Androgen profile in patients with polycystic ovary syndrome and its association with metabolic dysfunction
Aim. To optimize the diagnosis of biochemical hyperandrogenism (HA) and investigate its association with metabolic dysfunction in polycystic ovary syndrome (PCOS).Chernukha G.E., Naidukova A.A., Udovichenko M.A., Kaprina E.K., Ivanets T.Yu.
Materials and methods. The study comprised 437 women with PCOS and 160 healthy women. Clinical and laboratory evaluation consisted of pelvic ultrasonography (USG), analysis of androgen profile including serum levels of total testosterone, free testosterone, and androstenedione (TT, FT, A4), a two-hour glucose tolerance test (GTT) with monitoring of glucose and insulin levels, and the blood lipid profile.
Results. Measuring a complete serum androgen profile increases the detection rate of biochemical HA from 37.4 to 66.4%. The greatest contribution to the diagnosis of HA is made by determining serum A4 concentrations, which are elevated in every second patient with PCOS. HA with co-elevated serum TT and A4 is associated with a 1.5–2 fold increase in the rate of impaired glucose tolerance (IGT), hyperinsulinemia (HI), insulin resistance (IR), and dyslipidemia.
Conclusion. Patients with suspected PCOS should be tested for serum TT, FT, and A4; the measurement of A4 is most important. Co-elevated serum TT and A4 may be a useful predictor of developing IR, HI, IGT, and dyslipidemia.
Keywords
Polycystic ovary syndrome (PCOS) is the main form of hyperandrogenism (HA) in women accounting for 80–90% of all hyperandrogenic disorders [1]. PCOS diagnostic criteria are specified by the European Society of Human Reproduction and Embryology and the American Society for Reproductive Medicine (ASRM/ESHRE, Rotterdam, 2003). The diagnosis of PCOS is based on at least two of the following three criteria: ovulatory dysfunction (usually oligomenorrhea, OM), clinical or biochemical evidence of hyperandrogenism (HA), and polycystic ovaries (PCO) on ultrasound assessment (USG). These criteria generate four reproductive phenotypes: 3 androgenic - classic PC0S (HA + PCO + OM), anovulatory PC0S (HA + OM), ovulatory PC0S (HA + PCO) and nonhyperandrogenic PCOS (PCO + OM); not all endocrinological schools recognize these phenotypes. Thus, according to the criteria of the US National Institute of Health (NIH, 1990) and the guidelines of the Androgen Excess and PCOS Society (AE-PCOS Society, 2006), HA should be an obligatory diagnostic criterion for PCOS. It should be noted that there still is no consensus on the optimal testing for biochemical HA. In most clinical protocols, total serum testosterone (TT) is recommended as the first-line test for the diagnosis of HA in PCOS [2]. The Androgen Excess and PCOS Society indicate the priority of determining free testosterone (FT) and free androgen index (FAI). In the opinion of this expert group, testing for TT provides a limited contribution to the diagnosis of PCOS [3]. The position statement of European Society of Endocrinologists recommends measuring serum levels of TT and SHBG with the calculation of FAI, but FT assays should not be used due to their intrinsic inaccuracy. The same position statement suggests the measurement of serum androstenedione (A4) in cases of normal levels of TT and FT [4]. Androstenedione, the immediate precursor of testosterone that does not bind to SHBG, offers greater diagnostic accuracy for detecting HA compared to TT [5]. However, the International PCOS Network Recommendations 2018 consider testing for A4 in PCOS as a conditional second-line method, and the level of evidence to support this analysis is defined as low due to the small number of studies [6]. Therefore, the diagnostic value of serum A4 and its role in diagnosing PCOS diagnostic are not clearly defined. At the same time, it is important to note that underestimating the biochemical HA complicates the diagnosis of PCOS and may interfere with the determination of the phenotype in incomplete forms of the syndrome.
The accuracy of HA diagnostics is also important from the standpoint of its relationship with IR and HI, an increased risk of IGT, endothelial dysfunction, subclinical atherosclerosis, both in comparison with healthy women and women with the nonhyperandrogenic PCOS phenotype [7-9]. The optimal diagnosis of HA and the feasibility of predicting metabolic disorders based on the androgen profiles remains a subject of debate. This study aimed to optimize the diagnosis of biochemical HA and investigate its association with metabolic dysfunction in PCOS patients.
