Pregnancy is a natural model of metabolic syndrome: results of a dynamic study of physiological gestation
Objective. To identify the tendency for changes in carbohydrate, lipid metabolism, angiogenic, hormonal, hematological, inflammatory parameters and clarify what ‘normal pregnancy’ is in terms of fetal life support based on the monitoring of patients with uncomplicated pregnancy.Lipatov I.S., Tezikov Y.V., Shmakov R.G., Azamatov A.R., Martynova N.V.
Materials and methods. This was a comprehensive dynamic study of 40 healthy women with normal gestation and 30 healthy non-pregnant women.
Results. Insulin resistance and compensatory hyperinsulinemia, hyperleptinemia, atherogenic lipid profile, pro-inflammatory, pro-angiogenic and hypercoagulable state, sympathicotonia, hyperuricemia, visceral type accumulation, and endothelial-platelet interaction can develop in normal pregnancy.
Conclusion. Changes in normal pregnancy are aimed at the life support of the fetus with adverse atherogenic and diabetogenic changes in the maternal metabolism. The parameters of normal pregnancy are similar to the functional phase of the metabolic syndrome; thus, one can draw a conclusion that pregnancy is a natural model of the metabolic syndrome.
Keywords
The concept of ‘norm’ is constantly changing due to the dramatic progress in the study of the human body. Over the past decades, there have been changes in the normative indicators of human life expectancy, the duration of various periods of life, body mass index, blood pressure (BP), blood glucose and cholesterol, the duration of childbirth and the perinatal period; the possibility of achieving conception in late reproductive age has become a normal event, etc. [1]. Therefore, a clinician must be familiar with the parameters that characterize the ‘norms of non-pregnant women’ and know the principles of the development and course of the normal pregnancy, especially the dynamics of laboratory indicators and instrumental parameters. It is necessary to differentiate between physiological compensatory and adaptive changes(normal pregnancy) and pathological abnormalities that are suggestive of developing pregnancy complications; these issues are becoming more relevant in the context of an increasing prevalence of obstetric pathology with atypical and subclinical course [2, 3]. Homeostasis in pregnant women is characterized by the ability of the mother’s body to long-term compensation; at the same time, laboratory indicators remain at the borderline level of reference values for a long time; such category as ‘norms of compensated pathology’ reflects all these factors. [4].
During pregnancy, women are known toactivate an alternative type of energy supply, which causes metabolic changes characteristic of gestation [5, 6]. The course of pregnancy is accompanied by the development of insulin resistance (IR), especially muscle resistance, and its degree increases rapidly from the second trimester of gestation [7]. In response to the developing IR, pregnant women experience atherogenic changes in the lipid profile, which contribute to the energysupply of the mother’s body instead of the glucose supply necessary for the growing fetus. In the liver, the processes of glycogenolysis and gluconeogenesis are disinhibited due to impaired transmission of the insulin signal, and the activity of glycogenogenesis is reduced [5, 8]. As a result, changes in pregnancy are aimed at the growth and development of the fetus by increasing the supply of glucose, amino acids, and free fatty acids through the placenta and at maintaining the necessary functional state of the woman [6, 7]. Therefore, the study of gestational mechanisms of adaptation of the functional mother-fetus system allows us to identify general principles of the development of ‘pregnancy norms’, to distinguish them objectively from the ‘norms of compensated pathology’, and to stratify pregnant women by risk groups.
The objective of the study is to identify the tendency for changes in carbohydrate, lipid metabolism, hormonal, angiogenic, hematological, pro- and anti-inflammatory parameters and to clarify the ‘norms of pregnancy’ in terms of fetal life support based on clinical and laboratory monitoring of uncomplicated pregnancy.
Materials and methods
The study was conducted at the Perinatal Center of V.D. Seredavin Samara Regional Clinical Hospital and antenatal clinics in Samara in 2017–2019. For this purpose, a dynamic clinical and laboratory examination of 40 healthy pregnant women with a normal course of gestation (group I) was performed; the control group included 30 healthy non-pregnant women (group II). All the participants met the following criteria for inclusion in the study groups: early reproductive age of healthy non-pregnant and healthy pregnant women, term vaginal delivery to healthy newborns, informed consent to participate in the study,andconsent for the publication of the obtained data. Exclusion criteria were remarkable obstetric and gynecological history, overweight or obesity, presence of congenital, somatic, gynecological, infectious diseases, gestational and perinatal complications, and congenital fetal pathology. The pregnant women of group I were examined at 11–14 weeks, 18–21 weeks, and 30–34 weeks gestation. The women of group II were examined once on the 6th-10th day of the menstrual cycle.
