Molecular genetic and endocrine predictors of menstrual cycle regulation in patients with polycystic ovary syndrome undergoing metformin treatment
Objective: To identify clinical, laboratory and molecular genetic predictors of menstrual circle regulation in patients with polycystic ovary syndrome (PCOS) undergoing metformin treatment.Chernukha G.E., Naidukova A.A., Kaprina E.K., Miroshina E.D., Donnikov A.E.
Materials and methods: The study included 143 women with PCOS (mean age is 26.4±4.6 years, mean body mass index is 23.8 (4.8) kg/m2). The assessment of androgen profile and levels of AMH, LH, FSH was performed before and 6 months after the treatment. Also, 2-hour oral glucose tolerance test with insulin level examination and dual-energy X-ray absorptiometry were done. Single-nucleotide polymorphisms (SNPs) were genotyped using polymerase chain reaction and next generation sequencing for 45 loci. All patients were administered metformin (Glucophage Long) 1500 mg/day with dose titration for 6 months. Depending on the response to the therapy, the patients were divided into two groups: group 1 included 70 (53.1%) patients whose menstrual cycle was regulated, group 2 consisted of 48 (36.3%) patients without any effect of therapy; 14 (10.6%) patients with partial response to therapy were not included in the analysis of predicting the effectiveness of the treatment. Statistical analysis was carried out with SPSS Statistics 21. Parametric tests were used for the analysis of clinical data; odds ratio was calculated for the analysis of molecular genetic data.
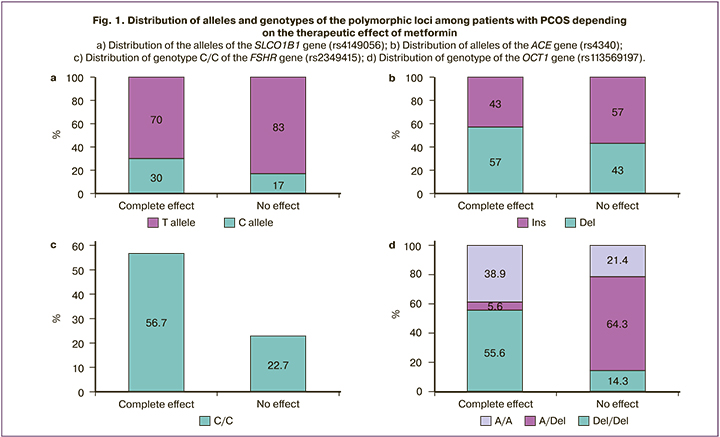
Results: The following independent predictors of the effectiveness of metformin therapy in PCOS were revealed: AMH level less than 13.3 ng/ml, total testosterone level less than 1.81 ng/ml, index of adipose tissue distribution A/G less than 0.90, as well as polymorphism of loci in the genes SLCO1B1 (rs4149056), ACE (rs4340), FSHR (rs2349415), OST1 (rs113569197). The model which was developed for predicting menstrual cycle regulation in patients with PCOS undergoing metformin therapy included the baseline level of AMH and rs2349415 SNPs of FSHR gene.
Conclusion: The most significant factors determining metformin effectiveness in PCOS patients were AMH level and genotype С/С of FSHR (rs2349415).
Keywords
Nowadays polycystic ovary syndrome (PCOS) is considered to be a polygenic endocrine disorder associated with reproductive and metabolic disorders which are caused mainly by insulin resistance (IR) and hyperinsulinemia. The incidence of PCOS reaches 18–20% in female population [1] and it exceeds 30% in the patients with type 2 diabetes mellitus, obesity and metabolic syndrome [2, 3]. The administration of insulin sensitizers can be due to the fact that IR is a key factor in the development of PCOS and its delayed complications. Such medications as biguanides (metformin), thiazolidinediones (pioglitazone, rosiglitazone) and inositols (D-chiro-inositol and myo-inositol) have been used in clinical practice. Metformin is the most widely used medication in clinical practice; it is effective and it is not associated with serious side effects, oncological and cardiovascular risks which are characteristic of thiazolidinediones. However, metformin may often cause dyspeptic disorders which can be controlled by prescribing prolonged treatment and titrating the medication dose.
According to the international evidence-based guideline for the assessment and management of PCOS and the recommendations of the Russian Society of Obstetricians and Gynecologists (2021), metformin should be administered only to patients with a body mass index (BMI) of more than 25 kg/m2 as a second-line therapy in case of intolerance to combined hormonal contraceptives or induction of ovulation in patients with resistance to clomiphene citrate [4]. However, the results of some studies indicate a high efficacy of metformin in patients without IR. The reproductive function of such patients may be restored due to the inhibitory effect of metformin on the function of granulosa and teka cells which is caused by normalization of the mitochondrial respiratory chain function and associated with a subsequent decrease in the secretion of anti-muller hormone (AMH) and androgens [5–7]. Metformin is also known to lead to an increase in the diversity of the intestinal microbiota and a change in its qualitative composition with a predominance of bacteria producing short-chain fatty acids; such a change contributes to a decrease in the permeability of the intestinal wall, control of the chronic inflammatory response and IR correction [8, 9].