Materials and methods
The study was conducted at the Department of Gynecological Endocrinology, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia. The study comprised 437 women with PCOS diagnosed by the Rotterdam criteria. To investigate the diagnostic accuracy of TT, FT, and A4, 160 somatically healthy women with normal ovarian morphology on USG, no signs of hirsutism, and reproductive dysfunction were examined. Both groups were comparable in age (mean 25.5 (4.6) and 25.2 (3.8) years, respectively, p = 0.68) and BMI (mean 25.2 (6.8) kg/m2 and 24.2 (5.6) kg/m2, respectively, p = 0.73).
Analysis of hormonal profile included serum levels of LH, FSH, TT and FT, A, SHBG, prolactin, and TSH, which were determined on the 2nd or 3rd day of a menstrual cycle by a fluorescence immunoassay with an Immulite 2000 automatic analyzer (Siemens, USA). The serum level of AMH was determined by ELISA (AMH GenII ELISA test system, Beckman Coulter, USA). Pelvic USG was performed on day 5-7 of the menstrual cycle with a 2000 Toshiba SSA-240 machine (Japan) using a 7.5 MHz transvaginal transducer to measure the ovarian volume. Ovulation was monitored by measuring plasma progesterone concentration and/or USG on day 20-24of menstrual cycle in 3 menstrual cycles.
As part of the study, a two-hour, oral glucose tolerance test (GTT) was performed with monitoring of fasting glucose and immunoreactive insulin level 1 and 2 hours after 75 g glucose load. The blood lipid profile included total serum cholesterol (TC), triglycerides (TG), and high-density lipoproteins (HDL) (Airon 200 analyzer, Biocon diagnostic kits); atherogenic coefficient (AC) and low-density lipoprotein (LDL) concentration were calculated using the A. N. Klimov’s (KE) and Friedwald equations, respectively.
Statistical analysis was performed using IBM SPSS Statistics, version 21. The distribution of continuous variables was tested for normality using the Kolmogorov-Smirnov test. Quantitative variables showing normal distribution were expressed as means and standard deviation M (SD), the comparison was performed using Student’s t-test. Differences in the mean values of several subgroups were analyzed by using ANOVA. Categorical variables were compared by the Pearson χ2 test. Correlation between two quantitative variables was analyzed using the Pearson correlation coefficient. The critical level of significance when testing statistical hypotheses was considered at p < 0.05.
 Results
Results
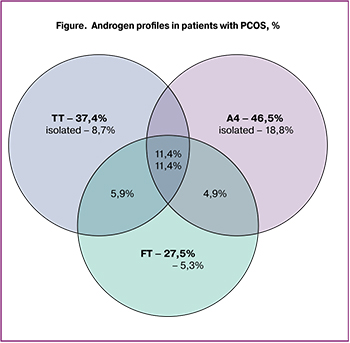
The assessment of androgen profiles showed that the measurement of serum levels of TT, FT, and A4 resulted in the diagnosis of HA in 37.4% (n = 164), 27.5% (n = 120), and 46.5% (n = 203) of patients, respectively (Fig. 1). Adding the measurement of FT level increases HA detection rate, traditionally diagnosed by the TT level, from 37.4 to 47.6%. When TT and FT levels are normal, the measurement of A4 allows the detection of biochemical HA in another 18.8% of patients. In general, measuring a complete serum androgen profile increases the detection rate of biochemical HA from 37.4 to 66.4%. Along with an increase in the level of androgens, 57.9% of patients showed a decrease in the level of SHBG (less than 50 nmol/L), which was associated with an increase in the androgens’ bioavailability.
To assess the contribution of testing for various androgens to the diagnosis of biochemical HA, we performed an ROC analysis, which included patients with androgenic PCOS phenotypes (n = 296) and patients without impaired reproductive function and signs of PCOS (n = 160).
A4 provided the ROC-curve with the largest area under the curve of 0.946 (95% CI: 0.927-0.965) followed by TT and FT with AUC of 0.837 (95% CI: 0.794-0.880) and 0.766 (95% CI: 0.719-0.812), respectively. The sensitivity and specificity of each model were determined according to the established reference values for the standardized test systems. For the TT threshold of 1.97 nmol/L, the sensitivity and specificity were 79.2% and 85.0%; for the FT threshold of 4.1 ng/ml, they were 68.0 and 88.1%, for the A4 threshold of 19.0 nmol/L they were 79.2 and 98.1%, respectively.