The women of both groups I and II were evaluated for metabolic indicators, such as venous blood glucose, uric acid, total cholesterol (TC), triglycerides (TG), high–density lipoproteins (HDL) in serum, with the calculation of atherogenicity index (AI=(TC-HDL)/ HDL) and the ratio TG/HDL, insulin resistance index (HOMA-IR = fasting glucose (mmol/L) x fasting insulin (pmol/L) x 0.138/22.5), and hematological indicators, such as number, fraction of immature and mean volume of platelets, their aggregation with collagen, number of white blood cells and neutrophils. The women were also assessed for hormones (insulin, leptin, placental lactogen (PL), cortisol in the blood, and norepinephrine in the urine); anti– inflammatory markers (interleukin (IL)-4, IL–10) and pro-inflammatory markers (index of leukocyte activation, tumor necrosis factor (TNF)-α, IL-6, C-reactive protein (CRP); vascular endothelial state (circulating endothelial cells (CEC), fibronectin (FN)); placental angiogenesis (placental growth factor (PlGF)) and decidualization of stromal cells (placental alpha-1-microglobulin (PAMG-1)). The dynamics of accumulation and regional fat distribution were determined using ultrasonography: thickness of subcutaneous fat layer (SFL) and preperitoneal fat layer (PFL) were measured and the abdominal wall fat index (AFI) was calculated (AFI=PFL/SFL). The type of daily BP variability, subjective sleep characteristics assessed by questionnaire of Y.I. Levin (1995), and episodes of night apnea were evaluated.
The study usedthe biochemical analyzer Architectc4000 (Abbotte, USA), the hematological analyzer SysmexXN-1000 (SysmexCorporation, Japan), laser aggregation analyzer ALAT-2 (Biola Ltd, Russia), and ultrasound system Voluson E6 (GE Healthcare, Austria). Concentrations of insulin, leptin, PL, cortisol, TNF-α, IL-6, IL-4, IL-10, CRP, PlGF, PAMG-1 in serum and FN in blood plasma, norepinephrine in daily urineweredeterminedby means of enzyme linked immunosorbent assay (ELISA). The number of CEC in the blood was calculated according to N.N. Petrishchev (2001).
Statistical analysis
Statistical processingof the numericaldata was performed using specialized software IBMSPSSStatistics 25 HCIMAGO 5.0, license no. 5725-A54. During statistical processing, normal distribution of values was assessed using the Kolmogorov–Smirnov test (Lilliefors modification) and Shapiro–Wilktest. The arithmetic mean (M) and standard deviation (SD) werecalculated for indicators with a normal distribution, and the statistical significance of differences was determined using univariate analysis of variance (ANOVA). In the case of nonparametric distribution, medians (Me) were calculated with an interquartile interval [Q1(25%);Q3(75%)],the Mann–Whitney U test with the Bonferroni correction was performed. The Wilcoxon paired test was used to compare dependent samples (dynamics of indicators during pregnancy). Spearman’s correlation analysis was performed to identify correlations. At a significance level of p less than 0.05, the results were considered statistically significant.
Results and Discussion
The average age of women was 27.4 (3.7) and 26.5 (4.0) years in groups I and II, respectively (p=0.69). The body mass index corresponded to the reference limits: 22.1 (0.8) kg/m2 in group I, and 22.4 (0.7) kg/ m2 in group II (p=0.77). All women had unremarkable gynecological, somatic, and allergic histories. In group I, there were 50% (20/40) of primigravidas, and 50% (20/40) of multigravidas and multiparas, their obstetric history was uneventful. All women of group I had term vaginal delivery without any complications. All newborns were born without asphyxia, with an average body weight of 3390 (280) g and body length of 52.7 (3.6) cm. The women were discharged from the hospital on 3–4 days, and lactogenesis was not impaired.
Mean systolic (118 (5) mmHg), diastolic (76 (4) mmHg), and mean (91 (5) mmHg) BP levels at the end of the 1st trimester significantly decreased by 18–21 weeks of gestation: 106 (4) mmHg, 69 (4) mmHg, and 82 (5) mmHg, respectively (psyst<0.001, pdiast=0.004, pmean=0.003); however, the levels were again close to the initial values at 30-34 weeks of gestation (psist=0.81, pdiast=0.89, and pmean=0.93). All pregnant women had a physiological type of daily BPvariability,dipper,characterized byadecrease in BP at night by 10-20%, compared to thedaily level [9, 10]. Physiological changes in the respiratory system during pregnancy can cause difficulty breathing and, most importantly, sleep disorders [11], with the development of gestational sleep apnea syndrome, episodes of which can lead to hypoxia and oxidative stress [12]. Gestational sleep apnea was not observed in pregnant women of group I; its absence reflects the moderate nature of changes and a sufficient reserve of adaptation. The analysis of subjective assessment of sleep characteristics showed that all women in group I scored >22 points, which corresponded to the normal level and reflected the balanced functioning of the central regulatory mechanisms. The levels and type of daily BP variability and sleep characteristics in healthy non-pregnant women corresponded to the normal indicators and were unremarkable.