One of the previous studies showed that every second patient with PCOS had her regular menstrual rhythm restored after taking metformin [10]. According to N. Schweighofer et al. (2014) metformin was effective only in every third patient with PCOS [11]. Though metformin therapy is not effective enough, a personalized approach to its administration has not been developed.
The objective of the study is to identify clinical, laboratory and molecular genetic predictors of menstrual circle regulation in patients with PCOS undergoing metformin treatment.
Materials and methods
The prospective study which was aimed at developing a model for predicting the effectiveness of metformin included 143 women with PCOS (mean age is 26.4±4.6 years, mean body mass index is 23.8 (4.8) kg/m2). The inclusion criterion was the presence of PCOS (Rotterdam Criteria, 2003). The informed consent to participate in the study was obtained from all the patients. The patients who had concomitant endocrine pathology and took hormone therapy for three months prior to the study were not eligible for inclusion in the study. Ultrasound examination of the pelvic organs was performed using with a 7.5-MHz transducer (SSA-240, Toshiba, Japan). On the second or third day of the menstrual cycle, there was an assessment of the levels of luteinizing hormone (LH), follicle-stimulating hormone (FSH), prolactin, thyroid-stimulating hormone, total and free testosterone, androstenedione, sex hormone binding globulin (SHBG), (using the immuno-chemiluminescent method, Immulite 2000, Siemens, USA) and AMH level (AMH Gen II ELISA, Beckman Coulter, USA). The metabolic status was assessed using a two-hour oral glucose tolerance test with 75 g of glucose, insulin secretion was detected three times on exertion. The patients were also assessed for their body composition using dual-energy X-ray densitometry (Lunar, USA).
The analysis of gene polymorphism was carried out in the Laboratory of Molecular and Genetic Methods at the National Medical Research Center for Obstetrics, Gynecology and Perinatology, Moscow, Russia. All patients were genotyped for 45 loci using polymerase chain reaction with the analysis of melting curves or targeted sequencing with the IonS5 system, Ion 520 and 530 chips. We identified polymorphisms associated with impaired androgen biosynthesis (CYP2C9* rs1057910, rs1799853; CYP17A1* rs743572; СYР19A1* rs936306), folliculogenesis (FSHR# rs12994034, rs17038027, rs6166, rs2349415, rs148976413; DENND1A# rs2479106, rs10818854; YAP1# rs1894116; AMН# rs10407022; LНCGR# rs12470652, rs13405728), carbohydrate metabolism (THADA# rs12478601, rs78635447, rs12468394; INSR* rs2059807, rs3815902; FTO* rs9939609, rs8050136; KCNJ11* rs5219; PPARGC1A* rs8192678; ОСТ1# (rs6282031, rs34130495, rs72552763, rs113569197; SLCO1B1* rs4149056; Rub5B/SUOX# rs705702; IRS1* rs1801278; TCF7L2* rs7903146, rs12255372; PPARA* rs4253778; LEP * rs7799039) and others (C9orf3# rs4385527; AGTR1* rs5186; GNB3* rs5443; IL1B* rs1143627; UCP2* rs660339; ATM* rs11212617, rs683369; END1* rs5370; ACE* rs4340; HGMA2# rs2272046).
All patients were administered metformin (Glucophage® Long, Merck Serono) 1500 mg/day with dose titration for 6 months. Due to low compliance or side effects, 11 patients were excluded from the study. The study was completed by 132 patients. Depending on the response to the therapy, the patients were divided into two groups: group 1 included 70 (53.1%) patients whose menstrual cycle was regulated, group 2 consisted of 48 (36.3%) patients without any effect of therapy; 14 (10.6%) patients with partial response to therapy (less than 5 menstruations for 6 months) were not included in the analysis of predicting the effectiveness of the treatment.
Statistical analysis
Statistical analysis was performed using the SPSS program (IBM Statistical Package for the Social Sciences, version 21). The Kolmogorov–Smirnov test showed Gaussian distribution of data, quantitative indicators are presented as the arithmetic mean and standard deviation (M(SD)). The quantitative parameters were compared using the Student’s t-test. The qualitative characteristics were compared using the Fisher criterion. The odds ratio (OR), as a symmetric value, was used to quantify risk in genetic studies. The odds ratio was determined with a 95% confidence interval (CI) which was calculated using the Wilson method. This method makes it possible to estimate confidence intervals for very small and very large frequencies, and it is acceptable for small-volume samples. The results were considered statistically significant at p-value<0.05.
