Diagnostic value of preactivated neutrophils in preeclampsia
Objective. To investigate the relationship of the levels of preactivated (primed) neutrophils in the peripheral blood of pregnant women with preeclampsia.Kharchenko D.K., Astashkin E.I., Kan N.E., Tyutyunnik N.V., Orekhova N.S., Boris D.A., Tyutyunnik V.L.
Subjects and methods. The investigation enrolled 14 women with preeclampsia (a study group, Group 1), 15 women with physiological pregnancy (a control group, Group 2), and 11 non-pregnant women (to obtain normative values). Formyl peptide, Ficoll-Hypaque (1.077 and 1.119 g/ml) gradients, lucigenin, Roswell Park Memorial Institute (RPMI) 1640 medium, Hanks medium, and fetal calf serum (Sigma-Aldrich) were determined in the peripheral blood. Neutrophils were isolated from the blood samples obtained from the ulnar vein (the anticoagulant heparin 35 IU/ml) using a two-step Ficoll-Hypaque gradient. Red blood cells were destroyed by hypotonic lysis. Neutrophils in the suspensions were at least 96%. The live cells tested with trypan blue were 94%. Suspensions containing 1×106 сells/ml were prepared. Formyl-methionyl-leucyl-phenylalanine (fMLP, 2 µM) was used as a stimulant. The formation of oxygen radicals was recorded in imp/sec, by using the luminophor lucigenin (30 µM) on a Biotox-7 chemiluminometer (Russia). The maximum radical formation amplitude, the time of its achievement, and the light sum were determined for a fixed time period.
Results. The cell suspensions from non-pregnant women showed a monotonic spontaneous increase in oxygen radical formation at a very low rate. The rate increased by 1.3 times in healthy pregnant women (p> 0.05). The rate of spontaneous oxygen radical generation rose sharply in pregnant women with preeclampsia. In this group, the formation of oxygen radicals reached maximum values and plateaued at 24±7 min. The spontaneous radical formation might be due to the stimulation of initially primed neutrophils as a result of their adhesion on the cell walls. To test this assumption, the standard stimulant fMLP that strongly stimulates the neutrophil generation of oxygen radicals were added to the suspensions from the women with preeclampsia. It should be noted that in both non-pregnant women and healthy pregnant women, fMLP significantly increased the level of radicals compared to the responses of neutrophils in women with preeclampsia.
Conclusion. The potentiated response to formyl peptide due to spontaneous neutrophil stimulation in pregnant women with preeclampsia suggests that their peripheral blood contain primed cells. This total response may suggest that the mechanism of stimulation of neutrophils due to their adhesion on the cell wall and to fMLP stimulation is different, additive in nature, and is carried out by different processes
Keywords
Preeclampsia as a complication of pregnancy is accompanied by a number of specific clinical changes including hypertension, proteinuria and multiple organ failure in patient with more severe impairment of the central nervous system [1-4]. All these changes are closely related to the systemic inflammatory response with the participation of blood phagocytes (neutrophils and monocytes) [2], forming and secreting numerous proinflammatory factors (inflammatory cytokines), proteolytic enzymes and oxygen radicals [5, 6]. However, it should be noted that stimulated phagocytes, and neutrophils particularly, are involved not only in the destruction and removal of dead and modified body’s own cells during aseptic inflammation, but also in such pathophysiological processes as blood pressure increase and damage to glomerular cells and kidney tubules [5-7]. One of the most important processes involving blood phagocytes is the “respiratory burst” associated with a rapid increase in oxygen consumption by these cells, which is a source of superoxide anions generated by the enzymatic NADPH oxidase system as a result of single-electron reduction of molecular oxygen [8]. The “respiratory burst” is preceded by a series of processes that transfer cells from a state of rest to a state of preactivation (priming) [9]. This preactivation develops under the influence of low concentrations of priming agents (10-10-10-9 M) creating favorable conditions for the stimulation of a “respiratory burst” as a result of the subsequent exposure of the resolving agent [10]. As a result of priming, a complex of processes develops, ending with the potentiation of the response to a stimulant [9-11]. The difference in current concentrations of priming agents and stimulants can be two to three orders of magnitude [9]. Primed and resting phagocytes closely approximated to each other morphologically. Primed neutrophils do not form significant amounts of oxygen radicals and do not secrete the cytotoxic compounds contained in the granules and can circulate in the bloodstream [11].
