Под бессимптомной бактериурией (ББ) подразумевают заболевание, характеризующееся отсутствием специфических симптомов острой инфекции мочевыводящих путей (ИМП) при наличии бактерий в моче [1, 2]. ББ является достаточно распространенной, и в развитых странах встречается в 2–15% всех беременностей, в некоторых развивающихся странах сообщается о показателях, превышающих 20% [3].
ББ чаще диагностируется в I триместре (до 75% случаев) и реже – во II и III триместрах беременности (в 25%) [3, 4]. Факторами риска бактериурии являются: мочевая инфекция в анамнезе, эндогенные очаги хронической инфекции, врожденные пороки развития мочевыводящих путей, воспалительные заболевания органов малого таза, сахарный диабет, низкий социально-экономический статус [1–3].
В то время как ББ у небеременных женщин, как правило, имеет доброкачественный характер, у беременных может вызывать значительные осложнения со стороны матери, плода и новорожденного, высокую вероятность манифестации в симптоматическую форму инфекции мочевого тракта. Препятствие оттоку мочи при беременности может привести к застою и увеличивает вероятность осложнения ББ циститом и пиелонефритом [2, 5, 6]. Механическая компрессия со стороны увеличивающейся матки, а также высокая секреция прогестерона, снижающая тонус мускулатуры мочевыводящих органов, являются основными причинами задержки мочи с развитием пузырно-мочеточникового рефлюкса, гидроуретера и гидронефроза. Различия в рН и осмоляльности мочи, а также вызванные беременностью глюкозурия и повышение кислотности могут способствовать росту бактерий и снижению устойчивости уроэпителия к инфекционной инвазии [7, 8]. Патогены мочевыводящих путей, вызывающие ББ, аналогичны тем, которые вызывают цистит и пиелонефрит. Считается, что лечение антибиотиками и элиминация бактерий предотвратят восходящую ИМП и развитие осложнений. Если ББ во время беременности не лечить, общепризнанно, что у 20–30% женщин развивается острый пиелонефрит, частота рецидива в течение года достигает 10% [3, 4, 7].
Большинство проведенных рандомизированных исследований и метаанализов доказывает ассоциацию ИМП у беременных с риском преждевременных родов, рождения детей с низкой массой тела, гипертензивных расстройств (в т. ч. преэклампсии), анемии, послеродового эндометрита [8, 9]. Вызванное патогенными микроорганизмами начало родовой деятельности до 37-й недели беременности опосредовано воспалительным процессом. Бактерии и их продукты распознаются рецепторами, такими как toll-подобные (TLR), индуцирующими выработку хемокинов, простагландинов и протеаз, что может привести к инициации родовой деятельности [10]. Связь между ББ и неблагоприятными исходами беременности была впервые предложена E.H. Kass в 1959 г., после публикации его оригинального рандомизированного плацебо-контролируемого исследования, показавшего, что лечение беременных с бактериурией предотвратило развитие пиелонефрита и до 20% преждевременных родов [11]. Быстро последовали другие исследования, и стало общепринятым, что выявление ББ во время беременности имеет важное значение, а ее лечение предупреждает развитие симптоматических ИМП. Данные факты отражены и в настоящих исследованиях, так, ретроспективный анализ, проведенный в Израиле [11, 12], показал связь между ББ и преждевременными родами (ОШ 1,9; 95% ДИ 1,7–2,0). В другом исследовании [12] также была выявлена значительная связь между бактериурией и преждевременными родами (ОШ 2,03; 95% ДИ 1,5–2,8).
Наиболее распространенным патогеном, ассоциированным с ББ, является Escherichia coli – встречается в 80% случаев. К другим возбудителям относятся: Klebsiella spp., Proteus spp., Enterobacter spp. и грамположительные микроорганизмы, включая Staphylococcus saprophyticus и Streptococcus agalactiae [1, 2, 5]. Бактерии колонизируют мочевыводящие пути, периуретральную область и влагалище. Уропатогенные грамотрицательные бактерии обладают специфическими факторами вирулентности, которые усиливают как колонизацию, так и инвазию мочевыводящих путей, например, P-фимбрии некоторых штаммов E. coli обеспечивают адгезию к уроэпителиальным клеткам. Штаммы E. coli, выделенные от беременных женщин с ББ, имеют сходный характер вирулентности со штаммами, выделенными от женщин с симптоматическими ИМП [5, 6]. Обнаружение Streptococcus agalactiae в моче в титре ≥104 КОЕ/мл у беременной связано с колонизацией влагалища, и лечение антибиотиками во время родов рекомендуется для предотвращения раннего начала инфекции новорожденных, вызванной данным патогенным микроорганизмом [6, 7].