When constructing a binary logistic regression model, the method of reverse selection was used. The presence or absence of cycle regulation after metformin therapy was considered as an outcome, and clinical and laboratory and molecular genetic characteristics of the patients were considered as predictors. The quality of regression model approximation at each subsequent step was evaluated using the likelihood function, namely, the negative double value of the logarithm of this function (-2LL). The quality of the obtained models was evaluated using ROC analysis of the area of the ROC curve. The area under the curve (AUC) was given with 95% confidence interval (CI). The part of the variance which could be explained with the help of logistic regression was calculated using the Nadelkerke R-Square method. A threshold value was traditionally considered as a 50% probability of outcome. Since it was a pilot study, and no data could be found in the literature for an adequate assessment of the expected effect, the sample size was not calculated.
Results
In order to study the effectiveness and predict the success of metformin therapy, we compared and analyzed the clinical and laboratory characteristics of patients with and without menstrual cycle regulation after metformin therapy (Table 1).
Before the administration of the therapy, oligomenorrhea was observed in 81.4% of patients with subsequent menstrual rhythm regulation and in 79.2% of patients without the response to metformin therapy (p>0.05); the frequency of amenorrhea was 18.6 and 20.8%, respectively (p>0.05).
The study groups did not differ in BMI, the rate of obesity, indicators of glycemia, fasting insulin, the frequency of glucose tolerance disorders, IR, hyperinsulinemia (p>0.05). Among the indicators of the hormonal profile in the group of patients whose menstrual cycle was regulated, there were lower baseline levels of AMH (p=0.001) and total testosterone (p=0.015); although no differences were found in the diversity of PCOS phenotypes (Table 1). However, patients whose menstrual cycle was regulated had a lower percentage of android adipose tissue (p=0.02) and the lowest value of index A/G (p=0.002) reflecting the ratio of android adipose tissue to gynoid one.
The ROC analysis showed that menstrual cycle regulation is most likely to occur at the initial level of AMH<13.3 ng/ml (AUC=0.70 (CI: 0.60–0.81)), the level of total testosterone<1.81 ng/ml (AUC=0.62 (CI: 0.52–0.73)) and index A/G<0.90 (AUC=0.69 (CI: 0.57–0.82)).
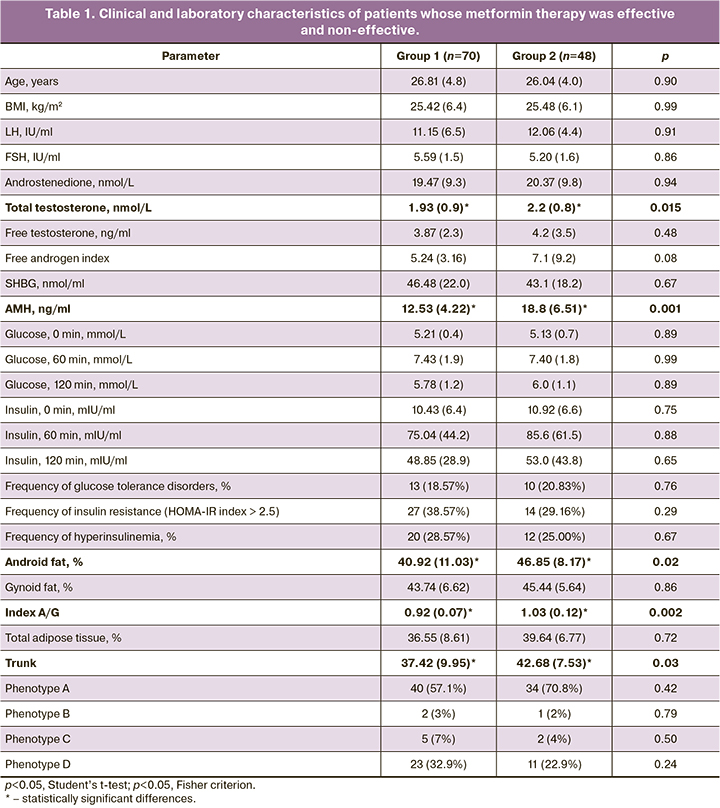
In order to develop a personalized approach to prescribing metformin therapy for PCOS, a comparative analysis was carried out on 45 polymorphic loci of patients, depending on the therapeutic effect. The genetic panel included loci associated with impaired folliculogenesis, carbohydrate metabolism and hyperandrogenism, according to the results of previous foreign molecular genetic studies. The obtained results indicate the association of the therapy effect with four polymorphic loci: SLCO1В1 (rs4149056), АСЕ (rs4340), FSHR (rs2349415), ОСТ1 (rs113569197) (Fig. 1). A comparative analysis of the frequency of SLCO1B1 allele (rs4149056) showed that C allele was more common (42/140, 30.0%) among patients with the full effect of metformin therapy than in the group without its effect (15/87, 17.0%), p=0.022. The frequency of allele T in SLCO1B1 gene (rs4149056) in the study groups was 72/87 (83%) and 98/140 (70.0%), respectively. Due to the small sample size, the mode of inheritance could not be established (Fig. 1a).
