Association of red blood cell distribution width and high sensitivity C-reactive protein with polycystic ovarian syndrome
Polycystic ovarian syndrome (PCOS) is a common endocrine disorder characterized by chronic anovulation and hyperandrogenism. C-reactive protein is an acute-phase reactant protein released into the blood within a few hours after tissue injury and inflammation. It is usually a screening marker for intravascular inflammation. Objective: To investigate the association between RDW-CV and high-sensitivity CRP (hsCRP) in PCOS. Materials and methods: In this case-control study, 100 women (50 with PCOS and 50 without PCOS as the control group) were enrolled. We measured the high-sensitivity C-reactive protein (hsCRP) level and red blood cell (RBC) distribution width (RDW-CV) in women with PCOS compared with normal women. Results: The mean age was 28.2 years for POCS and 29.9 years for healthy control. The mean (SD) body mass indexes were 27.5 (3.9) kg/m2 for PCOS and 27.7 (2.8) kg/m2 for healthy women. The significant result was found in mean (SD) LH level which was 4.8 (1.9) IU/L for POCS and 3.1 (1.2) IU/L for control group (p=0.001), while mean (SD) FSH levels were significantly high in normal group 6.9 (2.9) IU/L and lower in the PCOS women 5.5(1.2) IU/L. In addition, prolactin (p=0.3), Hb (p=0.7), E2 (p=0.6), and ET (p=0.6) levels were not significantly different between the two groups. RDW-CV levels were significantly higher in the PCOS women compared with the control individuals (13.4% vs. 11.9%), in addition, hsCRP levels were significantly higher in the PCOS women (1.9 mg/L vs. 0.9 mg/L) (p<0.0001). Conclusion: As a result, there is a significant increase in the levels of hsCRP and RDW-CV in women with PCOS, which may be indicators of cardiovascular disease risk in the future. Authors' contributions: Basima Shamkhi Al-Ghazali, Marwa Faisal Salman – conception and design of the study, statistical analysis; Basima Shamkhi Al-Ghazali, Marwa Faisal Salman, Ahmed Muhi Fahad – data collection and analysis, manuscript drafting; Basima Shamkhi Al-Ghazali – manuscript editing. Conflicts of interest: The authors have no conflicts of interest to declare. Funding: The source of funding – none (author self). Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the College of Medicine, Kufa University (ID: 0028, 04/03/2018). Patient Consent for Publication: All patients provided informed consent for the publication of their data. Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator. For citation: Basima Shamkhi Al-Ghazali, Marwa Faisal Salman, Ahmed Muhi Fahad. Association of red blood cell distribution width and high sensitivity C-reactive protein with polycystic ovarian syndrome. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2023; (7): 148-154 (in Russian) https://dx.doi.org/10.18565/aig.2022.163Basima Shamkhi Al-Ghazali, Marwa Faisal Salman, Ahmed Muhi Fahad
Keywords
Polycystic ovary syndrome (PCOS) is the most common metabolic and endocrine disorder amongst women of reproductive age; it is typified by oligo-ovulation or anovulation, signs of androgen excess and multiple small ovarian cysts [1]. It is a heterogeneous disorder of uncertain etiology. There is strong evidence reported a complex interactions between multifactorial reasons such as genetic, environmental, and behavioral factors [2]. Although, at least 70% of women remain undiagnosed [3]. PCOS affecting approximately (5–10%) of woman of reproductive age (12–45) years old [4].
Female with PCOS are predisposed to increased visceral adiposity that exert it’s effect in a paracrine and endocrine manner via the secretion of markers of inflammation like hsCRP, pro-inflammatory cytokines and various other markers of endothelial inflammation [5]. They have higher concentrations of serum hsCRP as compared to weight correlated women without PCOS [6]. hsCRP is closely related to an elevation in cardiovascular disorder and it is an independent cardiovascular risk factor [7]. hsCRP, especially its activated form in the blood vessel wall, stimulates the expression of various adhesion molecules in the endothelium [8]. Those molecules accelerate vascular inflammatory reactions and may accelerate the development of atherosclerosis [9]. hsCRP levels are closely correlated with the cardiovascular disorders risk; the higher the hsCRP, the greater the risk [10].
