Antiphospholipid syndrome and pregnancy
Antiphospholipid syndrome (APS) is a systemic process that affects all organs and tissues of the body and diagnosed upon fulfilment of clinical and biological criteria. The currently accepted clinical morbidities affect two organs: the vascular tree, leading to thrombotic manifestations, and the utero-placental unit, leading to pregnancy complications. Obstetric APS (oAPS) is an autoimmune disease leading to the synthesis of autoantibodies directly capable of activating key cells of vascular and/or placental pathophysiology. During pregnancy, placenta serves as the most important organ. Violations of the placenta function due to endothelial dysfunction, ischemia, and placenta microthrombosis are responsible for the development of obstetric complications: pre-eclampsia, HELLP-syndrome, placental abruption.This manuscript describes a data of a different clinical experience in the field of APS. APA directly or indirectly affects the implantation process and early embryonic stages. The thorough systematic review on histopathology in the placenta of oAPS women found, on the sincytiotrophoblast (sTB) side, a decreased trophoblast (TB) proliferation, increased TB death rates, a decreased syncytialisation process, an increased sTB death rate with increased cell debris, and areas of sTB denudation and of fibrin deposition. Experimental in vitro data confirmed that ab2GP1 Abs decrease fusion of TB cells, thus inhibiting sTB formation. Reduced eTB invasion was associated with decreased placenta anchorage, reduced transformation of maternal spiral arteries and reduced maternal flow to the placenta, mirroring the conditions in placenta-mediated late pregnancy complications such as preeclampsia.Our studies and over 20 years of clinical experience indicate the presence of etiopathogenetic relation between APS and obstetric complications and the high efficacy of prophylaxis with anticoagulants when it starts early, since the period of preconception.Gris J.-C., Makatsariya A.D., Bitsadze V.O., Khizroeva D.Kh., Khamani N.M.
Keywords
Definition
Antiphospholipid syndrome (APS) is diagnosed according to the clinical and biological criteria [1]. It can develop as the complication of the present systemic pathology (inflammatory, infectious, neoplastic diseases: secondary APS) or as an isolated entity (primary APS), which sometimes precedes the onset of a systemic disease.
The currently accepted clinical manifestations of APS affect two organs: the vascular tree, leading to thrombotic manifestations, and the utero-placental unit, leading to pregnancy complications.
Classification
Primary APS is diagnosed in the presence of clinical signs of the syndrome and the absence of symptoms of various connective tissue diseases, including systemic lupus erythematosus (SLE), rheumatoid arthritis, and autoimmune thrombocytopenic purpura. APS related to connective tissue diseases, various autoimmune diseases, and malignant tumors, infectious and drug-mediated is considered secondary APS.
In the presence of antiphospholipid antibodies (APA), the risk of thrombosis increases significantly.
Criteria for diagnosis
In 1999, diagnostic criteria for APS were proposed in Sapporo. However, in the future there were many works devoted to the clinical and laboratory manifestations of the syndrome, and therefore at the XI International Congress on APA (2005) the criteria for diagnosing APS proposed in Sapporo were revised (Table 1) [1].
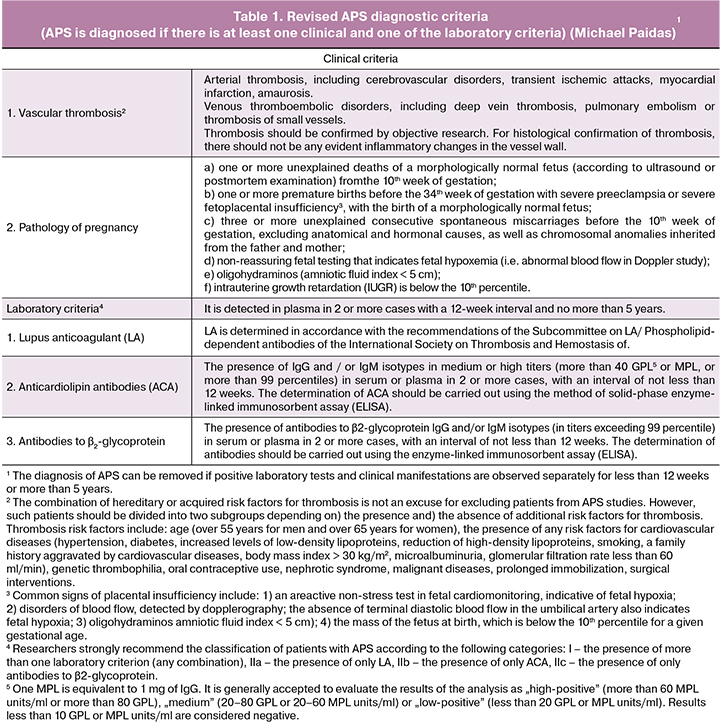
As Table 1 shows, the diagnosis of APS is defined in the presence of vascular thrombosis and pregnancy pathology. Vascular thrombosis can be arterial, venous, or that of small vessel, in any tissue or organ and must be objectively confirmed by imaging studies or histopathology, which can show no signs of inflammation in the vessel wall.