Priming mechanisms are complex and not fully understood. In a number of studies, it was suggested that placental impairment, hypoxic-ischemic changes in the placenta, death of cytotrophoblasts, spiral cells of the uterus and other types of cells play a central role in the occurrence and development of preeclampsia. It is accompanied by the secretion of proinflammatory cytokines into the blood, including platelet activating factor (PAF), tumor necrosis factor (TNF-a), formyl peptides formed not only by bacteria, but also by mitochondria (fMLP), endotoxins (LPS) and others, which initially prime neutrophils, and then accumulate and stimulate these cells [12-14]. With blood flow, the preactivated phagocytes spread throughout the body; they destroy the microvascular endothelial cell layer and form faulty cycles. The suppression of neutrophil apoptosis plays an important role in the cycles and, as a result, the survival time of these cells is increased [15]. It should be noted that priming itself seems to change the structure, composition and properties of both proteins (e.g., receptors) and lipids in the neutrophils membranes causing increase in the sensitivity of these cells to extracellular regulatory effects [16]. These changes are not pronounced and develop before a “respiratory burst” due to neutrophil adhesion which occurs not only on the endothelium and other cells types, but also on the components of the connective tissue and barriers of various types [17, 18].
The objective of the study is to determine the relationship of the preactivated (primed) neutrophils in the peripheral blood of pregnant women with preeclampsia.
Materials and Methods
The study included 14 women with preeclampsia (a study group), 15 women with a physiologically normal pregnancy (a control group) and 11 non-pregnant women (for obtaining standard values).
Groups were comparable in baseline clinical characteristics. All patients signed an informed consent to participate in this study, which was approved by the local ethics committee. Inclusion criteria in the groups were singleton pregnancy, delivery between 37 and 40 weeks 6 days gestation. The patients with preeclampsia were eligible for inclusion in the study group. Exclusion criteria were severe extragenital pathology, multiple pregnancy, fetal malformations, genetic and acute infectious diseases of the mother, intrauterine growth restriction.
Isolation of neutrophils. Venous blood was obtained from the ulnar vein of the patients. Heparin (30 IU / ml) was used as an anticoagulant. To remove red blood cells, a 3% dextran solution was added to the blood and kept at 37°C for 30 min. The blood was diluted with Hanks’ culture medium in a 1:1 ratio and layered on a two-step Ficoll-Hypaque gradient (1.077 g/ml and 1.119 g/ml) and centrifuged at 400 g × 30 min. Mononuclear (lymphocytes + monocytes) fraction and neutrophil fraction were collected. Erythrocytes in neutrophil suspension were disposed of using hypotonic lysis. The purity of the neutrophil suspension was not less than 96%, the part of live cells tested with trypan blue was 94-96%. Suspension of isolated neutrophils 2×106 cells/ml in RPMI-1640 medium with 1% FCS was prepared. The observations were carried out on neutrophil suspensions in Hanks’ colorless medium containing 20 mM HEPES buffer, pH 7.4 (1×106 cells/ml).
Lucigenin was used as a fluorochrome (final concentration in samples was 30 μM). The formation of oxygen radicals was recorded on a Biotoks-7 chemiluminometer (Russia) in imp/sec in a continuous mode.
Statistical analysis. Results were expressed as mean values ± standard error of the mean. When comparing, Student’s t-test was used. Results with р-value less than 0.05 were considered as statistically significant.
Results and Discussion
All the patients were comparable in age. Clinical and anamnestic characteristics, pregnancy and delivery features, the condition of newborns are presented in Table 1.
The analysis of the extragenital pathology structure showed that patients with preeclampsia were 1.5 times more likely to have ear, nose, throat (ENT) diseases, accounting for 12.2% and 9.1% (in groups, respectively). In addition, uterine myoma was detected two times more often in the study group, amounting to 12.2% and 5.5% (in groups, respectively), chronic inflammatory diseases of the pelvic organs were 1.5 times more often, accounting for 13.5% and 7.3% (in groups, respectively).
The pregnancy in patients with preeclampsia was 1.5 times more often complicated by the threatened miscarriage in the second trimester, accounting for 22.9% and 16.4% (in groups, respectively) (OR = 1.58; CI = 0.64-3.88).
A significant proportion of patients in the study group had an emergency delivery, in 44.1% of cases; in the control group this index was only 5.0% which is apparently due to the severity of preeclampsia. Emergency cesarean section was performed 3.8 times more often in the study group than in the control group (OR = 5.65; CI = 2.00-15.95).