Red cell distribution width (RDW-CV) is a recently suggested to be a novel biomarker reflecting a pro-inflammatory condition that has been shown to be predictive of adverse outcomes in multiple cardiovascular disease [11]. RDW-CV may exert a more direct effect in the pathway toward cardiovascular disease. The erythrocytes have a large amounts of free cholesterol. After any pathological change, the membrane may promote atherosclerosis by deposition of free cholesterol to plaques, thereby providing lipid rich membranes to foam cell formation, and by propagation of the inflammatory cascade [12, 13].
In this study, the association of RDW-CV and hsCRP as a pro-inflammatory marker in women with PCOS were studied.
Materials and methods
This is a case control study carried out on PCOS woman attending the fertility clinic of AL Sadder Teaching medical city and the consultant clinic of AL-Zahraa Teaching Hospital, AL-Najaf, Iraq from March 2018 to December 2019. The participation PCOS women in our study expressed their verbal consent and cooperation during period of research.
Fifty-women detected with PCOS by Rotterdam criteria which are hyperandrogenism, clinical (hirsutism or less commonly male pattern alopecia), biochemical (raised free androgen index (FAI) or free testosterone) and polycystic ovaries by ultrasound. Fifty-healthy control (normal ovulation without clinical or biochemical hyperandrogenemia). Inclusion criteria in this study are women in reproductive age group (17–38) years old and the exclusion criteria are:
- Hormonal or insulin-modifying therapy (IMT) or any other medications that affect metabolism, and reproduction, or inflammation like (Aspirin, statins, or any other medication for at least 2 months before blood examination).
- Any known disease, including diabetes, cardiovascular disease, thyroid disease, or current infectious disease.
- Oral contraceptive pill usage.
- Women with known hematological diseases such as hemolytic anemia, and thalassemia.
Detailed history documented including age, menstrual history, drugs history, medical history and smoking habit. Height, and weight were measured to calculate BMI. Trans-vaginal sonographic screening included: baseline u/s using a 7.5 MHz vaginal probe of Siemens US at day two of cycle to confirm ovarian morphology for PCOS, assessment of total follicular number and size for both ovaries, and measurement of endometrial thickness. A 10 ml of venous blood were collected from the antecubital veins of the participants during the follicular phase of early menstrual bleeding (third to sixth day) and centrifuged to collect serum. Sample was used to measure follicle stimulating hormone (FSH), luteinizing hormone (LH), prolactin, estradiol-2 (E2), hemoglobin (Hb), hsCRP, and RDW-CV.
In FSH measurement, the method as followed separated serum or plasma remain at 15–30°C less than 8 hours. Manufacturer recommends frozen specimens can be stored up to six months before testing. Frozen samples thawed only once. A minimum of 0.3 mL serum is needed for FSH. Sample volume for individual test is 55 µl and is run singly. Instrumentation used Abbott IMx System. The materials include: (1) One bottle (6.5 mL) anti-FSH-coated (mouse, monoclonal) microparticles in buffer with protein stabilizers. (2) One bottle (8.5 mL) anti-FSH (goat) alkaline phosphatase-conjugated in buffer with protein stabilizers. (3) One bottle (10 mL) 4-methylumbelliferyl phosphate, 1.2 mM in buffer. (4) One bottle (13 mL) wash buffer containing surfactant. (5) One bottle (4 mL) IMx FSH Mode 1 Calibrator (C). (6) Six bottles (4 mL each) of IMx FSH calibrators. (7) Three bottles (8 mL each) of IMx FSH controls.