Критерием диагностики ББ является количество бактерий >105 КОЕ/мл при микробиологическом исследовании мочи в двух последовательных образцах, собранной с максимальным соблюдением стерильности и доставленной в лабораторию в короткие сроки, что позволяет в наибольшей степени ограничить рост бактерий [2, 12, 14]. Обнаружение бактерий >105 КОЕ/мл в одном образце средней порции мочи является альтернативным вариантом диагностики, хотя вероятность того, что у женщины истинная бактериурия, составляет всего 80%; процент увеличивается до 95%, если две или более культуры при последовательном микробиологическом исследовании мочи являются положительными [13]. Поскольку эффективность экспресс-скрининговых тестов во время беременности недостаточно высокая, микробиологическое исследование мочи остается золотым стандартом диагностики ББ [13, 14].
Целью лечения ББ является элиминация возбудителя. Высеянный микроорганизм должен иметь чувствительность к выбранному антибиотику, продолжительность лечения должна быть наиболее короткой, кроме того, необходима приверженность к терапии препаратами, имеющими благоприятные фармакокинетические параметры [15, 16]. Следует отметить, что лечение должно быть безопасным во время беременности как для женщины, так и для развивающегося плода [1, 2, 5, 6]. С целью терапии ББ используются антибиотики разных групп. Фосфомицин и нитрофурантоин проявляют максимальную активность среди пероральных препаратов в отношении ББ. Также к препаратам выбора в эмпирической терапии ББ относятся цефалоспорины третьего поколения, чувствительность кишечной палочки к пероральным формам которых составляет >90%. Амоксициллин+клавулановая кислота и ампициллин могут применяться при установленной чувствительности, учитывая высокую резистентность кишечной палочки к данной группе антибактериальных препаратов [2, 4, 15, 16].
Повышение бактериальной резистентности патогенов мочевыводящих путей, частое рецидивирующее течение ББ могут затруднить выбор подходящего режима терапии, особенно в условиях нехватки ресурсов, где возможности для посева мочи и тестирования чувствительности к противомикробным препаратам ограничены [17, 18].
Одним из новых аспектов в лечении ИМП является использование иммуномодуляторов. Хорошо зарекомендовавшим себя на практике и в неоднократных клинических исследованиях является средство «Суперлимф», относящееся к группе стимуляторов репарации тканей, его использование в комплексной терапии хронического рецидивирующего цистита во время беременности показало высокие результаты по сравнению с монотерапией [19]. Преимуществом данного препарата является его противомикробная, противогрибковая и противовирусная активность. Суперлимф стимулирует функциональную активность клеток фагоцитарного ряда (моноцитов и нейтрофилов): активирует фагоцитоз, выработку цитокинов (ИЛ-1, ФНО), индуцирует противоопухолевую цитотоксичность макрофагов, регулирует миграцию клеток в очаг воспаления, увеличивает активность естественных киллеров. Препарат обладает антиоксидантной активностью, снижает развитие воспалительных реакций, стимулирует регенерацию и эпителизацию раневых дефектов [19–21].
Частые рецидивы ББ во время беременности, высокая резистентность патогенных микроорганизмов к антибактериальным препаратам обуславливают поиск новых схем лечения для достижения устойчивых клинических результатов. Комплексная терапия антибактериальными средствами с иммуномодулирующим препаратом «Суперлимф» может явиться эффективной комбинацией в получении высокого терапевтического эффекта.
Цель исследования: оценить эффективность терапии ББ у беременных, ее влияние на исходы беременности и течение перинатального периода.
Материалы и методы
Клиническое исследование включало 44 пациентки, перенесшие эпизод ББ в I триместре беременности. Пациентки отбирались в соответствии со следующими критериями включения:
- возраст от 18 до 40 лет;
- срок беременности от 6 до 12 недель;
- отсутствие пороков развития мочевыводящей системы, аутоиммунных заболеваний;
- отсутствие повышенной чувствительности к компонентам лекарственных препаратов;
- согласие пациентки на участие в клиническом исследовании;
- при бактериологическом исследовании мочи – выявление роста возбудителя >105 КОЕ/мл (исследование назначалось при первом посещении акушера-гинеколога).
Критерием исключения являлась клиническая симптоматика ИМП.
Методом произвольной выборки были сформированы 2 исследовательские группы:
- основная – группа 1, представлена 22 пациентками, получавшими лечение антибактериальным препаратом в сочетании с иммуномодулирующим средством «Суперлимф»;
- сравнения – группа 2, включающая 22 пациентки, получавшие терапию только антибактериальным препаратом.