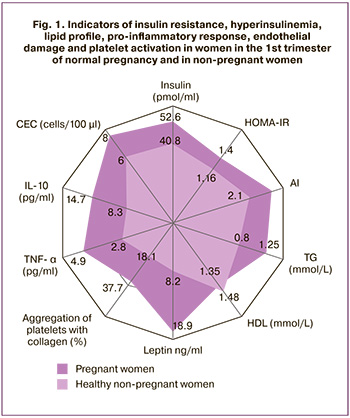 During gestation, the patients gained on average 1.5 [1.3;1.8] kg during the first trimester, 4.9 [4.5;5.3]kg at 18–21 weeks, and 11.8 [10.9;12.9] kg during the entire pregnancy. Subcutaneous fat layer (SFL) in non–pregnant women was measured as 9.4 [5.5;12.8] mm, peritoneal fat layer (PFL) was 9.2 [5.3;12.2] mm, and abdominal wall fat index (AFI) was 0.9 [0.8-1.0]. AFI in women of group I increased by 24% by the 3rd trimester of gestation, compared to the baseline value at 11–14 weeks (p=0.02). It should be noted that the increase in fat mass in the 1st, 2nd and 3rd trimesters of gestation in women of group I occurred not only due to SFL (9.5 [5.5;13.6] mm, 11.7 [7.3;15.3] mm, 13.5 [9.6;17.3] mm, respectively), but also by means of PFL (9.8 [6.0;13.1] mm, 10.3 [8.1;13.8] mm, 11.2 [8.8;14;6] mm, respectively), as PFL has a high metabolic activity. In this regard, it is interesting to develop an atherogenic lipid profile in pregnant women of group I (Table 1). At the same time, the average values of TC, TG, AI and the ratio of TG/HDL in pregnant women with normal gestation significantly increased during the gestation (p<0.001 for each of the indicators) and from the end of the 1st trimester they significantly exceeded the indicators in non-pregnant women (pTC=0.02, pTG<0.001, pAI=0.01,pTG/HDL=0.01) (Fig. 1). On the one hand, the presented changes are adaptive in nature and provide the mother’s body with the most energy-intensive substrates under the conditions of glucose redirection to the fetus due to IR of the tissues in a pregnant woman; on the other hand, they act as an alteration factor and maintain the state of IR, damaging endothelial cells and activating platelets, causing pro-inflammatory changes. However, in this situation, the accumulation of lipids in tissues of SFL and PFL compensates for the excessive damaging effect. This suggestion is confirmed by the strong positive correlation between PFL and the level of insulin, TNF-α, HOMA-IR, platelet aggregation (r from 0.81 to 0.94, p<0.001) and a positive correlation between PFL and the average platelet volume, the level of CEC (r from 0.62 to 0.73, p<0.05).
During gestation, the patients gained on average 1.5 [1.3;1.8] kg during the first trimester, 4.9 [4.5;5.3]kg at 18–21 weeks, and 11.8 [10.9;12.9] kg during the entire pregnancy. Subcutaneous fat layer (SFL) in non–pregnant women was measured as 9.4 [5.5;12.8] mm, peritoneal fat layer (PFL) was 9.2 [5.3;12.2] mm, and abdominal wall fat index (AFI) was 0.9 [0.8-1.0]. AFI in women of group I increased by 24% by the 3rd trimester of gestation, compared to the baseline value at 11–14 weeks (p=0.02). It should be noted that the increase in fat mass in the 1st, 2nd and 3rd trimesters of gestation in women of group I occurred not only due to SFL (9.5 [5.5;13.6] mm, 11.7 [7.3;15.3] mm, 13.5 [9.6;17.3] mm, respectively), but also by means of PFL (9.8 [6.0;13.1] mm, 10.3 [8.1;13.8] mm, 11.2 [8.8;14;6] mm, respectively), as PFL has a high metabolic activity. In this regard, it is interesting to develop an atherogenic lipid profile in pregnant women of group I (Table 1). At the same time, the average values of TC, TG, AI and the ratio of TG/HDL in pregnant women with normal gestation significantly increased during the gestation (p<0.001 for each of the indicators) and from the end of the 1st trimester they significantly exceeded the indicators in non-pregnant women (pTC=0.02, pTG<0.001, pAI=0.01,pTG/HDL=0.01) (Fig. 1). On the one hand, the presented changes are adaptive in nature and provide the mother’s body with the most energy-intensive substrates under the conditions of glucose redirection to the fetus due to IR of the tissues in a pregnant woman; on the other hand, they act as an alteration factor and maintain the state of IR, damaging endothelial cells and activating platelets, causing pro-inflammatory changes. However, in this situation, the accumulation of lipids in tissues of SFL and PFL compensates for the excessive damaging effect. This suggestion is confirmed by the strong positive correlation between PFL and the level of insulin, TNF-α, HOMA-IR, platelet aggregation (r from 0.81 to 0.94, p<0.001) and a positive correlation between PFL and the average platelet volume, the level of CEC (r from 0.62 to 0.73, p<0.05).