In LH test, we used a minimum of 230 µL of plasma/serum is needed for measurement of serum LH on the cobas e 411. A sample volume of 460 µL is preferred in order to allow for single sample repeat analyses. Serum collected using standard sampling tubes or tubes containing separating gel, or Li- , Na-, NH4 + heparin, K3-EDTA and sodium fluoride/potassium oxalate plasma are considered acceptable by the test manufacturer. All samples, calibrators, and QCs on the analyzer measured within 2 hours at 20–25°C prior to measurement. Specimens transported in 2.0 mL cryogenic vials with external screw-caps. These cryovials labeled in accordance with CDC and DLS policies and regulations. Equipment, instrumentation and supplies used are 1. Roche/Hitachi cobas e 411 analyzer (immunoassay analysis). 2. Ovation BioNatural 20-200 μL pipette (VistaLab Technologies, Brewster, NY) 3. Rotator to homogenize samples: Adams Nutator and Fisher Scientific hematology mixer 4. Sample cups (standard) 5. Purified water. 6. Calibrator: LH Calset II, Roche. 7. Control: PreciControl Universal, Roche. 8. Elecsys SysWash: Roche. 9. Assay Tips: Roche. 10. Assay Cups: Roche.
The E2 measure by assess all samples for acceptability using the criteria in label. Thaw all samples at room temperature: Frozen serum samples, QC samples, Internal Standard Working Solutions and Calibrators are allowed to reach room temperature and are homogenized by placing them on the rotator at medium speed for about 1.5 hrs. Placed pipette tips, all patient samples, QC samples and Calibrators on the Hamilton Microlab STAR Let Liquid Handler instrument. Placed all additional reagents on the instrument at the designated positions. Transfer 200 µL of each Calibrator (CC01-CC11), patient samples, QCs, and blanks into appropriate wells of a 96 2.0 mL deep-well plate. Transfered 100 µL of Internal Standard Working Solution to all patient samples, QCs, blanks and calibrators. Covered Sample Plate with ArctiSeal and allow serum and Internal Standard Working Solution to equilibrate using a multivortexer for approximately 45 minutes at room temperature. Centrifuged the Sample Plate for 3 minutes at room temperature and 2000 rpm. Recap sample and QC vials and store remaining samples and QCs at dedicated location in -70°C freezer. The equipment are Eppendorf Centrifuge (Eppendorf, Ramsey, MN), Hamilton Microlab STARLet Liquid Handler with 8-chanel and 96-chanel pipettors (Hamilton Company, Reno, NV), Water Bath- IsoTemp (Fisher Scientific), Glas-Col MultiPulse Vortexer (Glas-Col, Terre Haute, IN), Fisher Digital Multi-tube Vortex Mixer (Fisher Scientific), Eppendorf Repeater Plus Pipetter (Eppendorf, Ramsey, MN), Sato Label Maker CL612e and Label Making Software, 100-µL Positive displacement pipette (Gilson, Inc.), 96-Well, 2-ml square well plates (Seahorse Labware), 96-Well, 2-mL square well Round (Microliter Analytical Supplies INC), Robotic Reservoirs, Convoluted bottom (Thermo Scientific), ArctiSeal 96-Well Square Silicone w/ PTFE Spray Coating (Arctic White LLC), Eppendorf Combitips plus Pipet tips, 5 ml (Eppendorf, Ramsey, MN), Co-RE Tips, 480 standard volume tips (300 µL) with Filters (Hamilton Company, Reno, NV), Orbitron Rotator II (Boekel Scientific, Feasterville, PA), Eppendorf Swingbucket Rotor (Eppendorf, Ramsey, MN), Gilson Pipetman, Serial: W62622K (Gilson, Inc., Middleton, WI), D1000 100–1000 µL volume Diamond for pipetman, (Gilson, Middleton, WI).