The study of the postpartum period in the groups revealed no significant differences. The average volume of blood loss in the study group was 625.2 ± 166.6 ml, in the control group it was 489.1 ± 230.9 ml.
Perinatal outcomes were analyzed in all patients. All children were born alive. The mean Apgar score at the 1st minute in the study group was 7.2 ± 1.0, in the control group it was 7.9 ± 0.5, the mean score at the 5th minute was 8.4 ± 0.7 и 8.9±0.4, respectively.
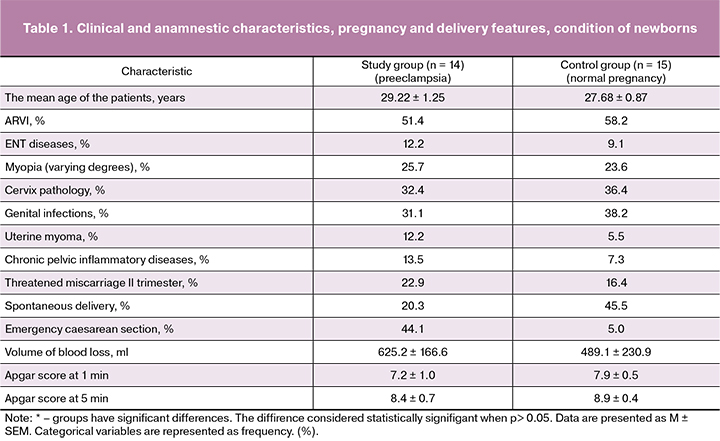
Determining the level of superoxide anions in suspensions of neutrophils obtained from the blood of non-pregnant women revealed its increase in the range of 1000-1500 imp/sec (Fig. 1-1). In preeclampsia, neutrophils generated oxygen radicals “spontaneously” and their level reached its maximum in 20-25 minutes (Fig. 1-2). The relation between the radicals “spontaneously” formed in preeclampsia patients and the radicals in non-pregnant women was 4 (6000 imp/sec : 1500 imp/sec = 4). Apparently, this “spontaneous” formation of oxygen radicals in preeclampsia patients is a result of stimulation of initially primed neutrophils due to interaction of cells with the cuvet and their adhesion.
In healthy pregnant women, neutrophils «spontaneously» generated superoxide anions, their concentration levels monotonously increased and on the plateau were on average (х ± s = 2090 ± 390; n = 15). This parameter was higher compared to one in non-pregnant women (х ± s = 1170 ± 442; n = 11), but significantly lower than the values observed in suspensions of neutrophils in preeclampsia patients (х ± s = 4540 ± 913; n = 14) (р < 0.05).
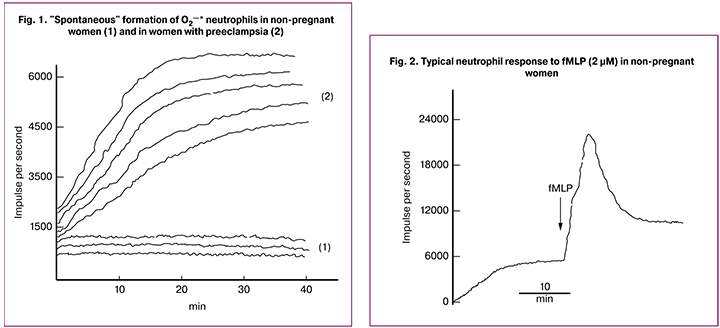
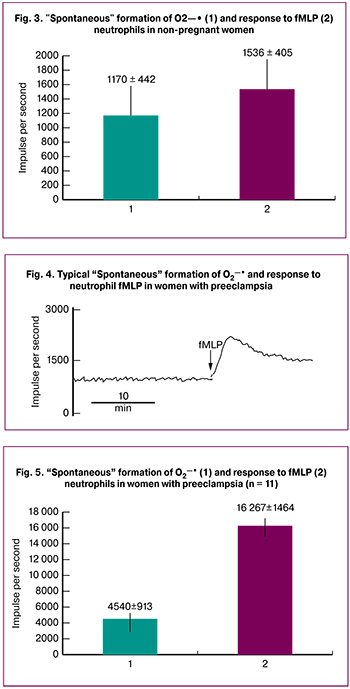 At the next stage of the study, formyl peptide (fMLP, 2 μM) produced by bacterial cells and released during mitochondrial death was added to the suspensions of cells from all three groups [19]. In addition, on the surface of neutrophils it interacts with two types of receptors coupled to G-proteins. With the help of protein kinases they transmit an activation signal to the components of the enzymatic NADPH-oxidase complex that generates superoxide anions [7, 14]. Our data suggest that formyl peptide increases the level of superoxides in the suspension of neutrophils in non-pregnant women to a small extent, namely by 1.3 times (Fig. 2, Fig. 3). The maximum response to formyl peptide during pregnancy was higher compared to one in non-pregnant women, but it never reached the values observed in preeclampsia patients.
