Bacterial vaginosis and antimicrobial activity during pregnancy
Objective. To compare the antimicrobial activity of vaginal discharge with the nature of bacterial vaginosis, vaginal pH, and microbiological indicators.Karapetyan T.E., Arzumanyan V.G., Tyutyunnik V.L., Kan N.E., Lomova N.A., Kanyukina A.A.
Subjects and methods. The investigation enrolled 53 pregnant women, of whom 34 were diagnosed with bacterial vaginosis and 19 were with normal vaginal microbiocenosis. The severity of bacterial vaginosis was estimated from the total scores using a combination of clinical symptoms: itching, burning, the nature and amount of discharge, pain during urination, dyspareunia, perianal dermatitis, swelling, hyperemia, and erosive lesions of the vaginal walls. Vaginal discharge samples were examined to determine the antimicrobial activity of the vaginal epithelium. The antibacterial activity of antimicrobial peptides was determined in the groups of pregnant women depending on the nature of clinical symptoms of bacterial vaginosis.
Results. Production of the endogenous antibiotics antimicrobial peptides provides adequate protection against infectious agents. The lowered activity of antimicrobial peptides leads to a quantitative increase in the facultative vaginal microflora and to the depletion of the obligate microflora, which contributes to the more pronounced clinical course of bacterial vaginosis.
Conclusion. To expand diagnostic parameters in the set of examinations of pregnant women with inflammatory and dysbiotic disorders in the lower reproductive tract, by including the indicators of systemic and local immunity along with microbiological monitoring and an integrated assessment of the vaginal microbiocenosis, will be able to personify therapeutic approaches for each individual patient.
Keywords
The introduction of modern diagnostic technologies into clinical microbiology made it possible to discover that the constancy of the species and quantity composition of normal microflora provides vaginal colonization resistance and prevents the population of the biotope by pathogenic microorganisms or the excessive growth of its own opportunistic pathogenic microflora and its translocation into loci not characteristic of these species. It results from the participation of lactobacilli in the biofilms creation when they adhere to the vaginal epithelial cells and the production of antimicrobial protein substances, bacteriocins, as well as lactic acid. Moreover, the vaginal and cervical epithelium secrete antimicrobial peptides (AMPs), which prevent colonization by exogenous microorganisms [1, 2].
There are more than 500 AMPs that protect epithelial tissues of humans and animals from the invasion of bacteria, fungi, protozoa, viruses. These peptides have an antimicrobial effect, therefore they are called natural antibiotics. They also play an important role in the development of inflammation, maintenance and regulation of the adaptive immune system [1, 2].
Several types of AMPs are found in the vagina, which are represented by defensins, cathelicidins, secretory leukocyte proteases inhibitors (SLPI), elafin and lysozyme. Currently, basic research on the role of defensins in the antimicrobial protection of the lower genital tract is being actively introduced into clinical medicine. Two groups of human defensins, namely α and ß are described. α-defensins are present in polymorphonuclear neutrophils and participate in the immunological response. ß-defensins show antiinfective activity against gram-negative bacteria and yeast-like fungi [3-5].
Antimicrobial substances of lactobacilli and antimicrobial polypeptides of the vaginal epithelium form a protective ecological barrier, which prevents colonization with microorganisms that are not characteristic of this biotope. Despite this, about 75% of women have a vaginal infection, and 5-10% of them suffer from recurrent bacterial vaginosis (BV) [6-8]. However, it is difficult to assess factors that influence more the realization of the immune response, the direct antibacterial and antiviral effect of AMP on pathogenic microorganisms or their immunomodulating effect.
It has been clinically proven that AMP inhibits the growth of gram-positive and gram-negative bacteria, fungi and some viruses, showing biological effectiveness in the reproductive tract in cooperation with other factors of local immunity.
By now two large subfamilies of the AMP class have been described: defensins and cathelicidins. Among the many AMPs, the producing cells and loci of α- and ß-defensins are lymphocytes, phagocytes and epithelial cells of the respiratory, gastrointestinal and urogenital tract. Defensins are among the first to protect the body at the local level in response to bacterial, mycotic and virus infection, as they are produced by the vagina and cervix epithelium.
Considering the search for new approaches in the diagnosis and therapy of BV during pregnancy, the study of the protective properties of antimicrobial peptides in response to infectious agents in BV is relevant.