The analysis of ACE gene polymorphism (rs4340) showed that Del allele (80/140, 57.0%) was more common in patients whose menstrual cycle was regulated than Ins allele (60/140, 43.0%) in comparison with patients without the effect of therapy. The patients who had no response to therapy had the frequency of Del allele 39/90 (43.0%) and Ins allele 51/90 (57.0%), p=0.04. The differences in the distribution of genotypes were not statistically significant (p=0.80, Fig. 1b).
The patients who had a response to metformin therapy showed the frequency of the C/C genotype 17/30 (56.7%) and carriers of T/* 13/30 (43.3%); the patients who had no effect of metformin therapy showed the frequency of the C/C genotype 5/22 (22.7%) and the genotypes of T/* 17/22 (77.2%), (p=0.01; OR=4.45 (95% CI: 1.30–15.23)). The women with the C/C genotype demonstrate a 4.5-fold greater chance of regulating the menstrual cycle due to metformin therapy compared to the ones with allele T (C/T and T/T genotypes, Fig. 1c).
The patients with a complete response to the therapy were more likely to have the Del/Del genotype (10/18, 55.6%) than the A/* genotypes (8/18, 44.5%) of the OST1 gene (rs113569197) in comparison with patients without the effect of therapy who showed the frequency of these genotypes as 2/14 (14.3%) and 12/14 (85.7), respectively, (p=0.02; OR=7.50 (95% CI: 1.29–43.69)).
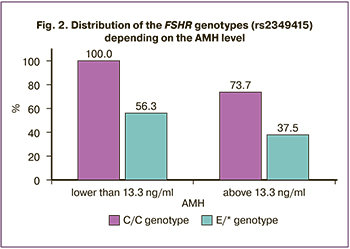
A mathematical model was created on the basis of the obtained data to predict the menstrual cycle regulation in patients with PCOS undergoing metformin treatment. According to the results of regression analysis, the FSHR genotype (rs2349415) and the initial level of AMH are factors determining the success of therapy (AUC=0.75 (95% CI: 0.66–0.85), sensitivity is 83%, specificity is 60%). The method of logistic regression was used to select a function that includes the initial serum AMH level and the FSHR genotype (rs2349415) and characterizes the likelihood of menstrual cycle regulation after the treatment. The part of the variance which could be explained with the help of logistic regression was 72.1% (it was calculated using the Nadelkerke R-Square method).
The coefficient values in the logistic regression and the verification of their significance are presented in Table 2.
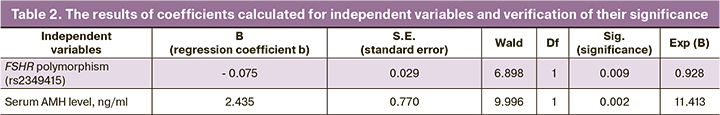
The classifying discriminant function is presented as:
Z=2.435×АМH – 0.075×FSHR + 4.69,
where FSHR is the number of C alleles in the locus -521T>C of the SLCOB1 gene; AMH is the serum AMH level before therapy.
The probability of the menstrual cycle regulation after metformin therapy (p) was determined by the formula p=1/(1+e-z). When the p value is greater than 0.5 (50% probability of outcome), the complete effect of metformin therapy can be assumed; when the p value is less than 0.5, the success of therapy is unlikely.
The accuracy of predicting the success of metformin therapy in PCOS using independent variables is 70.9%. The obtained model was evaluated with the help of the ROC-analysis method. Sensitivity was 83% (71–93%), specificity was 60% (48–80%), the area under the curve for this model was 0.755, 95% CI: 0.664–0.847 (p=3.5×10-13).
Discussion
Heterogeneity of clinical and endocrine-metabolic features of PCOS significantly complicates the diagnosis and search for optimal treatment regimens; isolation of reproductive phenotypes does not make it possible to personalize approaches to the management of patients. Traditionally, combined hormonal contraceptives are used as the first line therapy for PCOS; their administration contributes to the correction of biochemical hyperandrogenism, reducing the clinical manifestations of androgenization and menstrual cycle regulation. However, there are some patients with PCOS who have metabolic disorders that require appropriate correction or patients who cannot take hormonal contraceptives, who have contraindications to their use or side effects from their use, as well as those who are interested in achieving conception without the use of assisted reproductive technologies. Therefore, alternative therapy regimens are considered appropriate.