Pregnancy morbidities are defined as either three unexplained consecutive spontaneous miscarriages (embryonic losses) before the 10th week of gestation, independent of maternal anatomic or hormonal abnormalities and paternal and maternal chromosomal causes; or at least one death of a morphologically normal fetus at or before the 10th week of gestation; or at least one preterm birth of a morphologically normal neonate before the 34th week of gestation because of eclampsia or severe preeclampsia (PE) or recognized features of placental insufficiency.
Heart valve disease, livedo reticularis, thrombocytopenia and nephropathy are non-criteria features of APS, which may be present in isolation or in association with thrombosis and/or pregnancy morbidity.
The laboratory criteria include the presence of autoantibodies, the so-called “antiphospholipid antibodies” (aPL Abs); their primary targets are phospholipid-binding proteins, although antibodies directed against phospholipids have also been documented. The currently accepted subtypes are the lupus anticoagulant (LA), tested through coagulation assays, and two solid-phase autoantibodies: the anticardiolipin antibodies (aCL Abs) and the anti-ß2-glycoprotein I antibodies (aß2GP1 Abs), which can be of IgG and/or IgM isotypes. They must be found positive on two or more occasions at least 12 weeks apart, with IgG and IgM at significant titres, i.e. > the 99th percentile observed in the normal reference population.
APS is thus a highly heterogeneous syndrome, with initially isolated thrombotic APS cases (tAPS), initially isolated obstetrical APS cases (oAPS), initially mixed cases (mAPS), and some isolated cases progressing to mAPS over time. The clinical pathophysiology is only partially understood, but this heterogeneity suggests that multiple pathogenic processes may be involved. APS is thought to be the clinical consequence of an aPL Abs-mediated activation of key vascular and placental cells [2], as well as platelets, monocytes, endothelial cells, macrophages, and trophoblastic cells with their two differentiation modalities leading to formation of syncytiotrophoblasts and extravillous trophoblasts.
Background
In 1906 August Paul von Wasserman and colleagues reported on a complement fixation test for the detection of antibodies to syphilis. They developed a method for serological diagnosis of syphilis, based on the fixation of complement in the interaction of autoantibodies from the patients’ sera with syphilis and “syphilitic antigen”, isolated from the organs of animals. But only in 1941, Pangbourn proved that the chemical basis of this reaction is cardiolipin, contained in an alcohol extract of a bovine heart and used as an antigen in the Wasserman reaction.
This new nosological form, closely related to SLE, is genetically, clinically and serologically distinct from it. The emphasis in primary APS is placed on vascular disorders. Numerous clinical and morphological studies indicate that the basis of APS is a kind of vasculopathy associated with thrombotic occlusive lesions of the blood vessels. The absence in most cases of inflammatory or degenerative changes in the vascular wall proves the pathogenetic originality of vascular lesions in APS. These changes with APS have a generalized character; antibodies to phospholipids participate in their genesis.
In 1983 Graham Hughes described a new disorder distinct from SLE, the antiphospholipid syndrome (APS). It was revealed in patients with thrombosis, abortion, or cerebral diseases, associated with positivity for aCL Abs or LA [3].
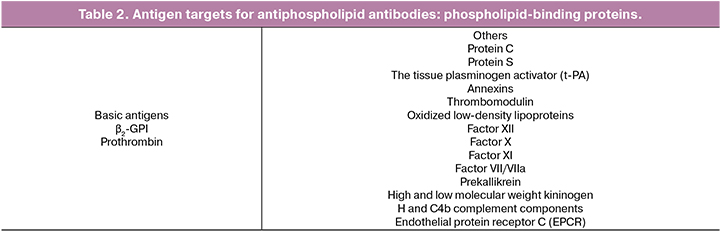
In the late 1990s, it was found that antiphospholipid antibodies did not recognize anionic phospholipids, as previously thought, but directed toward plasma proteins that are located on the anionic surfaces of phospholipids. The most important antigen targets of antiphospholipid antibodies are ß2-GPI and prothrombin.