One mml of blood in tube with anticoagulation substance, insert in computerize machine (CELL-DYN Ruby) and result as complete blood count include RDW-CV after about 1 minute. Regarding hsCRP, type of sample (serum or plasma) are: Take 5ml from sample and add to R1 (diluent), wait for 30 second. Then, add to it one drop of R2 (conjugate) and wait 20 second. After that, one drop from R3 add (wash) and wait 20 second than read in mg/L. Separated serum or plasma should be removed from the cells within one hour of collection. By used CRPHS reagent kit (300 tests): R1 reagent. TRIS buffer with bovine serum albumin and immunoglobulins (mouse), R2 reagent. Equipment are: Roche Cobas 6000 Chemistry Analyzer (Roche Diagnostics Corporation, Indianapolis), The Millipore Elix Gulfstream Clinical 35 System. Water purification is achieved by reverse osmosis, electrodeionization, bactericidal 254 nm UV lamp and 0.22 μm filtration.
The normal reference ranges for the indicators that are used in the laboratory are:
- hsCRP: a concentration of hsCRP less than 1 mg/L indicates a low risk of cardiovascular disease and its complications, 1–3 mg/L – moderate risk, more than 3 mg/L – high risk of vascular complications in healthy individuals and in patients with cardiovascular disease.
- FSH: Before puberty 0–4 IU/L, during puberty 0.3–10 IU/L, menstruating 4.7–21.5 IU/L, after menopause 25.8–134.8 IU/L, follicular phase 1–9 IU/L, mid-cycle 6–26 IU/L, luteal phase 1–9 IU/L.
- LH: postmenopause 16–66 IU/L, follicular phase 1–12 IU/L, ovulatory 16–104 IU/L, luteal phase 1–12 IU/L.
- Prolactin: Non-pregnant women: less than 25 ng/mL, pregnant women: 80 to 400 ng/mL.
- Hb: 12.1 to 15.1 g/dL.
- Estradiol: 30 to 400 pg/mL for premenopausal women, 0 to 30 pg/mL for postmenopausal women.
- Endometrial thickness (ET): during menstruation 2–4 mm, proliferative 5–7 mm, ovulatory 11 mm, secretory 16 mm, postmenopause ≤5 mm.
- RDW-CV: 11.0–15.0%.
Statistical analysis
Statistical analysis was done by using SPSS program version 20. Independent t-test for comparison of means of study groups. Glass's delta used to effect size measure. P-value equal or less than 0.05 were considered statistically significant.
Results
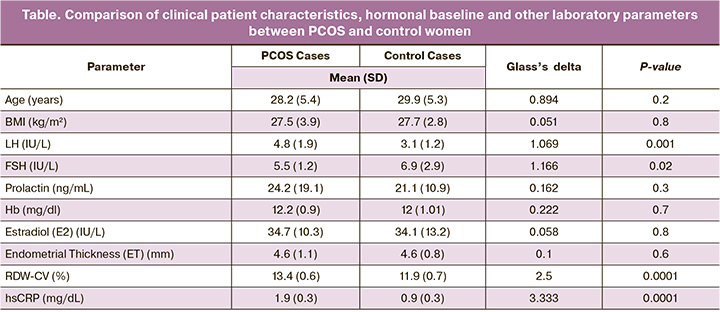
In this study, a 100 women were included, 50 women with PCOS and 50 were control. The mean age was 28.2 years for POCS and 29.9 years for healthy control. The mean (SD) body mass indexes were 27.5(3.9) kg/m2 for PCOS and 27.7(2.8) kg/m2 for healthy women. The significant result was found in mean (SD) LH level which was 4.8(1.9) IU/L for POCS and 3.1(1.2) IU/L for control group (P=0.001), while mean (SD) FSH levels were significantly high in normal group 6.9(2.9) IU/L and lower in the PCOS women 5.5(1.2) IU/L (P=0.02). In addition, prolactin (P=0.3), Hb (P=0.7), E2 (P=0.6) and ET (P=0.6) showed no significant differences between both groups. RDW-CV levels were significantly higher in the PCOS women compared with the control individuals (13.4% vs. 11.9%), in addition, hsCRP levels were significantly higher in the PCOS women (1.9 mg/L vs. 0.9 mg/L) (P<0.0001), (Table).