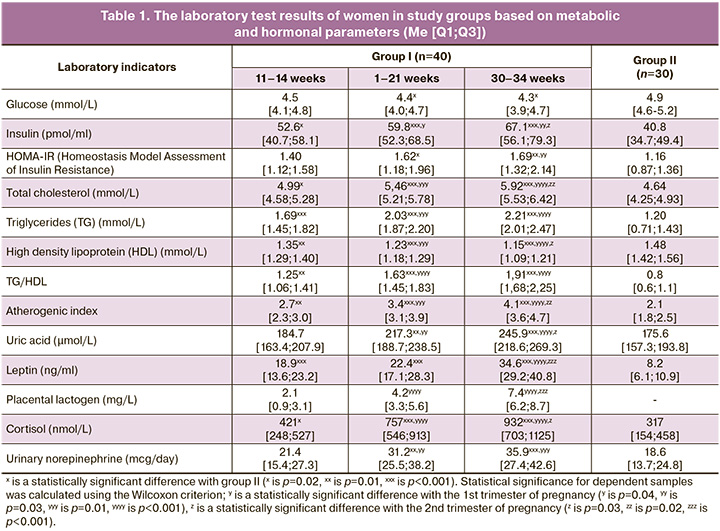
The course ofgestation is generallyknowntobe accompanied by the development of IR [6, 7]. The HOMA-IR analysis showed a statistically significant excess in the 2nd (p=0.02) and 3rd (p=0.01) laboratory tests in pregnant women in comparison with non-pregnantwomen.Despite this, the levelofvenous blood glucose during pregnancy was significantly lower compared to the indicator of women in group II (p=0.02 at 18–21 weeks; p=0.02 at 30–34 weeks), which results from glucose supply to the fetus dueto the difference in density gradients. Physiological IR contributes to the adequate energy supply of the developing embryo- and feto-placental complex; its anabolic activity is significantly higher than that of the mother’s body [7]. The development of physiological IR is also influenced by PAMG-1, which is synthesized by differentiated decidual cells and belongs to the class of low-molecular-weight proteins (IGFBP-1) that specifically bind insulin-like growth factors (IGF). PAMG-1 regulates both mitogenic and IGF activity. PAMG-1 contributes to the development of IR which is necessary for fetal energy supply by binding IGF in the systemic bloodstream. The regulation of mitogenic functions consists in limiting the tumor-like progress of the cytotrophoblast and providing a balanced flow of invasion waves. In this case, PAMG-1 acts as a natural ‘maternal’ regulator of trophoblast invasiveness. The data on the level of PAMG-1 showed its moderate increase(two-foldincrease) duringpregnancy(Table 2). Therefore, IR in normal pregnancy acts as an alteration factor in the mother’s body, impairing the usual glucose supply to the cells which is necessary for the growth and development of thefetus;it also causes diabetogenic metabolism and, in some ways, restricts the mother’s body in nutrients. This process is similar to the changes characteristic of the metabolic syndrome: due to IR, the supply of nutrients to the organs and tissues of thebodyislimited and the nutrients are redirected for the growth and development of adipose tissue; during pregnancy, the feto-placental complex is a kind of analog if adipose tissue. IR operates at the cellular and molecular levels, causing activation of the endothelial-platelet link, «diabetogenic» rearrangement of metabolism with an increase in the level offreefattyacids, and the development of moderate pro-inflammatory and hypercoagulation conditions. The increase in the level of insulin in pregnant women reflects the development of compensatory hyperinsulinemia which occurs during physiological adaptation to the above-described changes. At the same time, the average values of the indicator statistically significantly exceeded the values in non-pregnant women from the end of the 1st trimester (p=0.02) (Fig. 1). Compensatory hyperinsulinemia provides the necessary level of energy supply with glucose and amino acids of the mother’s body, deposits excessive free fatty acids in fat depots, and performs its vasodilating and vasoprotective functions. Hypersecretion of insulin is associated with activation of pancreatic β-cells, which is an adaptive response to physiological IR that develops under the influence of a wide range of placental contrinsular hormones. These hormones are factors of «physiological alterations» in pregnancy [5, 13].
One of the most important pregnancy hormones is PL, which has thegreatestcontrinsulareffect; its average level significantly increased with each subsequent laboratory examination: 11–14 weeks – 2.1 mg/L, 18–21 weeks – 4.2 mg/L (p1-2<0.001), 30–34 weeks – 7.4 mg/L (p2-3<0.001). In addition, significantly higher levels of leptin and cortisol wereobservedin pregnant women in the 1st trimester (pL<0.001; pK=0.01), 2nd trimester (pL<0.001; pK<0.001), and 3rd trimester (pL<0.001; pK<0.001), compared to group II. A similar phenomenon was observed when determining the level of norepinephrine in thedaily amount of urine; statistical significance was detected at 18–21 weeks (p=0.01) and 30–34 weeks (p<0.001) gestation, compared to non-pregnant women (Table 1). Elevated levels of these hormones are also known to occur in metabolic syndrome; hyperleptinemia and leptin resistance play a particularly significant role in the pathogenesis of this syndrome, and, in particular, in the progression of IR, endothelial dysfunction, pro-inflammatory state, and imbalance of vegetative status with a predominance of sympathicotonia [13, 14].