In addition to ß2-GPI and prothrombin, there are still many proteins-cofactors, which are a target for APA (Table 2).
Thus, the most contemporary view of APS is that of an autoimmune disease leading to the synthesis of autoantibodies directly capable of activating key cells of vascular and/or placental pathophysiology. The phospholipids disappear once the autoimmunisation is effective. Its traditional markers are, however, the consequence of a historical phospholipid-dependent artefact. So the question arises whether we should extend the general proposition to a thrombotic and/or obstetric syndrome, induced by any type of activating autoantibodies: the so-called aPL Abs are only their sub-variety.
Natural aß2GP1 Abs, role of ß2GP1 and influence of aß2GP1 Abs.
An important key to a better understanding of APS pathophysiology is the solution of the biology of β2-glycoprotein I. It consists of 5 domains, the 5th is positively charged and anionic phospholipid cells bind to it; the Ist domain contains the epitope GLY40-ARG43, which recognizes antibodies to β2-GPI, possesses LA activity and is strictly associated with thrombosis.
There is a known link between aPL and aβ2GP1 Abs, APS, and infectious agents. Among the infectious diseases associated with the presence of aβ2-GP1 Abs are viruses (parvovirus B19, cytomegalovirus, HIV, varicella-zoster virus, EBV, hepatitis B/C, adenovirus, HTLV1), bacteria (Streptococcus pyogenes, Staphylococcus aureus, Helicobacter pylori, Salmonella typhi, Mycobacterium leprae, Escherichia coli, Rickettsia typhi, Mycobacterium tuberculosis, Coxiella burnetti, Chlamydophila psittaci, Mycoplasma pneumoniae) and parasites (Plasmodium falciparum, Borrelia burgdorferi, leptospirosis, leishmania). ß2GPI-related synthetic peptides and infectious agents share high homology, with studies on experimental APS models demonstrating molecular mimicry between ß2GP1-related synthetic peptides, designed to provide epitopes for aß2GP1 Abs, and pathogen structures.
ß2GP1 is a scavenger molecule with specific binding site for the negatively charged phospholipid phosphatidylserine (PS) [4]. By binding to PS, ß2GP1facilitates clearance of particles and apoptotic bodies from the circulation. Cell-derived microparticles (MPs) are major sources of PS expression in the circulation. aß2GP1 Abs may thus cause impaired MPs clearance by masking ß2GP1 molecules; the consequence being an accumulation of cellular debris fuelling autoimmunity and inflammatory states.
ß2GP1 – aßGP1 as the basis for oAPS
An international multicentre study tested the association between circulating APA against domain I of ß2GP1 and APS morbidities [5]. They included patients from nine different centres who met the inclusion criteria of having aß2GP1 Abs in their plasma/serum. A sub-analysis was performed in the 201 pregnant women. Considering pregnancy complications, as a whole, only anti-ß2GP1 domain I IgG and aCL IgG were significant risk factors, not LA and not aß2GP1 IgG or IgM. Only anti-ß2GP1 domain I IgG was a risk factor for history of premature birth before the 34th week of gestation due to PE or placental insufficiency [5].
Other aPL Abs in oAPS
A case-control study performed in Italian patients with previous placenta-mediated complications found anti-prothrombin/phosphatidylserine Abs (aPT/PS Abs) to be independent risk factors for thrombosis and for LA, but not for pregnancy morbidities [6].
The practical application of non-conventional aPL Abs in patients with clinical oAPS has recently been investigated in a monocentric prospective French registry. After conventional aPL Abs, additional testing included aPT/PS IgG/IgM, anti-Annexin A5 IgG/IgM and antiphosphatidylethanolamine Abs. Patients with clinical criteria for oAPS but seronegative for conventional aPL Abs could have non-conventional aPL Abs, supporting findings that oAPS treatments, when proposed, have been associated with lower pregnancy loss rates [7].