Discussion
hsCRP has long been used as a marker of inflammation in the body [14]. High hsCRP levels are found practically in every inflammatory state, it may signal an elevated risk for all degenerative diseases, like cardiovascular disease (CVD), cancer, and DM [15]. The present study found that hsCRP levels are elevated in women with PCOS when compared with the controls non PCOS women (P=0.0001). High hsCRP levels may explain why some PCOS women may possibly be at an increased morbidity for the development of early-onset CVD [16].
Verit F. (2010) did a study on 52 normo-insulinemic PCOS women without metabolic syndrome and 48 control found that PCOS patients had increased C-RP (P<0.0001) and hsCRP was positively associated with BMI. Elevated hsCRP was associated with CVD risk factors in normo-insulinemic PCOS without metabolic syndrome. She recommend that these patients need more intensive screening or treatment for PCOS [17].
Other study done by Ramanand S. et al., on 30 healthy women and 88 PCOS women. They found that serum hsCRP is raised in Indian PCOS women reflecting association of low grade chronic inflammation; the obesity may aggravate these associations. A positive association between hsCRP and total cholesterol, and low density lipoprotein in the background of normal lipid profile is suggestive of precedence of chronic inflammation over dyslipidemia in PCOS [18]. Escobar-Morreale H. et al., found that women with PCOS exhibit elevation in circulating hsCRP that is independent of obesity. These findings corroborates existing molecular evidence of the chronic low-grade inflammation that may underpin the pathogenesis of PCOS [19].
Furthermore, Javedani M. et al., measured hsCRP in PCOS woman, their results showed that hsCRP was higher in PCOS compared to control, the difference was not statistically significant [20]. In addition, Oh J. et al., found that PCOS by itself is not correlated with increased hsCRP levels, but rather, documented risk factors for CVD such as BMI, blood pressure, and insulin sensitivity affect hsCRP levels in PCOS [21]. Iuhas C. et al., matched for age and BMI but without PCOS (control group), they found that hsCRP levels are elevated in PCOS group and are correlated with obesity, fat accumulation and not with the presence of PCOS itself [22].
The present study found that RDW-CV significantly increase in PCOS women when compare with non PCOS women (P<0.0001). RDW-CV is a numerical measure of the size variability of circulating erythrocytes. Recently, many authors reported that RDW-CV is a strong and independent predictor of adverse outcomes in population and its increment is also thought to be closely related to the risk of CVD morbidity [23, 24].
The results are consistent with a study done by Yilmaz Ö. et al., they found that RDW-CV levels were higher in PCOS women, and high-RDW-CV levels were independently related with PCOS [25].
Zalawadiya S. et al., found RDW-CV is commonly elevated in conditions associated with increased red blood cell destruction (hemolytic anemias) or defective erythropoiesis e.g., nutritional deficiencies of iron, folic acid, and vitamin B12 or blood transfusion. Similarly, they also confirmed a linear raised in RDW-CV with older age. Thus, increased RDW-CV might be a surrogate composite measure of different pathophysiologic processes (i.e., chronic inflammation, greater oxidative stress, nutritional deficiencies, and aging), might play an important role in the etio-pathogenesis of adverse cardiovascular events [26].
Conclusion
There is a significant increase in level of hsCRP and RDW-CV in women with PCOS which may be an indicators of cardiovascular disease risks in the future.
References
- Schrage J., Schaffer J. Polycystic ovarian syndrome and hyperandrogenism. In: Williams gynecology. 2nd ed. China: McGraw–Hill companies; 2008: 383-401.
- Bargiota A., Diamanti-Kandarakis E. The effects of old, new and emerging medicines on metabolic aberrations in PCOS. Ther. Adv. Endocrinol. Metab. 2012; 3(1): 27-47. https://dx.doi.org/10.1177/2042018812437355.
- Tomlinson J.A., Pinkney J.H., Evans P., Millward A., Stenhouse E. Screening for diabetes and cardio-metabolic disease in women with polycystic ovary syndrome. The British Journal of Diabetes & Vascular Disease. 2013; 13(3): 115-23. https://dx.doi.org/10.1177%2F1474651413495571.