The purpose of the study was to compare the antimicrobial activity of the vaginal discharge with the BV severity, the vaginal pH, and microbiological characteristics.
Material and methods
The study included 53 pregnant women, 34 of them were diagnosed with BV and 19 patients had normal vaginal microcenosis.
The presence and severity of BV were assessed according to Nugent quantitative scale (Nugent’s Diagnostic Criteria for Bacterial Vaginosis 1991, Table 1). A score of 7 shows BV. A score of 4 to 6 points was considered as an indicator of the intermediate type of microbiocenosis, and a score of 0 to 3 was considered as an indicator of the normal vagina microbiocenosis. On microscopic examination of gram-stained smears, the presence and abundance of lactobacilli, bacteroides, gram-positive cocci, and gardnerella were visually assessed. Abundance was expressed in scores on a 4-point scale, where 0 meant the absence of this group of microorganisms, 1 - single cells in the field of view, 2 – the moderate number of cells, 3 – the abundance of cells.
Pregnant women with verified BV diagnosis were subsequently divided into 3 subgroups, depending on the clinical manifestations severity. We developed a scoring scale for assessing BV clinical symptoms, which included such criteria as itching, burning, hyperemia, swelling, painful urination, dyspareunia, erosive lesions of the vaginal mucosa and vulva, the type of vaginal discharge. Based on the results of the questionnaire, the patient could score from 0 to 18 points. In accordance with the score, the 1st subgroup included patients with mild BV (scores 3-8), the 2nd subgroup included patients with moderate severity of BV (scores 9-12), and the 3d subgroup included patients with severe BV (scores 13-18). In each subgroup, a microbiological examination was carried out with an obligatory assessment of the vaginal pH and the elimination of possible infection with absolute pathogens.
To determine the antimicrobial activity of the vaginal epithelium, samples of the vaginal discharge were examined. The antimicrobial activity was determined as follows: cells of the 3-day test culture of Escherichia coli were incubated at 32 ° C with a ratio of 40 μl vaginal discharge / 10 μl bacterial suspension 104 CFU/ml. Samples were cultured from this mixture immediately after mixing and incubation for 2 hours. The result was calculated as the percentage of cells killed within the incubation process.
 The antimicrobial activity of AMP was determined in the groups of pregnant women, depending on the BV clinical symptoms manifestation type.
The antimicrobial activity of AMP was determined in the groups of pregnant women, depending on the BV clinical symptoms manifestation type.
Separation of proteins in the vaginal discharge samples was carried out by gradient SDS polyacrylamide gel (5-20%) electrophoresis. The samples were prepared under non-denaturing conditions, mixing 1 volume of sample with 2 volumes of buffer and applying 40 μl of sample per track. Staining was performed with Coomassie R-250. LMW (Amersham-Pharmacia) was used as molecular weight standards.
Statistical analysis of the results was carried out on PC using the SPSS Statistics 22.0 software for Windows. To determine the statistical significance of the differences, the exact two-sided Fisher test and the Mann-Whitney U test were used for unrelated aggregates. The odds ratio (OR) is given with a 95% confidence interval (CI).
Results
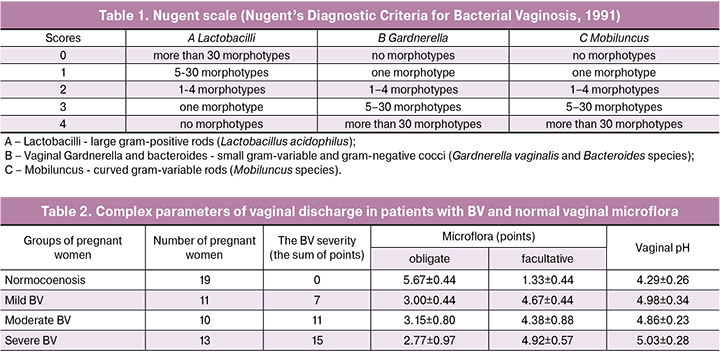
After selecting the patients and performing all necessary examinations, we assessed the microflora nature. Identified microorganisms were conditionally divided into two groups: “obligate microflora” (lactobacilli and bacteroides) and “facultative microflora” (cocci and gardnerella). The results of the complex evaluation are presented in Table 2.