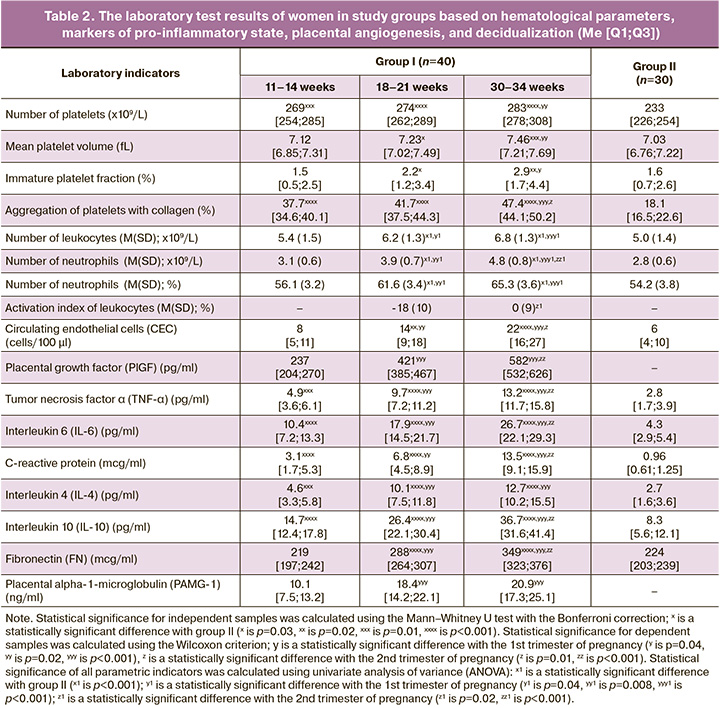
The analysis of laboratory results showed a statistically significant intergroup difference in the content of pro-inflammatory markers (Table 2). From the end of the 1st trimester, the average level of TNF-α in pregnant women was 1.25 times higher than one inhealthy non-pregnant women(p=0.01), IL-6 was2.4 times higher (p<0.001), and CRP increased 3.2 times (p<0.001); moreover, the assessment of the dynamics of the increase in indicators inthe 2nd and 3rd trimesters had significant differences compared to previous laboratory testing (TNF-α and IL-6,p1-2<0.001,CRP,p1-2=0.02;p2-3<0.001forall indicators). This pattern corresponds to an increase in the number of white blood cells and neutrophil fraction in women of group I, unlike women of group II (p<0.001). In order to assess the functional activity of leukocytes in pregnant women, the leukocyte activation index was calculated; it was determined after 20 weeks gestation. The average value of the indicator at18–21weeksofpregnancy wasminus 18(10)%, at 30–34 weeks – 0 (9)%; this value is within the normal criteria and corresponds to a balanced immune system. However, there were statistical differences between the values of the 2nd and 3rd trimesters, which reflect a moderate increase in the functional activity of white blood cells during normal pregnancy (p<0.001). The revealed changes indicate the activation of the immune response in the course of normal pregnancy in response to moderate inflammation of the decidual membrane during implantation of the fetal egg, trophoblast invasion, alteration of the spiral arteries in the development of the hemochorial type of placental structure, fetalization, migration, and subsequent aging of the placenta [15–17]. These pro-inflammatory shifts in homeostasis are compensated by an adaptive increase in anti-inflammatory cytokines due to the transformation of Th1 into a Th2-dependent type of immune response, the absence of classical HLA antigens on the surface of the trophoblast, the formation of a fibrin layer on its surface that hides specific antigens, and the programmed death of activated immunocompetent cells induced by cytiotrophoblast and syncytiotrophoblast through the induction of expression on the membrane of the Fas/ APO-1/CD95 receptor [15, 17]. We noted that the levels of anti-inflammatory cytokines IL-4 and IL-10 at the end of the 1st trimester of normal pregnancy were 1.7 and 1.8 times higher, respectively, compared to non-pregnant women (Fig. 1). At 18–21 weeks, the indicators significantly increased by 2.2 and 1.8 times, and by30–34 weeks ofgestation they increased by 1.3 and 1.4 times, respectively. The increased level of uric acid, which is, on the one hand, one of the most powerful soluble low-molecular-weight antioxidants, and, on the other hand, an activator of the endothelial-platelet link, contributes to inhibiting the systemic inflammatory response. In the 2nd and 3rd trimesters, thelevelsofuricacidinthebloodserumwere 1.2 (p=0.01) and 1.4 times (p<0.001), respectively, higher than those in non-pregnant women. Imbalance of cytokines, activation of the leukocyte link, and increasing hyperuricemia lead to the progression of the pro-inflammatory status, immunometabolic disorders, destabilization of endothelial function with subsequent gestational and perinatal complications; all these factors demonstrate a thin line between the ‘norms of pregnancy’ and the ‘norms of compensated pathology’. A characteristic feature of the presented changes in non-pregnant women is the involvement of these changes in the mechanisms of the development of the metabolic syndrome.
The endothelium of the vascularwallisthefirst barrier to alteration effects of developing IR, atherogenic fractions of the lipid profile, activated state of the immune system, increased levels of pro-inflammatory cytokines and free radicals. These factors cause hyperactivation and damage of endothelial cells with an increase in coagulation potential [8, 18–20]. To objectify this process, we determined the number of CEC in the blood. There was an increase in CEC in women of group I (8 cells/100 μl, 14 cells/100 μl and 22 cells/100 μl, respectively, according to the examination period), a significant difference in the values in the 2nd trimester (p=0.02) and 3rd trimester (p<0.001), compared to the women of group II. It was shown that at 18–21 weeks gestation in pregnant women of group I, the level of FN released as a result of endothelial activation, which is involved inthrombosis, begins to significantly exceed the indicator of healthy non-pregnant women (p<0.001). The obtained results of quantitative and functional assessment of platelets are consistent with the revealed results of hypercoagulation changes. The number and aggregation of platelets in pregnant women significantly increased from the end ofthe 1st trimester,compared togroup II (ptr=0.01, pagr<0.001),significantlyincreasingbytheend of gestation (ptr=0.02, pagr<0.001). Healthy pregnant women have an increase in the average platelet volume, which reflects its aggregation activity; there is also an increase in the fraction of immature platelets associated with increased consumption of mature forms. All these factors are indicative of activation of the platelet link. It should be noted that the literature widely covers similar endothelial and hemostatic changes in the functional phase of metabolic syndrome, but they become more severe and malignant in the progression of the syndrome [18, 21]. However, pregnancy triggers mechanisms that inhibit excessive endothelial damage and hypercoagulation potential: vasoprotective effect of compensatory hyperinsulinemia, proangiogenic state (increased synthesis of vascular endothelial growth factor (VEGF), activation of anticoagulant factors in the placenta (prostacyclin, annexin V, etc.), expression of heparin, proteins C and S on the surface of the trophoblast to preserve laminar flow in the intervillous space [15, 22]. Thus, according to the obtained data, there was a two-fold increase in the content of PlGF in women of group I, and PlGF is one of the VEGF family proteins synthesized only in pregnancy (from 237 to 582 pg/ml; p<0.001). It is noteworthy that the metabolic syndrome has also a high content of VEGF; however, if the level is high, the risk of metabolic complications is lower, and this reflects the protective role of the proangiogenic factor [23]. Figure 2 presents a generalized scheme showing similar changes in homeostasis in pregnancy and metabolic syndrome.