At the next stage of the study, formyl peptide (fMLP, 2 μM) produced by bacterial cells and released during mitochondrial death was added to the suspensions of cells from all three groups [19]. In addition, on the surface of neutrophils it interacts with two types of receptors coupled to G-proteins. With the help of protein kinases they transmit an activation signal to the components of the enzymatic NADPH-oxidase complex that generates superoxide anions [7, 14]. Our data suggest that formyl peptide increases the level of superoxides in the suspension of neutrophils in non-pregnant women to a small extent, namely by 1.3 times (Fig. 2, Fig. 3). The maximum response to formyl peptide during pregnancy was higher compared to one in non-pregnant women, but it never reached the values observed in preeclampsia patients.
In addition, during preeclampsia after the spontaneous formation of superoxides (Fig. 1, Fig. 2), formyl peptide (fMLP, 2 μM) induced a pronounced “respiratory burst” in neutrophil suspensions. Thus, after reaching the maximum values, the О2¾· level decreased and reached a constant level (Fig. 4).
The spontaneous formation of oxygen radicals in preeclampsia patients (Fig. 5) reached a plateau of 4540 ± 1464 imp/sec (n = 14), and the formyl peptide induced the formation of oxygen radicals to the maximum level of 16267 ± 1464 imp/sec (n = 14).
All these findings suggest that there are preactivated neutrophils in the blood of women with preeclampsia and they circulate in the blood stream through the body and are ready to be stimulated by various agents at any moment.
The synergistic enhancement of the oxygen radicals generation by neutrophils preexposed to low doses of various priming agents and the subsequent effect of the stimulator (priming effect) is important for functional responses of neutrophils under the conditions of systemic inflammation. Some studies have suggested that the phenomenon of neutrophil priming develops long before the onset of the clinical manifestation of preeclampsia and can be identified in patients’ blood samples with instrumental methods [9, 12]. Such priming occurs under the influence of different agents that are generated in case of the impaired placentation, including TNF-a, LPS, IL-8, leptin, particles of dead syncytiotrophoblasts, etc. [9-13].
The results obtained in our work show that some of the neutrophils isolated from the blood of women with preeclampsia are in a preactivated (primed) state. It should be noted that such cells not only synergistically responded to the standard stimulator fMLP, but also «spontaneously» generated oxygen radicals at a faster rate and a higher level compared with neutrophils obtained from the blood of non-pregnant and healthy pregnant women. As a working hypothesis, it was suggested that the “spontaneous” formation of oxygen radicals is a consequence of the stimulation of initially primed neutrophils resulting from their adhesion on the cuvet wall. This assumption is supported by previously obtained results which showed that the adhesion of neutrophils on the endothelial cells of microcirculatory vessels was accompanied by their mutual activation, expression of adhesion receptors and their ligands, as well as subsequent stimulation [18]. This process was suppressed with antibodies [17] and various chemical agents [18]. Moreover, it has been shown that adhesion and activation of neutrophils on plastics is not associated with adhesive cell receptors but occurs through other mechanisms and is approximately twice as high as compared to adhesion on the endothelium [17].
Several approaches are used to prevent the adverse effects of inadequately stimulated neutrophils on normal cells in surrounding tissues:
- Removal of radicals with direct antioxidants;
- Prevention of the generation of oxygen radicals formed by the enzymatic NADPH-oxidase complex, with the agents that affect its formation (mediated or indirect antioxidants);
- Decreased neutrophil intake in the affected area by suppressing their chemotaxis or interaction with the endothelial cells of microcirculatory vessels.
All these approaches are inevitably accompanied by the suppression of the protective effects of neutrophils; it plays an unfavorable role, especially with their prolonged implication. One of the new approaches is associated with the transfer of neutrophils from the state of preactivation (priming) to the state of rest. It is important to note that such a transfer does not suppress subsequent re-priming and fully retains the entire protective potential of neutrophils.