There was a weak correlation between TT, A4, FT and the age and BMI of PCOS patients (p > 0.05), while BMI correlated with SHBG and FAI (r = -0.525, p <0.001 and r = 0.444 p < 0.001, respectively). Correlation between BMI and FT was also weak (r = 0.124, p = 0.03). Levels of TT and A4 had a direct correlation with LH and AMH (r = 0.407 and r = 0.538 for TT and r = 0.539 and r = 0.612 for A, respectively, p <0.001).
We also analyzed the relationship of HA with metabolic dysfunction. Analyses of fasting glucose and GTT showed that 17.8% (n = 78), 32.5% (n = 142), 25.2% (n = 110), and 37, 5% (n = 164) of patients had IGT, IR, HI, and dyslipidemia, respectively. As seen from Table 1, dyslipidemia was most commonly observed in the form of isolated hypercholesterolemia (22.2%). Combined dyslipidemia was diagnosed in 10.3% of patients, and hypertriglyceridemia was rarely detected (less than 3%). TT and FT had no statistically significant correlations with glucose, insulin and lipid profile (p > 0.05). A4 had correlations with fasting insulin levels (r = 0.178, p = 0.03), 1 hour after oral glucose loading (r = 0.169, p = 0.01) the HOMA index (r = 0.182, p = 0.02), and cholesterol (r = 0.129, p = 0.02). A negative correlation was found between the levels of SHBG and fasting glucose (r = -0.182, p = 0.001), fasting insulin (r = -0.400, p = 0.001), 1 (r = -0.322, p = 0.001) and 2 hours (r = -0.281, p = 0.001) after oral glucose loading during GTT. The NOMA index correlated with the level of SHBG (r = -0.416, p = 0.001) and FAI (r = 0.315, p = 0.001). SHBG had correlations with the level of TG (r = -0.271, p = 0.01), HDL (r = 0.370, p = 0.001), LDL (r = -0.201, p = 0.05), and KE (r = - 0.284, p = 0.01).
Our findings suggested that SHBG and FAI were the most significant markers of metabolic dysfunction in PCOS. However, upon closer examination, it turned out that 64.5% of patients with elevated FAI had impaired lipid metabolism (22.6% were overweight, 41.9% had grade I – II obesity). Among patients with low levels of SHBG, 33.2% and 49.7% were overweight and obese, respectively. These observations and the correlation of SHBG and FAI with BMI in PCOS patients indicate that these markers had low diagnostic value in patients with normal BMI.
To assess the contribution of A4 and TT to the development of metabolic dysfunction in PCOS, we conducted a comparative analysis of the clinical and laboratory parameters of 246 patients with PCOS, who were divided into the following groups: high levels of TT and A4 (HT/HA) 28.8% (n = 126), high level of TT and normal A4 (HT/NA) 8.7% (n = 38), high level of A4 and normal TT (NT/HA) 17.6% (n = 77), and normal levels of TT and A4 (NT/NT) 32.3% (n = 141).
As seen from Table 1, the groups did not differ in age (p = 0.72), BMI (p = 0.25), rates of overweight (p = 0.41) and obesity (p = 0, 47). Analysis of the hormonal profile showed that higher mean levels of AMH and LH were characteristic of the HT/HA group and the lowest - of NT/NA group (p = 0.001). The mean levels of TT, A4, and FT in HT/HA group exceeded those in other androgenic groups (p = 0.001). The mean SHBG levels in HT/HA group (49.1 (37.4) nmol/L) were lower than in the NT/NA group (57.1 (29.6) nmol / L, p = 0.001). No differences in SHBG levels were found among other androgenic groups (p = 0.19). The data presented in the table also indicate higher levels of fasting glucose and insulin in the HT/ HA group than in the NT/ NA group (p = 0.03 and p = 0.01, respectively). There were no statistically significant differences between other groups with HA (p = 0.65).
