consistent with earlier studies [28–30]. Analysis of proteomic profile of the blood in pregnant women with fetal growth restriction and low for gestational age fetuses showed that there were not only similar pattern of changes but also significant differences between the groups depending on the phenotype of fetal growth restriction. The obtained results are consistent with the data reported by Priante E. et al. [24] and Miranda J. et al. [28]. They suggested that in low for gestational age fetuses, metabolic disorders with intact respiratory function of the placenta occur most often, leading to insufficient nutrition and long-term disorders in future (including metabolic disorders).
The developed models make it possible to make differential diagnosis with 80% sensitivity and 90% specificity between early-onset and late-onset of fetal growth restriction and low for gestational age fetuses.
Conclusion
The results of this study can be used in new approaches to diagnostic methods for different types of fetal growth restriction and small for gestational age fetus, as well as can be a starting point of future researches including potential therapeutic targets.
References
- McCowan L.M., Figueras F., Anderson N.H. Evidence-based national guidelines for the management of suspected fetal growth restriction: comparison, consensus, and controversy. Am. J. Obstet. Gynecol. 2018; 218(2S): 855-68. https://dx.doi.org/10.1016/j.ajog.2017.12.004.
- Министерство здравоохранения Российской Федерации. Недостаточный рост плода, требующий предоставления медицинской помощи матери (задержка роста плода). Клинические рекомендации (протокол лечения). М.; 2022. 71 с. [Ministry of Health of the Russian Federation. Insufficient growth of the fetus, requiring the provision of medical care to the mother (fetal growth retardation). Clinical Guidelines (treatment protocol). Moscow; 2022. 71 p. (in Russian)].
- Gordijn S.J., Beune I.M., Thilaganathan B., Papageorghiou A., Baschat A.A., Baker P.N. et al. Consensus definition of fetal growth restriction: a Delphi procedure. Ultrasound Obstet. Gynecol. 2016; 48(3): 333-9. https://dx.doi.org/10.1002/uog.15884.
- Haragan A., Himes K. Accuracy of ultrasound estimated fetal weight in small for gestational age and appropriate for gestational age grown periviable neonates. Am. J. Perinatol. 2018; 35(8): 703-6. https://dx.doi.org/10.1055/s-0037-1617433.
- Ганичкина М.Б., Мантрова Д.А., Кан Н.Е., Тютюнник В.Л., Хачатурян А.А., Зиганшина М.М. Ведение беременности при задержке роста плода. Акушерство и гинекология. 2017; 10: 5-11. [Ganichkina M.B., Mantrova D.A., Kan N.E., Tyutyunnik V.L., Khachaturyan A.A., Ziganshina M.M. Pregnancy management complicated by intrauterine growth restriction. Obstetrics and Gynecology. 2017; (10): 5-11. (in Russian)]. https://dx.doi.org/10.18565/aig.2017.10.5-11.
- Unterscheider J., Daly S., Geary M.P., Kennelly M.M., McAuliffe F.M., O'Donoghue K. et al. Optimizing the definition of intrauterine growth restriction: the multicenter prospective PORTO Study. Am. J. Obstet. Gynecol. 2013; 208(4): 290.e1-6. https://dx.doi.org/10.1016/j.ajog.2013.02.007.
- Gordijn S.J., Beune I.M., Ganzevoort W. Building consensus and standards in fetal growth restriction studies. Best Pract. Res. Clin. Obstet. Gynaecol. 2018; 49: 117-26. https://dx.doi.org/10.1016/j.bpobgyn.2018.02.002.
- Kononikhin A.S., Zakharova N.V., Semenov S.D., Bugrova A.E., Brzhozovskiy A.G., Indeykina M.I. et al. Prognosis of Alzheimer's disease using quantitative mass spectrometry of human blood plasma proteins and machine learning. Int. J. Mol. Sci. 2022; 23(14):7907. https://dx.doi.org/10.3390/ijms23147907.
- Токарева А.О., Чаговец В.В., Кононихин А.С., Стародубцева Н.Л., Франкевич В.Е., Николаев Е.Н. Алгоритм обработки масс-спектрометрических данных для получения диагностической панели молекулярных соединений на примере поиска маркеров метастазирования при раке молочной железы. Biomedical Chemistry: Research and Methods. 2021, 4(3): e00156. [Tokareva A.O., Chagovets V.V., Kononikhin A.S., Starodubtseva N.L., Frankevich V.E., Nikolaev E.N. Pipeline of mass-spectrometry data processing for diagnostic molecular marker panel obtaining using the example of search markers of breast cancer metastasis. Biomedical Chemistry: Research and Methods. 2021; 4(3): e00156. (in Russian)]. https://dx.doi.org/10.18097/BMCRM00156.