The main aPL Abs organ target in oAPS: the placenta
One of the main targets of APA during pregnancy is the placenta. Fetal loss syndrome, IUGR and PE are consequences of impaired placental function. Uteroplacental insufficiency is often referred to as vasculopathy of terminal spiral arteries that supply blood to the intervillous space. In fact, the fetal death in APS is preceded by IUGR, hypamnion, disorder of feto-placental blood flow, reflecting fetal hypoxia and placental dysfunction. APA directly or indirectly affects the implantation process and early embryonic stages. By the 21st day after ovulation, trophoblast villi are already vascularized enough and we can state the fact of establishment of uteroplacental blood flow. From that moment active contact with the mother’s plasma begins, as well as circulating aPL.
The pathological processes in tAPS and oAPS may, however, not be absolutely different. Results obtained using monoclonal aPL Abs from patients to characterise an APA-induced pregnancy loss in mice, showed that it could be prevented using antithrombotics such as hirudin and fondaparinux, supporting a role of thrombosis in pathology [8]. Long-term observational study “NOH-APS” conducted in women with the clinical criteria of oAPS also found that aPL Abs positive women were prone to developing an excess of venous thromboembolic events (1.46% (1.15%–1.82%) per year) and arterial thrombotic events, mainly strokes and transient ischaemic attacks (0.32% (0.18%–0.53%) per year) compared to factor V Leiden or prothrombin mutation positive women, and to women with a negative thrombophilia status [9].
Conventionally treated APS: risk factors for morbidities during pregnancy
The management of pregnant APS women by traditional therapeutic means requires an initial careful evaluation of clinical risks that may occur.
We consider that repeated aPL Abs testing throughout pregnancy is unnecessary.
The risk of pregnancy loss must be evaluated. A history of thrombosis, fetal death, autoimmune diseases, and an aPL Ab triple positivity is associated with a partial resistance to the conventional, antithrombotics-based prophylactic treatments given during a new pregnancy. A history of pure oAPS and a single aPL Ab positivity has better overall prognosis. The early variations of PIGF and sFlt1 concentrations in conventionally-treated newly pregnant oAPS women may help to detect patients at low risk of placenta-induced complications.
Treatment of APS women for prophylaxis of morbidities during pregnancy
For oAPS women with three or more pregnancy losses and no history of thrombosis, the 9th ACCP guidelines recommend antepartum administration of prophylactic or intermediate-dose unfractionated heparin (UFH) or prophylactic low-molecular-weight heparin (LMWH) combined with low-dose aspirin (LDA) (75–100 mg/d) over no treatment (Grade 1B) [10]. Weight-adjusted therapeutic doses of LMWHs are recommended in case of any thrombotic history, with regular anti-Xa activity monitoring [10]. Although combined therapy with LDA and LMWH is the mainstay of treatment for women with oAPS, the strength of the evidence for the efficacy of this approach remains controversial.
Our capacity to improve the management of pregnant oAPS women has to be evaluated [11].
The European collaborative study of 49 oAPS women with previous adverse obstetrical outcomes despite LMWH+LDA treatment, showed that additional treatments such as steroids (13%) and hydroxochloroquine (71%) can lower pregnancy loss rates from 76% to 14% and PE rates from 33% to 10%: this, however, was not a randomised controlled trial [12].
Data on the regular use of intravenous immune globulin treatments during pregnancy are contradictory. However, the group of Ware Branch reported the use of 1 g/kg for two days every four weeks in addition to a UFH+LDA treatment, in 17 high-risk women: no pregnancy loss recurrence was observed [13]. A few cases of plasma exchanges, and sometimes of immunoadsorption on extracorporeal affinity columns, have been described.
The use of hydroxychloroquine and statins has been gaining momentum. HCQ is described to restore trophoblast fusion affected by aPL Abs [14, 15].
Eculizumab, the terminal complement complex inhibitor, has mainly been used in cases of catastrophic APS, pregnancy being a rare precipitating factor [12].
The treatment of high-risk pregnant oAPS women remains a real challenge. Recommendations based on experts’ advice, but not backed by evidence, on treatment-refractory cases include to start LDA more than four weeks before conception, to start LMWH as soon as possible after conception, to increase dose of LMWH (from prophylactic to intermediate to full dose), to add hydroxychloroquine and low-dose prednisolone in the first trimester of gestation, and to add IV immune globulins to standard treatment. We need well-designed multicentre randomised controlled trials, taking in account the APS heterogeneity. The conception and development of new physiopathologically relevant treatments are warranted.