Insulin sensitizers, in particular, metformin, can be administered as the second-line therapy for PCOS. It should be noted that research on the study of IR as a pathogenetic mechanism of PCOS development was started in the 80s of the last century. Currently, metformin is administered to patients with PCOS if they have IR, hyperinsulinemia, impaired glucose tolerance and obesity. It is believed that the restoration of tissue sensitivity to insulin under the influence of metformin is associated with inhibition of cytochrome P450c17a in the ovaries, a decrease in the basal level of 17α-hydroxyprogesterone and androgen synthesis in the ovaries and adrenal glands [12]. After metformin therapy, there is also a regulation of synthesis of protein which binds insulin-like growth factor (IGFBP-1) by granulosa cells and a decrease in free biologically active IGF-1 fractions which may also contribute to the suppression of androgen synthesis by the theca cells [13]. Some of these effects are mediated by the stimulating effect of metformin on AMP-activated protein kinase (AMPK); its activity is reduced in patients with type 2 diabetes mellitus, metabolic syndrome and obesity [14]. Metformin is also known to have insulin-independent effects. They include inhibition of steroidogenesis by suppressing complex I of the mitochondrial respiratory chain, decreased expression of the StAR and SUR171a genes [15], increased activity of the hepatic kinase B1 (LKB1/AMRK) signaling pathway [16], decreased activity of mitogen-activated protein kinase (MARK -signaling pathway), in particular ERK1/2, normalization of the intestinal microbiota, as well as a direct effect on the secretion of gonadotropin-releasing hormone and LH [17]. These mechanisms may cause the normalization of the function of the theca cells, granulosa, cumulus and oocyte itself and explain the decrease in the level of AMH, correction of hyperandrogenism and reproductive function disorders, including the menstrual cycle regulation in patients with normal body weight even without IR [18, 19]. This can explain the effect of metformin which can regulate the menstrual cycle in patients with PCOS included in our study. Its effect did not depend on BMI, the presence and severity of IR, hyperinsulinemia and impaired glucose tolerance. According to some data, the effectiveness of metformin therapy in women with BMI less than 25 kg/m2 can reach 41.65% [20]; these findings are consistent with the results of our study. It is also worth noting that according to some data, metformin therapy at a dose of 1500 mg/day leads to a more noticeable decrease in the level of total testosterone and androstenedione in patients with normal BMI than with obesity [20].
Despite the extensive use of metformin in clinical practice, the issue of predicting the effectiveness of therapy at the stage of its administration has not been solved yet. The studies on this issue are few and quite contradictory. Thus, there is evidence in the literature that metformin therapy was more effective in severe hypersecretion of LH [21]; there are also indications that AMH level can determine the therapeutic effect of metformin in PCOS [7, 22]. The present study did not reveal the correlation of the initial level of LH with the regulation of the menstrual cycle; however, we obtained data on the high probability of cycle regulation at the level of total testosterone in the blood serum <1.81 ng/ml and the level of AMH<13.3 ng/ml. This may be due to both IR correction and suppression of granulosa cell activity.
The results of our study also showed that the such polymorphic loci as SLCO1B1 (rs4149056), OCT1 (rs113569197), FSHR (rs2349415), ACE (rs4340) can influence the outcomes of therapy. The literature that was available to us does not provide the data on the effect of SLCO1B1 polymorphism on the metabolism and action of metformin, however, according to the results of the genetic study performed by the Russian scientists, this locus is a candidate gene for PCOS [23]. A genome-wide study showed that carriers of the polymorphic locus SLCO1B1 (rs4149056) have a lower level of SHBG [24]. We revealed that allele C of the SLCO1B1 gene (rs4149056) is associated with the menstrual cycle regulation during six months of metformin therapy. Polymorphism of SLCO1B1 (rs4149056) is likely to lead to an increase in biologically active androgen fractions and hyperandrogenism development in PCOS by reducing the synthesis of SHBG. Metformin therapy is known to result in an increase in the level of SHBG and correction of the androgenic profile.
We have not been able to identify possible mechanisms determining the correlation of the ACE gene (rs4340) with the biological effects of metformin in PCOS; however, according to some foreign studies, this polymorphic locus is associated with hyperandrogenism and may increase the risk of PCOS [25].
The ovarian function is known to be interconnected with polymorphisms of the FSHR gene; variant loci can determine both a higher level of endogenous FSH and a longer duration of the follicular phase of the menstrual cycle, and a lower sensitivity to FSH, which requires higher doses of exogenous FSH in the protocols of assisted reproductive technologies [26, 27]. The results of the study showed that carriers of the C/C genotype of the FSHR gene (rs2349415) have nearly a five-fold increase in the chances of menstrual cycle regulation in comparison with carriers of the T allele. According to the results which were published previously, this polymorphic locus can determine a higher level of FSH and a lower level of estradiol [10]. However, its relationship with the outcomes of ovulation stimulation in patients with diseases of the reproductive system has not been studied yet.