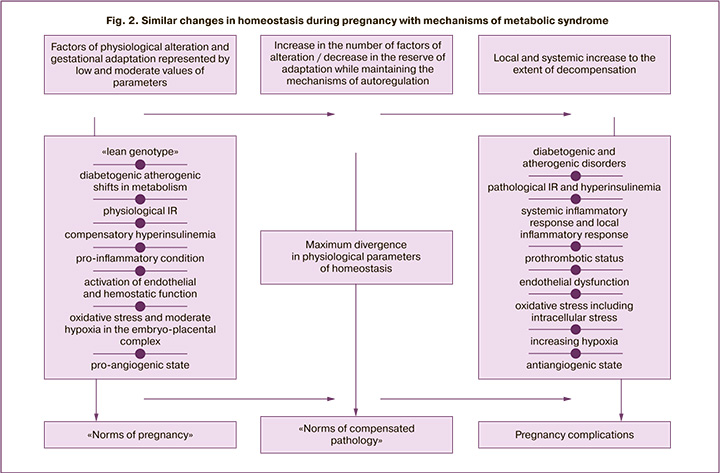
It should be noted that the balance between physiological IR and compensatory hyperinsulinemia contributes to an uncomplicated course of pregnancy with favorable outcomes. Failure of the adaptation characteristic of the normal pregnancy leads to the development of pathological IR and hyperinsulinemia, which are involved in the mechanisms of great obstetric syndromes.
Conclusion
The results of the study showed that the permanent nature of compensatory and adaptive mechanisms in pregnant women in response to the development of the embryo-placental and feto-placental complex is determined by the balance between «physiological damage» factors which occur within the «norms of pregnancy» (nidation, cytotrophoblast invasion and gestational reconstruction of spiral arteries with destruction of the vascular wall muscle elements, placentation, moderate inflammation of the decidual membrane, physiological IR, diabetogenic and atherogenic shifts in metabolism, endothelial damage, increased levels of pro-inflammatory cytokines and proteins of the acute phase of inflammation) and «mechanisms of gestational adaptation» (hormonal rearrangement, compensatory hyperinsulinemia, vasoprotective effect of angiogenic factors, transition to a Th2-dependent type of immune response with increased synthesis of anti-inflammatory cytokines, activation of the antioxidant system).
The changes in a woman’s body that are consistent with the «norms of pregnancy» are aimed at performing the evolutionally developed function of gestation, which consists in the primary energy and flexible life support of the developing fetus with adverse atherogenic and diabetogenic changes in the maternal metabolism.
IR and hyperinsulinemia which develop in normal pregnancyduetomanyfactors, namelyphylogenetically fixed action of contrinsular placental hormones and proteins, hyperleptinemia, increased atherogenic lipid fractions, pro-inflammatory and hypercoagulation conditions, activation of the endothelial-platelet link, sympathicotonia, hyperuricemia, visceral type of fat deposition, are remarkably similar to the pathogenetic mechanisms of the functional phase of the metabolic syndrome. This suggests that pregnancy is a natural model of metabolic syndrome.
These patterns of development of normal pregnancy with an emphasis on metabolic changes direct the attention of researchers to a comprehensive study of the pathogenetic role of changes associated with IR in the development of obstetric pathology.
References
- Томнюк Н.Д., Данилина Е.П. Терминологические понятия нормы и патологии в медицинской практике. Международный журнал прикладных и фундаментальных исследований. 2017; 7-2: 214-6. [Tomnyuk N.D., Danilina E.P. Terminology concepts of norm and pathology in medical practice. International journal of applied and basic research. 2017; 7-2:214-6. (in Russian)].
- Савельева Г.М., Шалина Р.И., Коноплянников А.Г., Симухина М.А. Преэклампсия и эклампсия: новые подходы к диагностике и оценке степени тяжести. Акушерство и гинекология: новости, мнения, обучение. 2018; 6(4): 25-30. [Savel'eva G.M., Shalina R.I., Konoplyannikov A.G., Simuhina M.A. Preeclampsia andeclampsia: new approaches in diagnosis and evaluation of severity. Obstetrics and Gynecology: News, Opinions, Training. 2018; 6(4):25-30. (in Russian)]. https://dx.doi.org/10.24411/2303-9698-2018-14002.