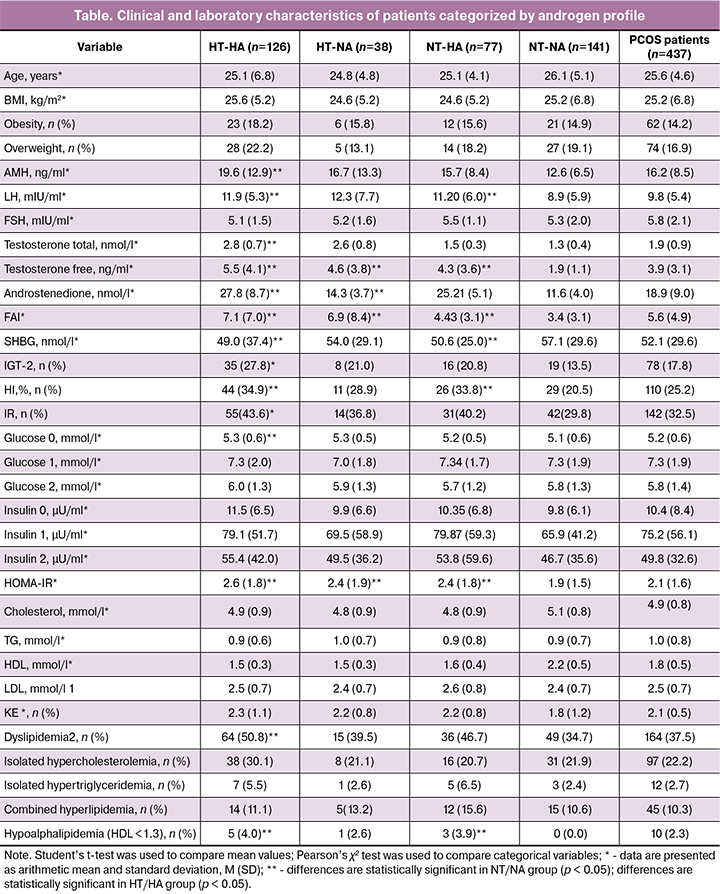
The rate of IGT (detected by fasting glucose and GTT) in the HT/HA group (27.8%) was statistically significantly higher than that in the NT/NA group (13.5%, p = 0.01), but did not significantly differ from its values in NT/HA and HT/NA groups (p = 0.49). In HT/HA and NT/HA groups, the HI rates were comparable (34.9% and 33.8%, respectively) and significantly higher than that in NT/NA group (p = 0.001 and p = 0.01, respectively). The mean NOMA-IR index in all groups with HA did not statistically significantly differ (p = 0.52) but was higher than in the NT/NA group (for the HT/HA group, p = 0.001, for HT/NA and NT/HA, p = 0.01).
There were no differences between groups in the levels of cholesterol, LDL, and TG (p> 0.05). The level of anti-atherogenic HDL in the NT/NA group was higher than in all androgenic groups (p = 0.03). The highest KE was in HT/HA group and the lowest in NT/NA group (p = 0.01). Dyslipidemia was found in every second and every third patient in HT/HA and NT/NA group, respectively (p = 0.02).
These findings suggest that a comprehensive assessment of the androgen profile is advisable, including not only the levels of TT and FT but also A4, which is more important in the diagnosis of PCOS. Metabolic disorders are fairly common among patients with HA, especially when HA co-occurs with elevated levels of TT and A4. In overweight and obese patients, low levels of SHBG can be considered as a predictor of metabolic dysfunction with HI and IR.
Discussion
The diversity of clinical manifestations of PCOS and insufficient diagnostic accuracy of tests for androgens creates difficulties in the diagnosis of the syndrome. Diagnosis of HA in patients with PCOS requires measuring serum levels of TT, SHBG, FAI, or FT. However, serum FT measured by ELISA is believed to be of insufficient diagnostic accuracy, and the serum level of TT is difficult to measure due to its physiologically low concentrations in women compared with men. Some commercial kits not even show TT at the limit of detection of the assay. Current literature is lacking studies investigating advantages of determining A4 compared to TT in PCOS [4]. However, clinicians, as a rule, do not test PCOS patients for A4 due to the ambiguity of information regarding this test.