Another locus associated with the menstrual cycle regulation during metformin therapy is the OCT 1 gene (rs113569197) (organic cation transporter). Previous studies showed that metformin is a substrate of OCT1 and OCT2 specific to the liver and kidneys [28]; the activity of these genes mediates its pharmacokinetics and pharmacodynamics [29]. Scientists from China showed the relationship of OCT1 polymorphism (rs683369 and rs628031) with the effectiveness of metformin, although they did not reveal the effect of the polymorphic locus on the risks of PCOS development [30]. Another study confirmed the risk of developing the syndrome in carriers of OCT1 (rs628031) which may be due to the high interethnic variability of the structure of this gene (http://ensembl.org). A mathematical model was created on the basis of the results of the study. The model makes it possible to predict the effectiveness of metformin therapy taking into account the C/C genotype of FSHR (rs2349415) and serum AMH levels in women with PCOS.
Conclusion
Thus, metformin therapy at a dose of 1500 mg / day makes it possible to restore the regular menstrual cycle in every second patient with PCOS without prescribing hormone therapy. There are four polymorphic loci that can be considered as molecular genetic markers. They are the genes FSHR (rs2349415), SLCO1B1 (rs4149056), ACE (rs4340), OCT1 (rs113569197). One can assume that the use of the mathematical model including the FSHR genotype (rs2349415) and serum AMH level of less than 13.3 ng/ml at the stage of choosing a therapy method will make it possible to identify a group of patients with a potentially high probability of menstrual cycle regulation in response to metformin.
References
1. Neven A.C.H., Laven J., Teede H.J., Boyle J.A. A summary on polycystic ovary syndrome: Diagnostic criteria, prevalence, clinical manifestations, and management according to the latest international guidelines. Semin. Reprod. Med. 2018; 36(1): 5-12. https://dx.doi.org/10.1055/s-0038-1668085.
2. Cassar S., Misso M.L., Hopkins W.G., Shaw C.S., Teede H.J., Stepto N.K. Insulin resistance in polycystic ovary syndrome: A systematic review and meta-analysis of euglycaemic-hyperinsulinaemic clamp studies. Hum. Reprod. 2016; 31(11): 2619-31. https://dx.doi.org/10.1093/humrep/dew243.
3. Kollmann M., laritsch P., Martins W.P., Guenther F., Schneider V., Herzog S.A. et al. Maternal and neonatal outcomes in pregnant women with PCOS: Comparison of different diagnostic definitions. Hum. Reprod. 2015; 30(10): 2396-403. https://dx.doi.org/10.1093/humrep/dev187.
4. Министерство здравохранения Российской Федерации. Клинические рекомендации. Синдром поликистозных яичников. 2021: 1-54. [Ministry of Health of the Russian Federation. Clinical guidelines. Polycystic Ovarian Syndrome. 2021: 1-54. (in Russian)].
5. Kai Y., Kawano Y., Yamamoto H., Narahara H. A possible role for AMP- activated protein kinase activated by metformin and AICAR in human granulosa cells. Reprod. Biol. Endocrinol. 2015; 13: 27. https://dx.doi.org/10.1186/ s12958-015-0023-2.
6. Wierman M.E., Auchus R.J., Haisenleder D.J., Hall J.E., Handelsman D., Hankinson S. et al. Editorial: The new instructions to authors for the reporting of steroid hormone measurements. J. Clin. Endocrinol. Metab. 2014; 99(12): 4375. https://dx.doi.org/10.1210/jc.2014-3424.
7. Григорян О.Р., Андреева Е.Н., Мельниченко Г.А., Дедов И.И. Влияние метформина на уровень антимюллерова гормона в терапии синдрома поликистозных яичников у женщин с ожирением. Проблемы репродукции. 2013; 1: 39-41. [Grigorian O.R., Andreeva E.N., Melnichenko G.A., Dedov I.I. The influence of metformin on amh level in obese patients with pcos. Russian Journal of Human Reproduction. 2013; 1: 39-41. (in Russian)].
8. Forslund K., Hildebrand F., Nielsen T., Falony G., Le Chatelier E., Sunagawa S. et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature. 2015; 528(7581): 262-266. https://dx.doi.org/10.1038/nature15766.
9. Vallianou N.G., Stratigou T., Tsagarakis S. Metformin and gut microbiota: their interactions and their impact on diabetes. Hormones (Athens). 2019; 18(2): 141-4. https://dx.doi.org/10.1007/s42000-019-00093-w.