A diagnosis that must not be overlooked: catastrophic APS (CAPS) in pregnancy
CAPS is a rare, life-threatening condition resulting in multi-organ ischaemia and failure, 3/4 of cases occurring in females, often of reproductive age: 4–6% of cases during the third trimester or puerperium [16]. More than half of the women who develop CAPS during pregnancy have a history of primary APS or of clinical criteria for tAPS or oASP; a third of cases are associated with SLE, and the rest have another autoimmune disease. Precipitating factors such as infection and surgical procedures are frequent.
Diagnosis is made on the basis of multi-organ thrombosis over one week affecting at least three organs or systems, including tPAS and oAPS criteria, positive aPL Abs on two occasions and histopathologic confirmation of small vessel occlusion. Vasculopathy is frequent, with a thrombotic microangiopathy component. Common presentations range from malaise, abdominal pain, dyspnea, and hypertension, to altered mental status and seizures [16]. Rapid deterioration can occur at any time, with detrimental outcomes both to the mother and fetus. Subsequent complications include hepatic infarction, bleeding, and diffuse alveolar haemorrhage. Misdiagnosis or delay in diagnosis are common during pregnancy, due to the similarities with hemolysis characterized by elevated liver enzymes, low platelets ( in HELLP syndrome), thrombotic thrombocytopenic purpura, and haemolytic uremic syndrome.
Over the last 15 years, mortality has decreased from one half to a third, due to earlier diagnosis and the use of aggressive triple therapy, associating anticoagulants (IV heparin), high dose steroids, and intravenous immune globulins (0.4 g/kg per day, 4–5 days) and/or plasma exchange (removal of 2–3 litres of plasma for a minimum of 3–5 days). The use of eculizumab is under investigation.
CONCLUSION
Despite some significant progress, nearly 40 years after the first description of some pregnancy morbidities in women positive for the aPL Abs, , oAPS is still insufficiently understood. APS is a non-specific mechanism of many diseases. The effects of APA are extremely diverse in contrast to the isolated form of genetic thrombophilia, which is apparently due to the high heterogeneity of antiphospholipid antibodies. The prognostic value of the clinical and biological markers is not yet definitely characterised. The debate is still too often obscured by studies of low methodological value; their paradoxical results are chaotic. The treatments available to us have obvious limitations and are sometimes controversial, but improvements frequently lack methodological and controlled studies. Only the emergence of truly pathophysiological treatments, comprehensively evaluated, will allow us to take the step towards this precise medicine that is required. In the meantime, we adopt our therapeutic managements with cautious pragmatism, trying to provide the best support to our patients minimising the side effects.
References
1. Miyakis S., Lockshin M.D., Atsumi T., Branch D.W., Brey R.L., Cervera R. et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J. Thromb. Haemost. 2006; 4(2): 295-306.
2. Arnout J. The pathogenesis of the antiphospholipid syndrome: a hypothesis based on parallelisms with heparin-induced thrombocytopenia. Thromb. Haemost. 1996; 75(4): 536-41.
3. Hughes G.R. Thrombosis, abortion, cerebral disease, and the lupus anticoagulant. Br. Med. J. (Clin. Res. Ed). 1983; 287(6399): 1088-9.
4. de Groot P.G., Meijers J.C. β(2)-Glycoprotein I: evolution, structure and function. J. Thromb. Haemost. 2011; 9(7): 1275-84.
5. de Laat B., Pengo V., Pabinger I., Musial J., Voskuyl A.E., Bultink I.E. et al. The association between circulating antibodies against domain I of beta2-glycoprotein I and thrombosis: an international multicenter study. J. Thromb. Haemost. 2009; 7(11): 1767-73.
6. Hoxha A., Mattia E., Tonello M., Grava C., Pengo V., Ruffatti A. Antiphosphatidylserine/prothrombin antibodies as biomarkers to identify severe primary antiphospholipid syndrome. Clin. Chem. Lab. Med. 2017; 55(6): 890-8.
7. Mekinian A., Bourrienne M.C., Carbillon L., Benbara A., Noémie A., Chollet-Martin S. et al. Non-conventional antiphospholipid antibodies in patients with clinical obstetrical APS: Prevalence and treatment efficacy in pregnancies. Semin. Arthritis Rheum. 2016; 46(2): 232-7.
8. Poindron V., Berat R., Knapp A.M., Toti F., Zobairi F., Korganow A.S. et al. Evidence for heterogeneity of the obstetric antiphospholipid syndrome: thrombosis can be critical for antiphospholipid-induced pregnancy loss. J. Thromb. Haemost. 2011; 9(10): 1937-47.