The process of priming is formed before the onset of clinical signs of systemic inflammation, which makes it possible to identify it with instrumental methods, such as recording the formation of oxygen radicals in combination with other methods for determining the level and activity of priming agents, including various cytokines.
Conclusion
Thus, it is possible to determine the risk of preeclampsia in pregnant women due to the identification of systemic inflammation markers and the formation of significant amounts of oxygen radicals during “respiratory burst” which result from the generation and stimulation of primed cells in the blood of pregnant women in vitro, while excluding other potentially possible causes of neutrophil activation. In addition, the identified high level of free radical oxidation and the presence of neutrophils preactivated to the respiratory burst, justifies the feasibility of developing preventive approaches aimed at reducing oxidative stress and preventing its adverse effects.
References
1. Савельева Г.М., Сухих Г.Т., Серов В.Н., Радзинский В.Е., ред. Акушерство. Национальное руководство. М.: ГЭОТАР-Медиа; 2015. 1080с. [Savelyeva G.M., Sukhikh G.T., Serov V.N., Radzinsky V.E. Obstetrics. National leadership. GEOTAR-Media, 2015; 1080 s. (in Russian)].
2. Ходжаева З.С., Холин А.М., Вихляева Е.М. Ранняя и поздняя преэклампсия: парадигмы патобиологии и клиническая практика. Акушерство и гинекология. 2013; 10: 4-11. [Khodzhaeva Z.S., Kholin A.M, Vikhlyaeva E.M. Early and late preeclampsia: paradigms of pathobiology and clinical practice. Obstetrics and gynecology, 2013; 10: 4-11. (in Russian)].
3. Tsukimori K., Fukushima K., Tsushima A., Nakano H. Geteration of reactive oxygen species by neutrophils and endothelial cell injury in normal and preeclamptic pregnancies. Hypertension. 2005; 46(4): 696-700. doi: 10.1161/01.HYP.0000184197.11226.71.
4. Tsukimori K., Nakano H., Wake N. Difference in neutrophil superoxide generation during pregnancy between preeclampsia and essential hypertension. Hypertension. 2007; 49(6): 1436-41. doi: 10.1161/HYPERTENSIONAHA.106.086751.
5. Mihu D., Razvan C., Malutan A., Mihaela C. Evaluation of maternal systemic inflammatory response in preeclampsia. Taiwan. J. Obstet. Gynecol. 2015; 54(2): 160-6. doi: 10.1016/j.tjog.2014.03.006.
6. Müller-Deile J., Schiffer M. Preeclampsia from a renal point of view: Insides into disease models, biomarkers and therapy. World J. Nephrol. 2014; 3(4): 169-81. doi: 10.5527/wjn.v3.i4.169
7. Palei A.C., Spradley F.T., Warrington J.P., George E.M., Granger J.P. Pathophysiology of hypertension in preeclampsia: a lesson in integrative physiology. Acta Physiol. (Oxford). 2013; 208(3): 224-33. doi: 10.1111/apha.12106
8. Ciz M., Denev P., Kratchanova M., Vasicek O., Ambrozova G., Lojek A. Flavonoids inhibit the respiratory burst of neutrophils in mammals. Oxid. Med. Cell. Longev. 2012; 2012: ID181295. doi:10.1155/2012/181295
9. Volk A.P.D., Barber B.M., Goss K.L., Ruff J.G., Heise Ch.K., Hook J.S., Moreland J.G. Priming of neutrophils and differentiated PLB-985 cells by pathophysiological concentration of TNF-a is partially oxygen dependent. J. Innate Immun. 2011; 3(3): 298-314. doi: 10.1159/000321439.
10. Zarbock A., Ley K. Neutrophil adhesion and activation under flow. Microcirculation. 2009; 16(1): 31-42. doi: 10.1080/10739680802350104.