The presented data show that the highest scores for obligate microflora were observed in the group of pregnant women with normal vaginal microcenosis, the lowest scores were in the group of pregnant women with severe BV. In turn, in the group of healthy pregnant women, the facultative microflora indicator was the lowest, and the group with severe BV had the highest one. Obligate and facultative microflora have shown the reverse and close correlation in all groups of pregnant women: the abundance of facultative microflora positively correlated with the BV severity, whereas the abundance of obligate microflora had an inverse correlation.
 Since the vaginal pH is the result of the lactic acid bacteria metabolism, the lowest pH was found in the group of healthy pregnant women, where an abundance of lactobacilli was observed, while the highest pH values were found in the group of patients with severe BV. Thus, the vaginal pH values directly depended both on the severity of the BV clinical course and on the quantitative assessment of the microflora of this locus.
Since the vaginal pH is the result of the lactic acid bacteria metabolism, the lowest pH was found in the group of healthy pregnant women, where an abundance of lactobacilli was observed, while the highest pH values were found in the group of patients with severe BV. Thus, the vaginal pH values directly depended both on the severity of the BV clinical course and on the quantitative assessment of the microflora of this locus.
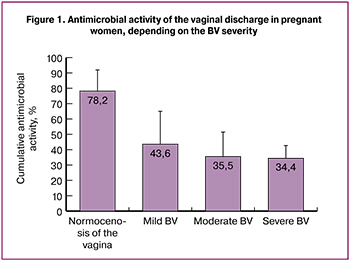
The study showed that the highest activity of AMP was found in the group of healthy pregnant women and was 78.2 ± 13.6% (Figure 1).
As the severity of the disease increased in infected pregnant women, the overall activity of AMP significantly decreased in comparison with the group of healthy pregnant women, accounting for 43.6 ± 21.2% in women with mild BV, 35.5 ± 15.6% with moderate BV and 34.4 ± 8.2% in pregnant women with severe BV (p<0.05). The correlation coefficient between the antimicrobial activity of AMP and the severity of the symptoms revealed a negative correlation (r = -0.936).
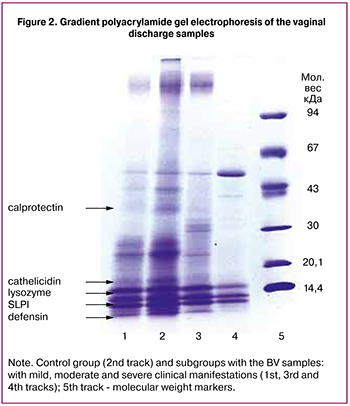
To determine the cause of the antimicrobial activity decrease in BV, we separated the proteins of the vaginal discharge in the gradient of polyacrylamide gel (Figure 2).
In the obtained samples, lines corresponding to antimicrobial peptides are determined. Track № 2 corresponds to the sample from the pregnant woman from the control group (antimicrobial activity 100.0%). Such polypeptides as calprotectin with a molecular weight about 37 kDa, cathelicidin hCAP18 (18 kDa), SLPI (about 12 kDa), lysozyme (14.5 kDa), and defensins (less than 5 kDa) are distinguishable on the track. Track № 1 corresponds to the mild severity of BV clinical manifestations (the sum of symptoms is 7 scores, antimicrobial activity is 66.7%). There is no calprotectin, other proteins are distinguishable, but the lines are not as intense. Track № 3 relates to the moderate severity of clinical manifestations (the sum of symptoms is 11 scores, antimicrobial activity is 21.6%), calprotectin is not detected and defensins are slightly distinguishable. Track № 4 corresponds to severe BV (the sum of symptoms is 15 scores, antimicrobial activity is 0.0%). There is no calprotectin, a weak lysozyme line is detected, there are no defensins.
Discussion
There is a pronounced expression of the innate immunity factors in the reproductive tract mucosa. There is a constant interaction between local defense factors and conditionally pathogenic vaginal microbiota.
The obligatory component of the vaginal microecosystem is the endogenous cationic AMPs. They are formed by the epithelium of barrier tissues and blood cells (neutrophils). The production of AMP is triggered by the interaction of microbial structures with Tolllike receptors (TLR) of cells, which is followed by the intensive formation of pro-inflammatory cytokines [9]. In addition to antimicrobial action, AMPs are involved in the inflammation regulation and in the acquired immunity formation.