- Stevens A.B., Brasuell D.M., Higdon R.N. Atypical preeclampsia – gestational proteinuria. J. Family Med. Prim. Care. 2017; 6(3): 669-71. https://dx.doi.org/ 10.4103/2249-4863.222029.
- Бунятян А.А., Мизиков В.М., ред. Анестезиология. Национальное руководство. М.: ГЭОТАР-Медиа; 2017. 656с. [Bunyatyan A.A., Mizikov V.M., ed. Anesthesiology: National guidance. Moscow: GEOTAR-Media Publ. 2017; 656. (in Russian)].
- Napso T., Yong H.E.J., Lopez-Tello J., Sferruzzi-Perri A.N. The role of placental hormones in mediating maternal adaptations to support pregnancy and lactation. Front. Physiol. 2018; 9: 1091. https://dx. doi.org/10.3389/fphys.2018.01091.
- Chen X., Stein T.P., Steer R.A., Scholl T.O. Individual free fatty acids have unique associations with inflammatory biomarkers, insulin resistance and insulin secretion in healthy and gestational diabetic pregnant women. BMJ Open Diabetes Res. Care. 2019; 7(1): e000632. https://dx. doi.org/10.1136/bmjdrc-2018-000632.
- Гордюнина С.В. Инсулинорезистентность при беременности (обзор литературы). Проблемы эндокринологии. 2013; 59(5): 61-6. [Gordyunina S.V. Pregnancy insulin resistance (literature review). Problemy endokrinologii/ Problems of Endocrinology. 2013; 59(5):61-6. (in Russian)].
- Rochlani Y., Pothineni N.V., Kovelamudi S., Mehta J.L. Metabolic syndrome: pathophysiology, management, and modulation by natural compounds. Ther. Adv. Cardiovasc. Dis. 2017; 11(8): 215-25. https://dx.doi.org/10.1177/1753944717711379.
- Мирошниченко А.И., Иванов К.М. Влияние ночного повышения артериального давления на ремоделирование сердца у пациентов с артериальной гипертонией. Аспирантский вестник Поволжья. 2019; 1-2: 65-9. [Miroshnichenko A.I., Ivanov K.M. The effect of nocturnal increase in blood pressure on remodeling of the heart in patients with arterial hypertension. Aspirantskii vestnik Povolzh'ya/Postgraduate bulletin of the Volga region. 2019; 1-2:65-9. (in Russian)]. https://dx.doi.org/10.17816/2072-2354.2019.19.1.65-69.
- Altikardes Z.A., Kayikli A., Korkmaz H., Erdal H., Baba A.F., Fak A.S. A novel method for dipper/non-dipper pattern classification in hypertensive and non-diabetic patients. Technol. Health Care. 2019; 27(Suppl. 1): 47-57. https://dx. doi.org/10.3233/THC-199006.
- Калачин К.А., Пырегов А.В., Шмаков Р.Г. Гестационное сонное апноэ. Связь беременности и преэклампсии с синдромом обструктивного апноэ сна. Альманах клинической медицины. 2019; 47(3): 266-75. [Kalachin K.A.,Pyregov A.V., Shmakov R.G. Gestational sleep apnea. The relationship of pregnancy and preeclampsia with obstructive sleep apnea syndrome. Al'manakh klinicheskoi meditsiny/Almanac of Clinical Medicine. 2019; 47(3):266-75. (in Russian)]. https://dx.doi.org/10.18786/2072-0505-2019-47-031.
- Karan S., Ginosar Y. Gestational sleep apnea: have we been caught napping? Int. J. Obstet. Anesth. 2016; 26: 1-3. https://dx.doi.org/10.1016/j. ijoa.2016.03.001.
- Kodogo V., Azibani F., Sliwa K. Role of pregnancy hormones and hormonal interaction on the maternal cardiovascular system: a literature review. Clin. Res. Cardiol. 2019; 108(8): 831-46. https://dx. doi.org/10.1007/s00392-019-01441-x.
- Nolan C.J., Prentki M. Insulin resistance and insulin hypersecretion in the metabolic syndrome and type 2 diabetes: Time for a conceptual framework shift. Diab. Vasc. Dis. Res. 2019; 16(2): 118-27. https://dx.doi.org/10.1177/1479164119827611.
- Тезиков Ю.В., Липатов И.С., Калинкина О.Б., Гогель Л.Ю., Белоконева Т.С., Мартынова Н.В., Жернакова Е.В., Юсупова Р.Р., Мингалиева Л.К. Стратификация беременных на ранних сроках гестации путем объективизации факторов «физиологической альтерации», механизмов гестационной адаптации и эмбриоплацентарной дисфункции. Наука и инновации в медицине. 2016; 4(4): 6-13. [Tezikov Yu.V., Lipatov I.S., Kalinkina O.B., Gogel' L.Yu., Belokoneva T.S., Martynova N.V. et al. Stratification of pregnant women at early gestational ages by means of objectivation of "physiological alteration" factors, mechanisms of gestational adaptation and fetoplacental dysfunction. Nauka i innovatsii v meditsine/ Science & Innovations in Medicine. 2016; 4:6-13. (in Russian)].