- Goldenberg N., Glueck C. Medical therapy in women with polycystic ovarian syndrome before and during pregnancy and lactation. Minerva Ginecol. 2008; 60(1): 63-75.
- Carmina E., Bucchieri S., Esposito A., Del Puente A., Mansueto P., Orio F. et al. Abdominal fat quantity and distribution in women with polycystic ovary syndrome and extent of its relation to insulin resistance. J. Clin. Endocrinol. Metab. 2007; 92(7): 2500-5. https://dx.doi.org/10.1210/jc.2006-2725.
- Makedos A., Goulis D.G., Arvanitidou M., Mintziori G., Papanikolaou A., Makedou A., Panidis D. Increased serum C-reactive protein levels in normal weight women with polycystic ovary syndrome. Hippokratia. 2011; 15(4): 323-6.
- Park R., Detrano R., Xiang M., Fu P., Ibrahim Y., LaBree L., Azen S. Combined use of computed tomography coronary calcium scores and C-reactive protein levels in predicting cardiovascular events in nondiabetic individuals. Circulation. 2002; 106(16): 2073-7. https://dx.doi.org/10.1161/01.cir.0000033819.29662.09.
- Lagrand W.K., Visser C.A., Hermens W.T., Niessen H.W., Verheugt F.W., Wolbink G.J., Hack C.E. C-reactive protein as a cardiovascular risk factor: more than an epiphenomenon? Circulation. 1999; 100(1): 96-102.https://dx.doi.org/10.1161/01.cir.100.1.96.
- Pasceri V., Willerson J.T., Yeh E.T. Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation. 2000; 102(18): 2165-8.https://dx.doi.org/10.1161/01.cir.102.18.2165.
- Jialal I., Stein D., Balis D., Grundy S.M., Adams-Huet B., Devaraj S. Effect of hydroxymethyl glutaryl coenzyme a reductase inhibitor therapy on high sensitive C-reactive protein levels. Circulation. 2001; 103(15): 1933-5.https://dx.doi.org/10.1161/01.cir.103.15.1933.
- Tonelli M., Sacks F., Arnold M., Moye L., Davis B., Pfeffer M.; for the Cholesterol and Recurrent Events (CARE) Trial Investigators. Relation between red blood cell distribution width and cardiovascular event rate in people with coronary disease. Circulation. 2008; 117(2): 163-8. https://dx.doi.org/10.1161/CIRCULATIONAHA.107.727545.
- Kolodgie F.D., Gold H.K., Burke A.P., Fowler D.R., Kruth H.S., Weber D.K. et al. Intraplaque hemorrhage and progression of coronary atheroma. N. Engl. J. Med. 2003; 349(24): 2316-25. https://dx.doi.org/10.1056/NEJMoa035655.
- Arbustini E., Morbini P., D'Armini A.M., Repetto A., Minzioni G., Piovella F. et al. Plaque composition in plexogenic and thromboembolic pulmonary hypertension: the critical role of thrombotic material in pultaceous core formation. Heart. 2002; 88(2): 177-82. https://dx.doi.org/10.1136/heart.88.2.177.
- Tillett W.S., Francis T. Serological reactions in pneumonia with a non-protein somatic fraction of pneumococcus. J. Exp. Med. 1930; 52(4): 561-71.https://dx.doi.org/10.1084/jem.52.4.561.
- Trichopoulos D., Psaltopoulou T., Orfanos P., Trichopoulou A., Boffetta P. Plasma C-reactive protein and risk of cancer: a prospective study from Greece. Cancer Epidemiol. Biomarkers Prev. 2006; 15(2): 381-4.https://dx.doi.org/10.1158/1055-9965.EPI-05-0626.
- Boulman N., Levy Y., Leiba R., Shachar S., Linn R., Zinder O., Blumenfeld Z. Increased C-reactive protein levels in the polycystic ovary syndrome: a marker of cardiovascular disease. J. Clin. Endocrinol. Metab. 2004; 89(5): 2160-5.https://dx.doi.org/10.1210/jc.2003-031096.