- Tokareva A.O., Chagovets V.V., Kononikhin A.S., Starodubtseva N.L., Frankevich V.E., Nikolaev E.N. Comparison of the effectiveness of variable selection method for creating a diagnostic panel of biomarkers for mass spectrometric lipidome analysis. JMS. 2021; 56(3): e4702. https://dx.doi.org/10.1002/jms.4702.
- Anwar M.A., Dai D.L., Wilson-McManus J., Smith D., Francis G.A., Borchers C.H. et al. Multiplexed LC-ESI-MRM-MS-based assay for identification of coronary artery disease biomarkers in human plasma. Proteomics Clin. Appl. 2019; 13(4): e1700111. https://dx.doi.org/10.1002/prca.201700111.
- Bhardwaj M., Gies A., Weigl K., Tikk K., Benner A., Schrotz-King P. et al. Evaluation and validation of plasma proteins using two different protein detection methods for early detection of colorectal cancer. Cancers (Basel). 2019; 11(10): 1426. https://dx.doi.org/10.3390/cancers11101426.
- Sovio U., White I.R., Dacey A., Pasupathy D., Smith G.C.S. Screening for fetal growth restriction with universal third trimester ultrasonography in nulliparous women in the Pregnancy Outcome Prediction (POP) study: a prospective cohort study. Lancet. 2015; 386(10008): 2089-97. https://dx.doi.org/10.1016/S0140-6736(15)00131-2.
- Miranda J., Rodriguez-Lopez M., Triunfo S., Sairanen M., Kouru H., Parra-Saavedra M. et al. Prediction of fetal growth restriction using estimated fetal weight vs a combined screening model in the third trimester. Ultrasound Obstet. Gynecol. 2017; 50(5): 603-11. https://dx.doi.org/10.1002/uog.17393.
- MacDonald T.M., Hui L., Robinson A.J., Dane K.M., Middleton A.L., Tong S. et al. Cerebral-placental-uterine ratio as novel predictor of late fetal growth restriction: prospective cohort study. Ultrasound Obstet. Gynecol. 2019; 54(3): 367-75. https://dx.doi.org/10.1002/uog.20150.
- Vollgraff Heidweiller-Schreurs C.A., De Boer M.A., Heymans M.W., Schoonmade L.J., Bossuyt P.M.M., Mol B.W.J. et al. Prognostic accuracy of cerebroplacental ratio and middle cerebral artery Doppler for adverse perinatal outcome: systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2018; 51(3): 313-22. https://dx.doi.org/10.1002/uog.18809.
- Morales-Roselló J., Buongiorno S., Loscalzo G., Abad García C., Cañada Martínez A.J., Perales Marín A. Does uterine Doppler add information to the cerebroplacental ratio for the prediction of adverse perinatal outcome at the end of pregnancy? Fetal. Diagn. Ther. 2020; 47(1): 34-44. https://dx.doi.org/10.1159/000499483.
- Alles J., Fehlmann T., Fischer U., Backes C., Galata V., Minet M. et al. An estimate of the total number of true human miRNAs. Nucleic Acids Res. 2019; 47(7): 3353-64. https://dx.doi.org/10.1093/nar/gkz097.
- Sayed D., Abdellatif M. MicroRNAs in development and disease. Physiol. Rev. 2011; 91(3): 827-87. https://dx.doi.org/10.1152/physrev.00006.2010.
- Hu X.Q., Zhang L. MicroRNAs in uteroplacental vascular dysfunction. Cells. 2019; 8(11):1344. https://dx.doi.org/10.3390/cells8111344.
- Kajdy A., Modzelewski J., Cymbaluk-Płoska A., Kwiatkowska E., Bednarek-Jędrzejek M., Borowski D. et al. Molecular pathways of cellular senescence and placental aging in late fetal growth restriction and stillbirth. Int. J. Mol. Sci. 2021; 22(8): 4186. https://dx.doi.org/10.3390/ijms22084186.
- Blitz M.J., Rochelson B., Vohra N. Maternal serum analytes as predictors of fetal growth restriction with dierent degrees of placental vascular dysfunction. Clin. Lab. Med. 2016; 36(2): 353-67. https://dx.doi.org/10.1016/j.cll.2016.01.006.
- Crovetto F., Triunfo S., Crispi F., Rodriguez-Sureda V., Roma E., Dominguez C. et al. First-trimester screening with specific algorithms for early- and late-onset fetal growth restriction. Ultrasound Obstet. Gynecol. 2016; 48(3): 340-8. https://dx.doi.org/10.1002/uog.15879.