В качестве антибактериального препарата всем беременным назначался фосфомицин – антибиотик широкого спектра действия, производное фосфоновой кислоты, разрешенный к применению в I триместре беременности. Схема приема: двукратно, по 3 г с интервалом 24 ч на ночь после мочеиспускания. Иммуномодулирующая терапия проводилась препаратом «Суперлимф» 10 ЕД ректально по 1 суппозиторию 2 раза в сутки в течение 10 дней. Курс лечения начинался одновременно с антибактериальной терапией.
Всем пациенткам было проведено стандартное исследование, согласно клиническим рекомендациям, проведен анализ клинико-анамнестических показателей. Эффективность лечения оценивалась на 11-й день от начала проведения терапии, анализировалось микробиологическое исследование мочи, межрецидивный период. Все пациентки динамически наблюдались в течение всей беременности после проведенного лечения с целью оценки частоты осложнений, а также исходов родов и особенностей перинатального периода в обеих группах в зависимости от примененной схемы лечения.
Статистический анализ
Полученные результаты проходили статистическую обработку на персональном компьютере при помощи программы Microsoft Office 2021, с использованием стандартного пакета Excel. Для статистического анализа применяли программу StatSoft (STATISTICA), в расчете средних величин использовались непараметрические методы.
Результаты и обсуждение
Во всех исследуемых группах отмечалась высокая приверженность пациенток к проводимому лечению, ни в одном из случаев не были отмечены побочные реакции на фоне приема лекарственных средств.
При анализе микробиологического исследования мочи в обеих группах отмечался рост одного или двух возбудителей в титре ≥105 КОЕ/мл. В группе 1 наиболее часто диагностировалась Escherichia coli – в 80,5% случаев, Klebsiella spp. встречалась в 7,9%, рост Proteus spp. – в 6,5% и Enterobacter spp. – в 5,1%; в группе 2 Escherichia coli встречалась в 79,7% случаев, рост Klebsiella spp. отмечался в 8,2%, Enterobacter spp. – в 6,5% и Proteus spp. – в 5,6%. В таблице 1 представлены результаты исследования до и после лечения на 11-й день для каждой группы.
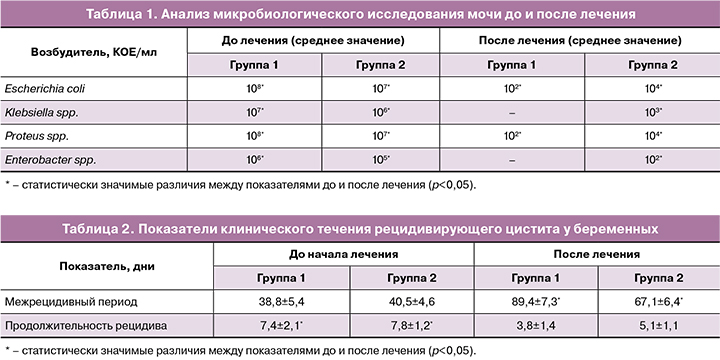
Лечение в группах 1 и 2 имело положительный результат. Однако наибольший эффект был достигнут в группе 1 на фоне комбинированной терапии антибактериальным препаратом и препаратом «Суперлимф».
При оценке общего анализа мочи всех беременных (n=44) были выявлены бактерии в 98% случаев, лейкоциты в большом количестве – в 27%. На 11-й день от начала лечения бактерии диагностировались в 10% случаев, лейкоциты – в 5%.
При наблюдении всех пациенток в течение беременности после проведенного лечения оценивались межрецидивный период и длительность обострения. Данные полученных результатов отображены в таблице 2.
Как видно из данных, представленных в таблице 2, проведенная терапия способствовала значимому снижению частоты и продолжительности рецидивов у пациенток обеих групп. Стоит отметить, что длительность межрецидивного периода была значительно выше, а продолжительность рецидива короче в группе 1 (фосфомицин+Суперлимф) по сравнению с группой 2.
В периоде наблюдения на фоне пролеченной ББ у пациенток в обеих группах отмечалось развитие симптоматических ИМП: в основной группе у 1 пациентки развился эпизод впервые выявленного острого цистита, у 1 беременной – рецидив хронического цистита и 1 случай острого пиелонефрита; в группе 2 эпизод впервые выявленного острого цистита был также у 1 пациентки, однако в 4 случаях ББ привела к рецидиву хронического цистита, еще у 3 беременных возник острый пиелонефрит. Приведенные данные еще раз подтверждают высокую эффективность комплексной терапии ББ и снижение вероятности возникновения симптоматической ИМП на фоне комбинированного лечения.