- Verit F.F. High sensitive serum C-reactive protein and its relationship with other cardiovascular risk factors in normoinsulinemic polycystic ovary patients without metabolic syndrome. Arch. Gynecol. Obstet. 2010; 281(6): 1009-14. https://dx.doi.org/10.1007/s00404-009-1226-6.
- Ramanand S.J., Ramanand J.B., Raparti G.T., Ghanghas R.R., Halasawadekar N.R., Patil P.T. et al. High sensitivity C-reactive protein (hs-CRP) and clinical characteristics, endocrine, metabolic profile in Indian women with PCOS: a correlation. Int. J. Reprod. Contracept. Obstet. Gynecol. 2014;3(1): 118-26.
- Escobar-Morreale H.F., Luque-Ramírez M., González F. Circulating inflammatory markers in polycystic ovary syndrome: a systematic review and meta-analysis. Fertil. Steril. 2011; 95(3): 1048-58.e1-2. https://dx.doi.org/10.1016/j.fertnstert.2010.11.036.
- Javedani M., Sheibani H., Madadi Y., Yoonesi L. Relationship between c-reactive protein and carotid artery intima media thickens in polycystic ovarian syndrome patients. Acta Medica Mediterranea. 2015; 31(7): 1393-8. Available at:http://eprints.iums.ac.ir/id/eprint/5245
- Oh J.Y., Lee J.A., Lee H., Oh J.Y., Sung Y.A., Chung H. Serum C-reactive protein levels in normal-weight polycystic ovary syndrome. Korean J. Intern. Med. 2009; 24(4): 350-5. https://dx.doi.org/10.3904/kjim.2009.24.4.350.
- Iuhas C., Costin N., Mihu D. High-sensitivity C-reactive protein in patients with polycystic ovary syndrome. Romanian Journal of Diabetes Nutrition and Metabolic Diseases. 2012; 19(4): 389-96. Available at: https://rjdnmd.org/index.php/RJDNMD/article/view/256
- Perlstein T.S., Weuve J., Pfeffer M.A., Beckman J.A. Red blood cell distribution width and mortality risk in a community-based prospective cohort. Arch. Intern. Med. 2009; 169(6): 588-94. https://dx.doi.org/10.1001/archinternmed.2009.55.
- Patel K.V., Ferrucci L., Ershler W.B., Longo D.L., Guralnik J.M. Red blood cell distribution width and the risk of death in middle-aged and older adults. Arch. Intern. Med. 2009; 169(5): 515-23. https://dx.doi.org/10.1001/archinternmed.2009.11.
- Yilmaz Ö., Mehmet C., Kelekci S., Temur M. Association between red blood cell distribution width and polycystic ovary syndrome. Endocr. Res. 2015; 40(4): 181-7. https://dx.doi.org/10.3109/07435800.2014.987398.
- Zalawadiya S.K., Veeranna V., Niraj A., Pradhan J., Afonso L. Red cell distribution width and risk of coronary heart disease events. Am. J. Cardiol. 2010; 106(7): 988-93. https://dx.doi.org/10.1016/j.amjcard.2010.06.006.
Received 12.07.2022
Accepted 04.07.2023
About the Authors
Basima Shamkhi Al-Ghazali, Department of Gynecology, College of Medicine, Kufa University, Iraq, https://orcid.org/0000-0002-5149-2848Marwa Faisal Salman, Department of Gynecology, Al-Najaf Health Directorate, Ministry of Health, Al-Najaf, Iraq.
Ahmed Muhi Fahad, Department of Cardiothoracic and Vascular Surgery, Al-Sadder Teaching Medical City, Al-Najaf Health Directorate, Ministry of Health, Al-Najaf, Iraq, +9647832495436, ayam.mohammad@yahoo.com, https://orcid.org/0000-0003-3748-681X
Corresponding author: Ahmed Muhi Fahad, ayam.mohammad@yahoo.com