- Priante E., Verlato G., Giordano G., Stocchero M., Visentin S., Mardegan V. et al. Intrauterine growth restriction: new insight from the metabolomic approach. Metabolites. 2019; 9(11): 267. https://dx.doi.org/10.3390/metabo9110267.
- Dessì A., Ottonello G., Fanos V. Physiopathology of intrauterine growth retardation: from classic data to metabolomics. J. Matern. Fetal Neonat. Med. 2012; 25(Suppl 5): 13-8. https://dx.doi.org/10.3109/14767058.2012.714639.
- Favretto D., Cosmi E., Ragazzi E., Visentin S., Tucci M. et al. Cord blood metabolomic profiling in intrauterine growth restriction. Anal. Bioanal. Chem. 2012; 402(3): 1109-21. https://dx.doi.org/10.1007/s00216-011-5540-z.
- Bahado-Singh R.O., Yilmaz A., Bisgin H., Turkoglu O., Kumar P., Sherman E. et al. Artificial intelligence and the analysis of multi-platform metabolomics data for the detection of intrauterine growth restriction. PLoS One. 2019; 14(4): e0214121. https://dx.doi.org/10.1371/journal.pone.0214121.
- Miranda J., Simões R.V., Paules C., Cañueto D., Pardo-Cea M.A., García-Martín M.L. et al. Metabolic profiling and targeted lipidomics reveals a disturbed lipid profile in mothers and fetuses with intrauterine growth restriction. Sci. Rep. 2018; 8(1): 13614. https://dx.doi.org/10.1038/s41598-018-31832-5.
- Paules C., Youssef L., Miranda J., Crovetto F., Estanyol J.M., Fernandez G. et al. Maternal proteomic profiling reveals alterations in lipid metabolism in late-onset fetal growth restriction. Sci. Rep. 2020; 10(1): 21033. https://dx.doi.org/10.1038/s41598-020-78207-3.
- Youssef L., Erlandsson L., Åkerström B., Miranda J., Paules C., Crovetto F. et al. Hemopexin and α1-microglobulin heme scavengers with differential involvement in preeclampsia and fetal growth restriction. PLoS One. 2020; 15(9): e0239030. https://dx.doi.org/10.1371/journal.pone.0239030.
Received 22.12.2023
Accepted 20.03.2024
About the Authors
Maria V. Volochaeva, PhD, Senior Researcher, Department of Regional Cooperation and Integration; Physician, 1 Maternity Department, Academician V.I. KulakovNational Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4, +7(919)968-72-98, volochaeva.m@yandex.ru, https://orcid.org/0000-0001-8953-7952
Alisa O. Tokareva, PhD, specialist, Laboratory of Clinical Proteomics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4, +7(495)531-44-44 (доб. 3113), alisa.tokareva@phystech.edu,
https://orcid.org/0000-0001-5918-9045
Alexey S. Kononikhin, PhD, Senior Researcher, Laboratory of Clinical Proteomics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russia, 117997, Russia, Moscow, Ac. Oparina str., 4; Senior Researcher, Laboratory of Mass Spectrometry, Skolkovo Institute
of Science and Technology, +7(495)531-44-44 (доб. 3113), a_kononihin@oparina4.ru, https://orcid.org/0000-0002-2238-3458
Evgenii N. Kukaev, PhD, Senior Researcher, Laboratory of Clinical Proteomics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4; Researcher, Semenov Research Center of Chemical Physics,
+7(495)531-44-44 (доб. 3113), e_kukaev@oparina4.ru, https://orcid.org/0000-0002-8397-3574
Victor L. Tyutyunnik, Professor, Dr. Med. Sci., Leading Researcher of the Center for Scientific and Clinical Research, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4, +7(903)969-50-41, tioutiounnik@mail.ru,
Researcher ID: B-2364-2015, SPIN-код: 1963-1359, Authors ID: 213217, Scopus Author ID: 56190621500, https://orcid.org/0000-0002-5830-5099
Natalia E. Kan, Professor, Dr. Med. Sci., Deputy Director of Science, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4, +7(926)220-86-55, kan-med@mail.ru, Researcher ID: B-2370-2015, SPIN-код:
5378-8437, Authors ID: 624900, Scopus Author ID: 57008835600, https://orcid.org/0000-0001-5087-5946
Natalia L. Starodubtseva, PhD, Head of the Laboratory of Clinical Proteomics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4, +7(495)531-44-44 (доб. 3113), n_starodubtseva@oparina4.ru,
https://orcid.org/0000-0001-6650-5915
Corresponding author: Maria V. Volochaeva, volochaeva.m@yandex.ru