10. Найдукова А.А., Ананьев Е.В., Чернуха Г.Е. Влияние метформина на репродуктивную функцию женщин с различными фенотипами СПКЯ. Акушерство и гинекология. 2017; 10: 55-61. [Naidukova A.A., Ananyev E.V., Chernukha G.E. Effect of metformin on the reproductive function of women with different phenotypes of polycystic ovary syndrome. Obstetrics and Gynecology. 2017; 10: 55-61. (in Russian)]. https://dx.doi.org/10.18565/ aig.2017.10.55-61.
11. Schweighofer N., Lerchbaum E., Trummer O., Schwetz V., Pieber T., Obermayer- Pietsch B. Metformin resistance alleles in polycystic ovary syndrome: Pattern and association with glucose metabolism. Pharmacogenomics. 2014; 15(3): 305-17. https://dx.doi.org/10.2217/pgs.13.223.
12. Nestler J.E., Jakubovich D.J. Decreases in ovarian cytochrome P450c17 alpha activity and serum free testosterone after reduction of insulin secretion in polycystic ovary syndrome. N. Engl. J. Med. 1996; 335(9): 617-23. https://dx.doi.org/10.1056/NEJM199608293350902.
13. Huhtala M.S., Tertti K., Juhila J., Sorsa T, Ronnemaa T. Metformin and insulin treatment of gestational diabetes: effects on inflammatory markers and IGF- binding protein-1 - secondary analysis of a randomized controlled trial. BMC Pregnancy Childbirth. 2020; 20(1): 401. https://dx.doi.org/10.1186/s12884- 020-03077-6.
14. Shpakov A.O. Improvement effect of Metformin on female and male reproduction in endocrine pathologies and its mechanisms. Pharmaceuticals (Basel). 2021; 14(1): 42. https://dx.doi.org/10.3390/ph14010042.
15. Attia G.R., Rainey W.E., Carr B.R. Metformin directly inhibits androgen production in human thecal cells. Fertil. Steril. 2001; 76(3): 517-24. https://dx.doi.org/10.1016/s0015-0282(01)01975-6.
16. Jiang Z.Z., Hu M.W., Ma X.S., Schatten H., Fan H.Y., Wang Z.B., Sun Q.Y. LKB1 acts as a critical gatekeeper of ovarian primordial follicle pool. Oncotarget. 2016; 7(5): 5738-53. https://dx.doi.org/10.18632/oncotarget.6792.
17. Roland A.V., Moenter S.M. Prenatal androgenization of female mice programs an increase in firing activity of gonadotropin-releasing hormone (GnRH) neurons that is reversed by metformin treatment in adulthood. Endocrinology. 2011; 152(2): 618-28. https://dx.doi.org/10.1210/en.2010-0823.
18. Tosca L., Chabrolle C., Uzbekova S., Dupont J. Effects of metformin on bovine granulosa cells steroidogenesis: Possible involvement of adenosine 5‘ monophosphate-activated protein kinase (AMPK). Biol. Reprod. 2007; 76(3): 368-78. https://dx.doi.org/10.1095/biolreprod.106.055749.
19. Tosca L., Rame C., Chabrolle C., Tesseraud S., Dupont J. Metformin decreases IGF1-induced cell proliferation and protein synthesis through AMP-activated protein kinase in cultured bovine granulosa cells. Reproduction. 2010; 139(2): 409-18. https://dx.doi.org/10.1530/REP-09-0351.
20. Maciel G.A.R., Soares Junior J.M., Alves da Motta E.L., Abi Haidar M., de Lima G.R., Baracat E.C. Nonobese women with polycystic ovary syndrome respond better than obese women to treatment with metformin. Fertil. Steril. 2004; 81(2): 355-60. https://dx.doi.org/10.1016/j.fertnstert.2003.08.012.
21. Maciel G.A.R., Hayashida S.A., da Costa L.C., Marcondes J.A., da Fonseca A.M., Soares J.M. Jr, Baracat E.C. Influence of LH and high-density lipoprotein cholesterol (HDL-C) on metformin response in women with polycystic ovary syndrome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011; 157(2): 180-4. https://dx.doi.org/10.1016/j.ejogrb.2011.03.028.
22. Neagu M., Cristescu C. Anti-Mullerian hormone--a prognostic marker for metformin therapy efficiency in the treatment of women with infertility and polycystic ovary syndrome. J. Med. Life. 2012; 5(4): 462-4.
23. Чернуха Г.Е., Найдукова А.А., Каприна Е.К., Донников А.Е. Молекулярно-генетические предикторы формирования синдрома поликистозных яич-ников и его андрогенных фенотипов. Акушерство и гинекология. 2021; 4: 120-7. [Chernukha G.E., Naidukova A.A., Kaprina E.K., Donnikov A.E. Molecular genetic predictors of polycystic ovary syndrome and its androgenic phenotypes. Obstetrics and Gynecology. 2021; 4: 120-7. (in Russian)]. https://dx.doi.org/10.18565/aig.2021.4.120-127.