11. Miralda I., Uriarte S.M., McLeish K.R. Multiple phenotypic changes define neutrophil priming. Front. Cell. Infect. Microbiol. 2017; 7: 217. doi: 10.3389/fcimb.2017.00217
12. Germain A.J., Sacks G.P., Soorana S.R., Sargent I.L., Redman C.W. Systemic inflammatory priming in normal pregnancy and preeclampsia: the role of circulating syncytiotrophoblast microparticles. J. Immunol. 2007; 178(9): 5949-56. doi: 10.4049/jimmunol.178.9.5949
13. Moreland J.G., Davis A.P., Matsuda J.J., Hook J.S., Bailey G., Nauseef W.M., Lamb F.S. Endotoxin priming of neutrophils requires NADPH oxidase-generated oxidants and is regulated by the anion transporter ClC-3. J. Biol. Chem. 2007; 282(47): 33958-67. doi: 10.1074/jbc.M705289200
14. Mittal M., Siddiqui M.R., Tran K., Reddy S.P., Malik A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox. Signal. 2014; 20(7): 1127-67. doi: 10.1089/ars.2012.5149
15. Rolfo A., Giuffrida D., Nuzzo A.M., Pierobon D., Cardaropoli S., PiccoliE. et al. Pro-inflammatory profile of preeclamptic placental mesenchymal stromal cells: New insights into the etiopathogenesis of preeclampsia. PLoS One. 2013; 8(3): e59403. doi: 10.1371/journal.pone.0059403
16. Husemann J., Obstfeld A., Febbraio M., Kodama T., Silverstein S.C. CD11b/CD18 mediates production of reactive oxygen species by mouse and human macrophages adherent to matrixes containing oxidized LDL. Arterioscler. Thromb. Vasc. Biol. 2001; 21(8): 1301-5.
17. Forsyth K.D., Levinsky R.J. Role of the LFA-1 adhesion glycoprotein in neutrophil adhesion to endothelium and plastic surfaces. Clin. Exp. Immunol. 1989; 75(2): 265-8.
18. Maruyama N., Tansho-Nagakawa S., Miyazaki C., Shimomura K., Ono Y., Abe S. Inhibition of neutrophil adhesion and antimicrobial activity by diluted hydrosol prepared from Rosa damascene Biol. Pharm. Bull. 2017; 40(2): 161-8. doi:10.1248/bpb.b16-00644
19. Wenceslau C.F., McCarthy C.G., Goulopoulou S., Szasz T., NeSmith E.G., Webb R.C. Mitochondrial-derived N-formyl peptides: novel links between trauma, vascular collapse and sepsis. Med Hypotheses. 2013; 81(4): 532-5. doi: 10.1016/j.mehy.2013.06.026.
Received 17.02.2018
Accepted 02.03.2018
About the Authors
Kharchenko, Daria K., postgraduate student of the National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Health of Russia (117997, Moscow, Ac. Oparina, 4 str.). Tel.: +7-915-165-87-00. E-mail: drkharchenko@mail.ruAstashkin, Evgeny I., PhD, MD, professor of the department of pathology First Moscow State Medical University named after I.M. Sechenov of the Ministry of Health of Russia, (Sechenov University) (119991, Moscow, Trubetskaya str. 8/2.). Tel. +7-916-062-05-30. E-mail: ast-med@mail.ru
Kan, Natalia E., PhD, MD, the head of the obstetric department of the National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Health of Russia (117997, Moscow, Ac. Oparina, 4 str.). Tel.: +7-926-220-86-55. E-mail: kan-med@mail.ru.
Number Researcher ID B-2370-2015. ORCID ID 0000-0001-5087-5946.
Tyutyunnik, Nataliya V., postgraduate student of the National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Health of Russia (117997, Moscow, Ac. Oparina, 4 str.). Tel.: +7-903-144-50-05. E-mail: tysia07@bk.ru
Orekhova, Natalia S., PhD, senior researcher of the laboratory of extreme SIC First Moscow State Medical University named after I.M. Sechenov of the Ministry of Health
of Russia, (Sechenov University) (119991, Moscow, Trubetskaya str. 8/2.). Tel. +7-915-361-19-58. E-mail: ast-med@mail.ru
Boris, Dayana A., postgraduate student of the National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov
Ministry of Health of Russia (117997, Moscow, Ac. Oparina, 4 str.). Tel.: +7-915-081-89-97. E-mail: dayana_boris@mail.ru
Tyutyunnik, Victor L., PhD, MD, the head of the obstetric physiological department of the National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Health of Russia (117997, Moscow, Ac. Oparina, 4 str.). Tel.: +7-903-969-50-41. E-mail: tioutiounnik@mail.ru.
Number Researcher ID B-2364-2015. ORCID ID 0000-0002-5830-5099
For reference: Kharchenko D.K., Astashkin E.I., Kan N.E., Tyutyunnik N.V.,Orekhova N.S., Boris D.A., Tyutyunnik V.L.: Diagnostic value of preactivated neutrophils in preeclampsia. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2018; (11): 24-30. (in Russian)
https://dx.doi.org/10.18565/aig.2018.11.24-30