У всех пациенток оценивались акушерские и перинатальные исходы. Произошло 44 родоразрешения, при этом в основной группе частота своевременных родов составляла 95,4% (n=21), преждевременных – 5,6% (n=1), в группе сравнения своевременные роды произошли в 77,3% (n=17), преждевременные – в 22,7% случаев (n=5).
Во всех группах исследования беременность и роды протекали с осложнениями: преждевременное излитие околоплодных вод являлось одним из наиболее частых и составляло 18% (n=4), 36,4% (n=8) по группам соответственно; хроническая гипоксия плода в основной группе была в 13,6% (n=3) случаев, в группе сравнения – в 31,8% (n=7); многоводие встречалось в 22,7% (n=5), 27,3% (n=6) по группам соответственно; для группы 1 процент встречаемости острой гипоксии плода составил 9% (n=2), для группы 2 – 22,7% (n=5); слабость родовой деятельности не наблюдалась ни в одном из случаев основной группы, в группе 2 – в 18,2% (n=4); отслойка нормально расположенной плаценты в группе 1 наблюдалась у 4,5% (n=1), в группе 2 составляла 13,6% (n=3). Объем кровопотери при родах в большинстве случаев был в пределах нормативных значений и в среднем составлял для группы 1 379,5±38,6 мл, для группы 2 – 409±40,5 мл. Общая длительность своевременных родов в среднем составила 9 ч 20 минут±1 ч 40 минут, преждевременных родов – 8 ч 25 минут±1 ч 35 минут. Исходя из вышеизложенных данных, в группе 1 исследования значимо реже (р<0,05) отмечались осложнения беременности и родов по сравнению с группой 2.
Частота оперативного родоразрешения путем кесарева сечения соответственно по группам составила 18,2% (n=4), 36,4% (n=8) (р<0,05), что значимо ниже в основной группе.
На следующем этапе исследования были проанализированы перинатальные исходы у 45 детей от матерей из двух групп (у одной пациентки в группе 2 родилась двойня). Гестационный возраст новорожденных был в сроке от 32 до 41 недели беременности. Состояние детей оценивали при помощи стандартизированной шкалы Апгар, значения которой варьировались от 6 до 9 баллов в группе 1 и от 5 до 9 баллов – в группе 2, асфиксия плода наблюдалась в 9% (n=2), 22,7% (n=5) случаев по группам соответственно. Учитывая наибольшее число преждевременных родов в группе 2, процент недоношенных детей здесь был также выше и составил 22,7% (n=5). Дети с признаками внутриутробной инфекции значимо чаще (р<0,05) рождались в группе 2 – 27,3% (n=6) по сравнению с группой 1 – 13,6% (n=3). Масса доношенных новорожденных в среднем была 3346±74 г, недоношенных – 2245±66 г.
Анализируя результаты, полученные в ходе проведенного исследования, можно сделать заключение, что эффективность комплексного лечения ББ антибактериальным препаратом в комбинации со свечами «Суперлимф» была наиболее высокой, что подтверждалось значимым снижением роста патогенных микроорганизмов при микробиологическом исследовании, существенным удлинением периода между рецидивами ББ и уменьшением их длительности в группе 1. Наиболее благоприятные акушерские и перинатальные исходы также соответствовали основной группе исследования.
Заключение
Результаты, полученные в ходе проведенного исследования, подтверждают высокую эффективность комплексного лечения ББ у беременных антибактериальным препаратом с иммуномодулирующим средством «Суперлимф». Действие антибактериальных препаратов хорошо изучено и известно, однако частые рецидивы ББ во время беременности, высокая резистентность патогенных микроорганизмов к антибиотикам обуславливают поиск новых схем лечения для достижения устойчивых клинических результатов. Так, благодаря действию препарата «Суперлимф» на клетки, участвующие в иммунном ответе, происходит активация нейтрофилов, моноцитов, макрофагов, вследствие чего значительно быстрее происходит элиминация патогенных микроорганизмов. В ходе исследования не было обнаружено побочного и токсического эффектов препарата, отмечалась высокая приверженность пациенток к лечению. Исходя из полученных данных, препарат «Суперлимф» можно рекомендовать к использованию в терапии ББ у беременных в сочетании с антибактериальным препаратом, что может считаться эффективной комбинацией в достижении высокого терапевтического эффекта.