To optimize the diagnosis of HA, we investigated the androgen status of patients with PCOS and its association with metabolic dysfunction. It turned out that the traditional measurement of serum TT allows the detection of HA only in every 3rd patient, and the additional measurement of serum FT detects HA in every 4th patient with PCOS. Therefore, the measurement of both TT and FT increases the detection rate of HA to 47.6%. An increase in A4 level was diagnosed in almost every 2nd (46.5%) patient with PCOS. An isolated increase in A4 level was detected in 18.8% of patients, while an isolated increase in TT and FT was observed only in 8.7% and 5.2% of patients, respectively. According to some studies, HA is detected more often by TT than by A4 [10]. In the present study, about a third of patients had increased levels of both TT and A4 (HT/HA group), every 5th patient had an increased level of A4 and normal TT (NT/HA group), and only every 10th patient had an increased level of TT and normal level of A4 (HT/NA group). These findings suggest that the determination of serum A4 makes a significant contribution to the diagnosis of PCOS, allowing the detection of HA in every 5th patient with a normoandrogenic PCOS phenotype established by the level of TT and FT. According to some authors, the measurement of A4 identified HA in 30-60% of patients with PCOS, including about 10% of patients with phenotype D diagnosed by the level of TT [11]. Several other studies also reported a greater diagnostic value of A4 in comparison with TT in the diagnosis of HA, but they used liquid chromatography and tandem mass spectrometry. This study showed the benefits of a comprehensive assessment of androgen status, which resulted in an increase in the detection rate of HA from 37.4% (diagnosed only by TT) to 66.4%. This approach seems to be most appropriate for examining women with ovulatory dysfunction and polycystic ovarian morphology on USG in the absence of clinical HA.
PCOS is also considered as a reproductive metabolic syndrome associated with IR, IGT, and an increased risk of cardiovascular and gestational complications [13, 14]. The incidence rates and severity of metabolic disorders correlate with overweight and obesity. However, 30-40% of patients with PCOS, even with a normal BMI have impaired glucose metabolism. Incidence rates of IR and IGT in this category of patients are 5.7 and 3.4 times higher, respectively than among healthy subjects of similar age and BMI [13]. An increase in the incidence of IGT, IR, and compensatory HI in non-obese women with PCOS is largely associated with androgen excess. Animal studies have shown that androgen excess can lead to the formation of IR by inducing serine phosphorylation in insulin receptors (IRS), resulting in reduced tyrosine kinase activity and impaired insulin signaling [15, 16]. Most clinical trials have reported the association between HA and IR, while some others have not identified this relationship. This does not allow the prediction of cardiovascular and gestational complications of the syndrome. Despite the existing pathophysiological basis of the association between HA and IR, there is no consensus on the feasibility of predicting metabolic disorders based on the patient’s androgen profile. In the present study, IR, HI, and IGT were found to be associated with low serum SHBG levels, which was mainly observed in patients with lipid disorders. In this context, logic dictates that serum level of SHBG may be considered as a marker of IR and HI in overweight and obese patients.
Serum levels of TT and FT had no statistically significant correlations with concentrations of glucose, insulin, HOMA index, and rates of IGT, IR, HI, and dyslipidemia. These observations may be attributed to the relatively low rates of obesity and overweight and/or young age of the study participants, that is, those parameters that significantly contribute to the development of the metabolic syndrome. At the same time, the A4 level was found to correlate with insulin levels (detected by fasting glucose and GTT at 1 h) and the HOMA index. There is also research evidence on the association between an isolated increase in A4 level and its combination with an increased level of TT and the insulin sensitivity index (ISI) [17]. A more detailed analysis showed that patients in the HT/HA group had a higher level of fasting glucose and insulin, a higher rate of IR and HI than patients in the NT/NA group. The study findings indicate the highest metabolic risks in women with combined HA in terms of TT and A4. So, the rate of IGT in this group was twice higher, and the rates of IR and dyslipidemia were 1.5 times higher than those of patients with normal androgen profiles. The rates of glucose and lipid metabolism disorders in the NT/HA and HT/NA groups were higher than in the NT/NA group; however, the differences did not reach statistical significance. Similar data were reported by O’Reilly, who found that HA detected by A4 or its combination with elevated TT was associated with increased the likelihood of developing IR and IGT.
Interestingly, this association was found not only in patients with HA but also in PCOS patients with normal androgen levels compared to healthy women [17]. In our study, the rate of IGT in the NT/NA group was 13.5%, which is higher than that in the Russian population (8.3%) reported by a multicenter epidemiological study (2007) [18]. These observations suggest that latent metabolic dysfunction occurs even in patients with a nonhyperandrogenic PCOS phenotype.