24. Coviello A.D., Haring R., Wellons M., Vaidya D., Lehtimdki T, Keildson S. et al. A genome-wide association meta-analysis of circulating sex hormone-binding globulin reveals multiple loci implicated in sex steroid hormone regulation. PLoS Genet. 2012; 8(7): e1002805. https://dx.doi.org/10.1371/journal.pgen.1002805.
25. Cintra M.T.R., Balarin M.A.S., Tanaka S.C.S.V., Silva V.I.M.D., Marqui A.B.T., Resende E.A.M.R. et al. Polycystic ovarian syndrome: Rs1799752 polymorphism of ACE gene. Rev. Assoc. Med. Bras. 2018; 64(11): 1017-22. https://dx.doi.org/10.1590/1806-9282.64.11.1017.
26. Laven J.S.E. Follicle stimulating hormone receptor (FSHR) polymorphisms and polycystic ovary syndrome (PCOS). Front. Endocrinol. (Lausanne). 2019; 10: 23. https://dx.doi.org/10.3389/fendo.2019.00023.
27. Simoni M., Tempfer C.B., Destenaves B., Fauser B.C. Functional genetic polymorphisms and female reproductive disorders: Part I: Polycystic ovary syndrome and ovarian response. Hum. Reprod. Update. 2008; 14(5): 459-84. https://dx.doi.org/10.1093/humupd/dmn024.
28. Wang D.S., Jonker J.W., Kato Y., Kusuhara H., Schinkel A.H., Sugiyama Y. Involvement of organic cation transporter 1 in hepatic and intestinal distribution of metformin. J. Pharmacol. Exp. Ther. 2002; 302(2): 510-5. https://dx.doi.org/10.1124/jpet.102.034140.
29. Zolk O. Disposition of metformin: Variability due to polymorphisms of organic cation transporters. Ann. Med. 2012; 44(2): 119-29.
30. Chang H.H., Hsueh Y.S., Cheng Y.W., Ou H.T., Wu M.H. Association between polymorphisms of OCT1 and metabolic response to metformin in women with polycystic ovary syndrome. Int. J. Mol. Sci. 2019; 20(7): 1720. https://dx.doi.org/10.3390/ijms20071720.
Received 17.12.2021
Accepted 25.02.2022
About the Authors
Galina E. Chernukha, Dr. Med. Sci., Professor, Chief Researcher, Department of Gynecological Endocrinology, V.I. Kulakov NMRC for OG&P,Ministry of Health of Russia, +7(916)311-05-21, g_chernukha@oparina4.ru, https://orcid.org/0000-0002-9065-5689, 117997, Russia, Moscow, Ac. Oparina str., 4.
Andrey E. Donnikov, M.D., Ph.D., Head of the Laboratory of Molecular Genetic Methods, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(495)438-49-51, a_donnikov@oparina4.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.
Alina A. Naydukova, Ph.D. Student, Department of Gynecological Endocrinology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(916)675-00-97, aleeshka@mail.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.
Elena K. Kaprina, Ph.D. Student, Department of Gynecological Endocrinology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(916)129-41-18, kaprina_elena@mail.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.
Ekaterina D. Miroshina, Junior Researcher, Department of Gynecologic Endocrinology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(917)533-48-00, emiroshina.md@gmail.com, 117997, Russia, Moscow, Ac. Oparina str., 4.
Authors’ contributions: Chernukha G.E., Donnikov A.E., Naidukova A.A. - developing the concept and design of the study; Naidukova A.A., Kaprina E.K., Miroshina E.D. - collecting and processing the material; Donnikov A.E., Naidukova A.A. - statistical data processing; Naidukova A.A., Miroshina E.D. - writing the text; Chernukha G.E., Donnikov A.E. - editing. Conflicts of interest: The authors declare that they have no competing interests.
Funding: The study “Improvement of management tactics of patients with various PCOS phenotypes based on the assessment of molecular genetic, morphological, immunological and endocrine-metabolic parameters”, AAAA15-115123110128-4 dated December 31, 2015, supervised by G.E. Chernukha.
Ethical Approval: The study was reviewed and approved by the local Ethics Committee of Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology (Ref. No.9 of 17.10.2014).
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Chernukha G.E., Naidukova A.A., Kaprina E.K., Miroshina E.D., Donnikov A.E. Molecular genetic and endocrine predictors of menstrual cycle regulation in patients with polycystic ovary syndrome undergoing metformin treatment.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2022; 3: 80-88 (in Russian)
https://dx.doi.org/10.18565/aig.2022.3.80-88