Imbalance of congenital immunity components, namely violation of ß-defensin 1 (HBD1) and TLR gene expression in the epithelial cells of the lower urogenital tract and in the placenta is a marker for the intrauterine infection [9, 10].
Reduction of the AMP concentration in the vaginal discharge in pregnant women with BV was detected earlier [11], however, the study of these data in accordance with varying disease severity has not been conducted. Based on our results, we can conclude that the most important factors of the vagina antibacterial protection in these patients are calprotectin, defensins, and lysozyme.
The study of the antimicrobial activity of the lower reproductive tract discharge in BV patients showed a decrease in the defensin concentration in this group of patients compared to healthy women and patients with vulvovaginal candidiasis [12, 13]. The defensins level was normalized after the course of therapy. The decrease of antimicrobial peptides in BV patients is caused by the unique ability of Gardnerella vaginalis to alter the local immune response. There are no lipopolysaccharides on the gardnerella cell surface, and peptidoglycans are found in low concentrations, and therefore do not lead to the proper activation of Toll-like receptors and the defensins production.
Thus, the production of endogenous antibiotics, which are AMPs, provides proper protection against infectious agents. The decrease in AMP activity leads to a quantitative increase in the facultative vaginal microflora and the depletion of the obligate microflora, which contributes to a more pronounced clinical course of BV.
Conclusion
The development of diagnostic modalities for examination of pregnant women with inflammatory and dysbiotic disorders in the lower reproductive tract, including the indicators of systemic and local immunity, microbiological monitoring with an integral assessment of the vaginal microbiocenosis, will allow to personify therapeutic approaches for each patient.
References
1. Nelson D.B., Rockwell L.C., Prioleau M.D., Goetzl L. The role of the bacterial microbiota on reproductive and pregnancy health. Anaerobe. 2016; 42: 67-73.
2. Cavera V.L., Volski A., Chikindas M.L. The natural antimicrobial subtilosin A synergizes with Lauramide Arginine Ethyl Ester (LAE), ε-poly-L-lysine (Polylysine), clindamycin phosphate and metronidazole, against the vaginal pathogen Gardnerella vaginalis. Probiotics Antimicrob. Proteins. 2015; 7(2): 164-71.
3. Анкирская А.С., Муравьева В.В. Опыт микробиологической диагностики оппортунистических инфекций влагалища. Клиническая микробиология и антимикробная химиотерапия. 2001; 3(2): 190-4. [Ankirskaya A.S., Muravyeva V.V. Experience of microbiological diagnosis of opportunistic vaginal infections. Klinicheskaya mikrobiologiya i antimikrobnaya himioterapiya. 2001: 3(2): 190-4. (in Russian)]
4. Doerflinger S.Y., Throop A.L., Herbst-Kralovetz M.M. Bacteria in the vaginal microbiome alter the innate immune response and barrier properties of the human vaginal epithelia in a species-specific manner. J. Infect. Dis. 2014; 209(12): 1989-99.
5. Mitchell C., Gottsch M.L., Liu C., Fredricks D.N., Nelson D.B. Associations between vaginal bacteria and levels of vaginal defensins in pregnant women. Am. J. Obstet. Gynecol. 2013; 208(2): 132. e1-7.
6. Rittenschober-Böhm J., Waldhoer T., Schulz S.M., Stihsen B., Pimpel B., Goeral K. et al. First trimester vaginal ureaplasma biovar colonization and preterm birth: results of a prospective multicenter study. Neonatology. 2018; 113(1): 1-6.
7. Hoffman M.K., Bellad M.B., Charantimath U.S., Kavi A., Nagmoti J.M., Nagmoti M.B. et al. A comparison of colorimetric assessment of vaginal pH with nugent score for the detection of bacterial vaginosis. Infect. Dis. Obstet. Gynecol. 2017; 2017: 1040984.
8. Nwankwo T.O., Aniebue U.U., Umeh U.A. Syndromic diagnosis in evaluation of women with symptoms of vaginitis. Curr. Infect. Dis. Rep. 2017; 19(1): 3.