Conclusion
Based on the study results, it can be concluded that patients with suspected PCOS should be tested for biochemical HA by assessing a complete serum androgen profile, which provides almost a 2-fold higher diagnostic accuracy than the traditional measurement of serum TT. The greatest contribution to the diagnosis of HA is made by determining serum A4 concentrations, which are elevated in every second patient with PCOS. HA with co-elevated serum TT and A4 represents a useful predictor of metabolic risk in PCOS patients.
References
- Azziz R., Sanchez L.A., Knochenhauer E.S., Moran C., Lazenby J., Stephens K.C., Taylor K., Boots L.R. Androgen Excess in Women: Experience with Over 1000 Consecutive Patients. JCEM. 2004; 89(2): 453–462. https://doi.org/10.1210/jc.2003-031122
- The Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and longterm health risks related to polycystic ovary syndrome. Fertil Steril. 2004; 81(1):19-25. doi: 10.1016/j.fertnstert.2003.10.004
- Azziz R., Carmina E., Dewailly D., Diamanti-Kandarakis E., Escobar-Morreale H.F., Futterweit W., Janssen O.E., Legro R.S., Norman R.J., Taylor A.E., Witchel S.F.; Androgen Excess Society. Positions statement: criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an Androgen Excess Society guideline. J. Clin. Endocrinol. Metab. 2006; 91(11): 4237-45. DOI: 10.1210/jc.2006-0178
- Conway G., Dewailly D., Diamanti-Kandarakis E., Escobar-Morreale H.F., Franks S., Gambineri A., Kelestimur F., Macut D., Micic D., Pasquali R., Pfeifer M., Pignatelli D., Pugeat M., Yildiz B.; ESE PCOS Special Interest Group. The polycystic ovary syndrome: a position statement from the European Society of Endocrinology. Eur. J. Endocrinol. 2014; 171(4): P1-29. doi: 10.1530/EJE-14-0253
- Azziz R., Carmina E., Dewailly D., Diamanti-Kandarakis E., Escobar-Morreale H.F., Futterweit W., Janssen O.E., Legro R.S., Norman R.J., Taylor A.E., et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009; 91:456–88. doi:10.1016/j.fertnstert.2008.06.035)
- Teede H.J., Misso M.L., Costello M.F., Dokras A., Laven J., Moran L., Piltonen T., Norman R.J. International PCOS Network. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum Reprod. 2018; 33(9): 1602–18. doi: 10.1093/humrep/dey256
- Jones H., Sprung V.S., Pugh C.J., Daousi C, Irwin A., Aziz N., Adams V.L., Thomas E.L., Bell J.D., Kemp G.J., Cuthbertson D.J. Polycystic ovary syndrome with hyperandrogenism is characterized by an increased risk of hepatic steatosis compared to nonhyperandrogenic pcos phenotypes and healthy controls, independent of obesity and insulin resistance. J Clin Endocrinol Metab. 2012; 97(10): 3709–16. doi:10.1210/jc.2012-1382
- Чернуха Г.Е., Блинова И.В., Купрашвили М.И. Эндокринно-метаболические характеристики больных с различными фенотипами синдрома поликистозных яичников. Акушерство и гинекология. 2011; 2: 70-76.[Chernukha G.E., Blinova I.V., Kuprashvili M.I. Endocrine and metabolic characteristics of patients with different phenotypes of polycystic ovary syndrome. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2011; 2: 70-76.(in Russian)].
- Cakir E1, Doğan M, Topaloglu O, Ozbek M, Cakal E, Vural MG, Yeter E, Delibasi T. Subclinical atherosclerosis and hyperandrogenemia are independent risk factors for increased epicardial fat thickness in patients with PCOS and idiopathic hirsutism. Atherosclerosis. 2013; 226: 291–295. doi: 10.1016/j.atherosclerosis.2012.11.004.