9. Gris J.C., Bouvier S., Molinari N., Galanaud J.P., Cochery-Nouvellon E., Mercier E. et al. Comparative incidence of a first thrombotic event in purely obstetric antiphospholipid syndrome with pregnancy loss: the NOH-APS observational study. Blood. 2012; 119(11): 2624-32.
10. Bates S.M., Greer I.A., Middeldorp S., Veenstra D.L., Prabulos A.M., Vandvik P.O. VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012; 141(2, Suppl.): e691S-736S.
11. Akinshina S., Makatsariya A., Bitsadze V., Khizroeva О., Khamani N. Thromboprophylaxis in pregnant women with thrombophilia and a history of thrombosis. J. Perinat. Med. 2018; Jun 27. pii: /j/jpme.ahead-of-print/jpm-2017-0329/jpm-2017-0329.xml.
12. Mekinian A., Alijotas-Reig J., Carrat F., Costedoat-Chalumeau N., Ruffatti A., Lazzaroni M.G. et al.; on the behalf of the SNFMI and the European Forum on Antiphospholipid Antibodies. Refractory obstetrical antiphospholipid syndrome: Features, treatment and outcome in a European multicenter retrospective study. Autoimmun. Rev. 2017; 16(7): 730-4.
13. Branch D.W., Peaceman A.M., Druzin M., Silver R.K., El-Sayed Y., Silver R.M. et al. A multicenter, placebo-controlled pilot study of intravenous immune globulin treatment of antiphospholipid syndrome during pregnancy. The Pregnancy Loss Study Group. Am. J. Obstet. Gynecol. 2000; 182(1, Pt 1): 122-7.
14. Mekinian A., Lazzaroni M.G., Kuzenko A., Alijotas-Reig J., Ruffatti A., Levy P. et al.; SNFMI and the European Forum on Antiphospholipid Antibodies. The efficacy of hydroxychloroquine for obstetrical outcome in anti-phospholipid syndrome: Data from a European multicenter retrospective study. Autoimmun. Rev. 2015; 14(6): 498-502.
15. Lefkou E., Mamopoulos A., Dagklis T., Vosnakis C., Rousso D., Girardi G. Pravastatin improves pregnancy outcomes in obstetric antiphospholipid syndrome refractory to antithrombotic therapy. J. Clin. Invest. 2016; 126(8): 2933-40.
16. Khizroeva J., Bitsadze V., Makatsariya A. Catastrophic antiphospholipid syndrome and pregnancy. Clinical report. J. Matern. Fetal Neonatal Med. 2018; Jan 8.
Received 26.10.2017
Accepted 22.12.2017
About the Authors
Jean-Christophe Gris, M.D., Ph.D., Professor of Department of Haematology, University hospital, Nîmes, University of Montpellier, France;Professor of Obstetrics and Gynecology, Department of obstetrics and gynecology #2, I.M. Sechenov First Moscow State Medical University, Moscow, Russia
Alexander Makatsariya, M.D, PhD, corresponding member of the Russian Academy of Sciences, Professor, Head of the Department of obstetrics and gynecology #2,
I.M. Sechenov First Moscow State Medical University. 119048, Russia, Moscow, Trubetskaya str. 8, bld. 2. Tel.: +74957885840. E-mail: gemostasis@mail.ru
Viktoria Bitsadze, MD, Professor of the Russian Academy of Sciences, Professor of the Department of obstetrics and gynecology #2, I.M. Sechenov First Moscow
State Medical University. 119048, Russia, Moscow, Trubetskaya str. 8, bld. 2. Tel.: +79262313829. E-mail: vikabits@mail.ru
Dzamilya Khizroeva, MD, Professor of the Department of obstetrics and gynecology #2, I.M. Sechenov First Moscow State Medical University.
119048, Russia, Moscow, Trubetskaya str. 8, bld. 2. Tel.: +79153619073. E-mail: jamatotu@gmail.com
Nadine Khamani, postgraduate student of the Department of Obstetrics and Gynecology #2, I.M. Sechenov First Moscow State Medical University.
119048, Russia, Moscow, Trubetskaya str. 8, bld. 2. Tel.: +74957885840. E-mail: nadinka@list.ru
For citations: Gris J.-C., Makatsariya A.D., Bitsadze V.O., Khizroeva D.Kh., Khamani N.M. Antiphospholipid syndrome and pregnancy. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2018; (10): 5-11. (in Russian)
https://dx.doi.org/10.18565/aig.2018.10.5-11