9. Redelinghuys M.J., Ehlers M.M., Bezuidenhoudt J.E., Becker P.J., Kock M.M. Assessment of Atopobium vaginae and Gardnerella vaginalis concentrations in a cohort of pregnant South African women. Sex. Transm. Infect. 2017; 93(6): 410-5.
10. Haahr T., Ersbøll A.S., Karlsen M.A., Svare J., Sneider K., Hee L. et al. Appreciable uncertainty regarding benefits and risks in the treatment of bacterial vaginosis to prevent preterm birth. Acta Obstet. Gynecol. Scand. 2017; 96(2): 251-2.
11. Lamont R.F., Keelan J.A., Larsson P.G., Jørgensen J.S. The treatment of bacterial vaginosis in pregnancy with clindamycin to reduce the risk of infection-related preterm birth: a response to the Danish Society of Obstetrics and Gynecology guideline group’s clinical recommendations. Acta Obstet. Gynecol. Scand. 2017; 96(2): 139-43.
12. Catallozzi M., Fraiz L.D., Hargreaves K.M., Zimet G.D., Stanberry L.R., Ratner A.J. et al. Pregnant women’s attitudes about topical microbicides for the prevention and treatment of bacterial vaginosis during pregnancy. Int. J. STD AIDS. 2017; 28(9): 881-6.
13. Хрянин А.А., Решетников О.В. Бактериальный вагиноз. Новая парадигма. Акушерство и гинекология. 2016; 4: 133-9. [Khryanin A.A., Reshetnikov O.V. Bacterial vaginosis: A new paradigm. Akusherstvo i ginekologiya/Obstetrics and Gynecology. 2016; (4): 133-139. (in Russian)] http://dx.doi.org/10.18565/aig.2016.4.133-139
Received 13.12.2017
Accepted 22.12.2017
About the Authors
Karapetyan, Tamara E., MD, senior researcher of the obstetrics department, National Medical Research Center of Obstetrics, Gynecology and Perinatology namedafter Academician V.I. Kulakov, Ministry of Health of Russia.
117997, Russia, Moscow, Ac. Oparina str. 4. Tel.: +79851400618. E-mail: t_karapetyan@oparina4.ru. ORCID ID 0000-0003-0025-3182
Arzumanyan, Vera G., PhD, MD, I.I. Mechnikov Research Institute of Vaccines and Sera, Russian Academy of Medical Sciences.
105064, Russia, Moscow, Malyj Kazennyj pereulok, 5A. Tel.: +74959174900. E-mail: mech.inst@mail.ru
Tyutyunnik, Victor L., PhD, MD, the head of the obstetric physiological department, National Medical Research Center of Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov, Ministry of Health of Russia. 117997, Russia, Moscow, Ac. Oparina str. 4. Tel.: +79039695041. E-mail: tioutiounnik@mail.ru.
Researcher ID B-2364-2015. ORCID ID 0000-0002-5830-5099
Kan, Natalia E., PhD, MD, the head of the obstetric department, National Medical Research Center of Obstetrics, Gynecology and Perinatology named
after Academician V.I. Kulakov, Ministry of Health of Russia.
117997, Russia, Moscow, Ac. Oparina str. 4. Tel.: +79262208655. E-mail: kan-med@mail.ru. Researcher ID B-2370-2015. ORCID ID 0000-0001-5087-5946
Lomova, Natalia A., PhD, researcher of the obstetric department, National Medical Research Center of Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov, Ministry of Health of Russia.
117997, Russia, Moscow, Ac. Oparina str. 4. Tel.: +79161442162. E-mail: n_lomova@oparina4.ru. ORCID ID 0000-0002-6090-586X
Kanyukina, Anna A., a student of A.I. Evdokimov Moscow State University of Medicine and Dentistry.
127473, Russia, Moscow, Delegatskaya str. 20/1. Tel.: +79057312894. E-mail: ya.friend@yandex.ru
For citations: Karapetyan T.E., Arzumanyan V.G., Tyutyunnik V.L., Kan N.E., Lomova N.A., Kanyukina A.A. Bacterial vaginosis and antimicrobial activity during pregnancy. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2018; (8): 85-90. (in Russian)
https://dx.doi.org/10.18565/aig.2018.8.85-90