- Капустин Р.В., Аржанова О.Н. Субклиническое воспаление как фактор развития инсулинорезистентности во время беременности. Российский вестник акушера-гинеколога. 2017; 17(1): 27-36. [Kapustin R.V., Arzhanova O.N. Subclinical inflammation as a factor for the development of insulin resistance during pregnancy. Russian Bulletin of the Obstetrician-Gynecologist. 2017; 17(1):27-36. (in Russian)]. https://dx.doi.org/10.17116/rosakush201717127-36.
- Bränn E., Edvinsson Å., Rostedt Punga A., Sundström-Poromaa I., Skalkidou A. Inflammatory and anti-inflammatory markers in plasma: from late pregnancy to early postpartum. Sci. Rep. 2019; 9(1): 1863. https://dx.doi.org/10.1038/s41598-018-38304-w.
- Хромылев А.В., Макацария А.Д. Ожирение, метаболический синдром и тромбофилия. Акушерство и гинекология. 2017; 10: 27-33. [Khromylev A.V., Makatsariya A.D. Obesity, metabolic syndrome and thrombophilia. Akusherstvo i Ginekologiya/Obstetrics and gynecology. 2017; 10: 27-33. (in Russian)]. https://dx.doi.org/10.18565/aig.2017.10.27-33.
- Echeverria C., Eltit F., Santibanez J.F., Gatica S., Cabello-Verrugio C., Simon F. Endothelial dysfunction in pregnancy metabolic disorders. Biochim. Biophys. Acta Mol. Basis Dis. 2020; 1866(2): 165414. https://dx.doi.org/10.1016/j.bbadis.2019.02.009.
- Moore T.A., Ahmad I.M., Schmid K.K., Berger A.M., Ruiz R.J., Pickler R.H. et al. Oxidative stress levels throughout pregnancy, at birth and in the neonate. Biol. Res. Nurs. 2019; 21(5): 485-94. https://dx.doi.org/10.1177/1099800419858670.
- Серов В.Н. Метаболический синдром (нейрообменно-эндокринный синдром). Medica Mente. Лечим с умом. 2015; 1: 16-9. [Serov V.N. Метаболический синдром (нейрообменно-эндокринный синдром). Medica mente. Lechim s umom/Medica mente. Treat wisely. 2015; 1:16-9. (in Russian)].
- Тимохина Е.В., Стрижаков А.Н., Зафириди Н.В., Губанова Е.С. Инновационный подход к прогнозированию и терапии преэклампсии – мировой опыт. Акушерство и гинекология. 2019; 5: 5-10. https://dx.doi.org/10.18565/aig.2019.5.5-10. [Timohina E.V., Strizhakov A.N., Zafiridi N.V., Gubanova E.S. An innovative approach to predicting and treating preeclampsia – global experience. Akusherstvo i Ginekologiya/Obstetrics and gynecology. 2019; 5:5-10. (in Russian)]. https://dx.doi.org/10.18565/aig.2019.5.5-10.
- Шепель Р.Н., Драпкина О.М. Новые векторы в диагностике метаболического синдрома: оценка уровня сосудистого эндотелиального фактора роста, пентраксина-3 и трансформирующего фактора роста бета. Кардиоваскулярная терапия и профилактика. 2019; 18(6): 57-61. [Shepel R.N., Drapkina O.M. New directions in metabolic syndrome diagnosis: assessment of vascular endothelial growth factor, pentraxin-3 and transforming growth factor beta levels. Kardiovaskulyarnaya terapiya i profilaktika/ Cardiovascular Therapy and Prevention. 2019; 18(6):57-61. (in Russian)]. https://dx.doi.org/10.15829/1728-8800-2019-6-57-61.
Received 14.05.2020
Accepted 21.05.2020
About the Authors
Igor S. Lipatov, MD, professor, professor of obstetrics and gynecology chair number 1 of Samara State Medical University.E-mail: i.lipatoff2012@yandex.ru. ORCID: 0000-0001-7277-7431. 89, Chapaevskaya str., Samara, Russia, 443099.
Yury V. Tezikov, MD, professor, Head of obstetrics and gynecology chair number 1 of Samara State Medical University.
E-mail: yra.75@inbox.ru. ORCID: 0000-0002-8946-501X.
89, Chapaevskaya str., Samara, Russia, 443099.
Roman G. Shmakov, MD, RAS Professor, chief medical officer of Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia. E-mail: mdshmakov@mail.ru. ORCID: 0000-0002-2206-1002.
4, Oparina str., Moscow, 117997, Russia.
Amir R. Azamatov, resident of obstetrics and gynecology chair number 1 of Samara State Medical University. E-mail: azamatov.amir@yandex.ru.
ORCID: 0000-0003-0372-6889. 89, Chapaevskaya str., Samara, Russia, 443099.
Nadezhda V. Martynova, teaching assistant of obstetrics and gynecology chair number 1 of Samara State Medical University.
E-mail: og1samsmu@mail.ru. ORCID: 0000-0002-2107-1508. 89, Chapaevskaya str., Samara, Russia, 443099.
For citation: Lipatov I.S., Tezikov Yu.V., Shmakov R.G., Azamatov A.R., Martynova N.V. Pregnancy as a natural model of metabolic syndrome: results of a dynamic study of normal gestation.
Akusherstvo i Ginekologiya / Obstetrics and gynecology. 2020; 9: 88-96 (in Russian)
https://dx.doi.org/10.18565/aig.2020.9.88-96