- Lerchbaum E., Schwetz V., Rabe T., Giuliani A., Obermayer-Pietsch B. Hyperandrogenemia in Polycystic Ovary Syndrome: Exploration of the Role of Free Testosterone and Androstenedione in Metabolic Phenotype. PLoS One. 2014; 9(10): e108263. doi: 10.1371/journal.pone.0108263
- Georgopoulos N.A., Papadakis E., Armeni A.K., Katsikis I., Roupas N.D., Panidis D. Elevated serum androstenedione is associated with a more severe phenotype in women with polycystic ovary syndrome (PCOS). Hormones (Athens). 2014; 13(2): 213-21. DOI: 10.1007/BF03401335
- Ruan X., Li M., Mueck A.O. Why does Polycystic Ovary Syndrome (PCOS) Need Long-term Management? Curr Pharm Des. 2018; 24(39): 4685-92. doi: 10.2174/1381612825666190130104922
- Zhu S., Zhang B., Jiang X., Li Z, Zhao S., Cui L., Chen Z.J. Metabolic disturbances in non-obese women with polycystic ovary syndrome: a systematic review and meta-analysis. Fertil Steril. 2019;111(1):168-177. doi: 10.1016/j.fertnstert.2018.09.013
- Ананьев Е.В. Синдром поликистозных яичников и беременность. Акушерство и гинекология. 2017; 9: 5-11.[Ananyev E.V. Polycystic ovary syndrome and pregnancy.Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2017; (9): 5–11. (in Russian)]. http://dx.doi.org/10.18565/aig.2017.9.5-11
- Ciaraldi T.P., Aroda V., Mudaliar S., Chang R.J., Henry R.R. Polycystic ovary syndrome is associated with tissue-specific differences in insulin resistance. J. Clin. Endocrinol. Metab. 2009; 94(1):157–63. DOI: 10.1210/jc.2008-1492
- Højlund K., Glintborg D. Andersen N.R., Birk J.B., Treebak J.T., Frøsig C., Beck-Nielsen H., Wojtaszewski J.F. Impaired insulin-stimulated phosphorylation of Akt and AS160 in skeletal muscle of women with polycystic ovary syndrome is reversed by pioglitazone treatment. Diabetes. 2008; 57(2):357–66. DOI: 10.2337/db07-0706
- O’Reilly M.W., Taylor A.E., Crabtree N.J., Hughes B.A., Capper F., Crowley R.K., Stewart P.M., Tomlinson J.W., Arlt W. Hyperandrogenemia Predicts Metabolic Phenotype in Polycystic Ovary Syndrome: The Utility of Serum Androstenedione. J Clin Endocrinol Metab. 2014; 99(3): 1027–36. doi: 10.1210/jc.2013-3399
- Мамедов М.Н., Поддубская Е.А. Диагностика и лечение ранних нарушений углеводного обмена в общетерапевтической практике (методические рекомендации). ФГУ ГНИ ЦПМ. М.; 2011. 36 с. [Mamedov M.N., Poddubskaya E.A. Diagnostika i lechenie rannix narushenij uglevodnogo obmena v obshheterapevticheskoj praktike (metodicheskie rekomendaczii). FGU GNI CzPM. M.; 2011. 36 s.(In Russian)].
Received 15.04.2019
Accepted 19.04.2019
About the Authors
Galina E. Chernukha, MD, professor, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. KulakovMinistry of Health of Russia. 117997, Russia, Moscow, Ac. Oparina str. 4. Tel.: +79859996000. E-mail: c-galina1@yandex.ru ORCID ID 0000-0002-9065-5689
Alina A. Naydukova, postgraduate student, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov
Ministry of Health of Russia. 117997, Russia, Moscow, Ac. Oparina str. 4. Tel.: +7(916)6750097. E-mail: aleeshka@mail.ru
Maria A., Udovichenko postgraduate student, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov
Ministry of Health of Russia. 117997, Russia, Moscow, Ac. Oparina str. 4. Tel.: +7 (925)4802955. E-mail: mariia911@yahoo.com
Elena K. Kaprina postgraduate student, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov
Ministry of Health of Russia. 117997, Russia, Moscow, Ac. Oparina str. 4. Tel.: +7(916)1294118. E-mail: kaprina_elena@mail.ru
Tatyana Y., MD, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Health of Russia.
117997, Russia, Moscow, Ac. Oparina str. 4. Tel.: +7(910)4042669. E-mail t_ivanets@oparina4.ru
For citation: Chernukha G.E., Naidukova A.A., Udovichenko M.A., Kaprina E.K., Ivanets T.Yu. Androgen profile in patients with polycystic ovary syndrome and its association with metabolic dysfunction. Akusherstvo i Ginekologiya/ Obstetrics and gynecology. 2019; 11: 122-28.(In Russian).
https://dx.doi.org/10.18565/aig.2019.11.122-128



